Abstract
The neuropeptide oxytocin has recently been shown to modulate covert attention shifts to emotional face cues and to improve discrimination of masked facial emotions. These results suggest that oxytocin modulates facial emotion processing at early perceptual stages prior to full evaluation of the emotional expression. Here, we used functional magnetic resonance imaging to examine whether oxytocin alters neural responses to backwardly masked angry and happy faces while controlling for attention to the eye vs the mouth region. Intranasal oxytocin administration reduced amygdala reactivity to masked emotions when attending to salient facial features, ie, the eyes of angry faces and the mouth of happy faces. In addition, oxytocin decreased neural responses within the fusiform gyrus and brain stem areas, as well as functional coupling between the amygdala and the fusiform gyrus specifically for threat cues from the eyes. Effects of oxytocin on brain activity were not attributable to differences in behavioral performance, as oxytocin had no impact on mere emotion detection. Our results suggest that oxytocin attenuates neural correlates of early arousal by threat signals from the eye region. As reduced threat sensitivity may increase the likelihood of engaging in social interactions, our findings may have important implications for clinical states of social anxiety.
INTRODUCTION
Understanding emotional cues from facial expressions allows for inferences on biologically relevant events within the environment (eg, potential threat) and is fundamental to human social interactions. Within the last years, intranasal administration of the neuropeptide oxytocin was found to affect social behavior and cognition (Heinrichs et al, 2009; Meyer-Lindenberg et al, 2011). Most notably, oxytocin enhances facial emotion recognition in healthy individuals (for a recent meta-analysis, see Shahrestani et al, 2013), as well as in disorders characterized by impairments in social cognition such as autism (Domes et al, 2014) or developmental prosopagnosia (Bate et al, 2014). Preliminary results from imaging studies indicate that oxytocin alters signaling within several parts of the emotional brain (for recent reviews, see Bethlehem et al, 2013 and Kanat et al, 2013). In particular, oxytocin affects amygdala-dependent social information processing (Domes et al, 2013a, 2014; Hurlemann et al, 2010), and attenuates amygdala reactivity to emotional cues in men (Domes et al, 2007; Kirsch et al, 2005; Petrovic et al, 2008).
In addition, oxytocin modulates visual attention to signals conveyed by the eyes. For example, intranasal oxytocin enhances attentional orienting based on emotional cues from the eyes (Tollenaar et al, 2013) and increases fixations to the eye region via modulatory influences on the amygdala (Gamer et al, 2010). Impaired use of information from the eye region was reported for patients with amygdala lesions (Adolphs et al, 2005), as well as for clinical conditions characterized by social deficits, such as autism (Kliemann et al, 2010) or anxiety (Horley et al, 2003). Owing to its beneficial effects on social cognition and behavior, intranasal oxytocin administration has been discussed as a therapeutic adjunct in the treatment of these conditions (Heinrichs and Domes, 2008; Meyer-Lindenberg et al, 2011).
Preliminary evidence suggests that oxytocin may influence facial emotion processing at early perceptual stages prior to full stimulus evaluation. For example, intranasal oxytocin enhances covert attention to happy faces (Domes et al, 2013b) and facilitates attentional disengagement from masked angry faces (Ellenbogen et al, 2012). Furthermore, oxytocin improves recognition of dynamic facial expressions at lower intensity levels (Lischke et al, 2012; Prehn et al, 2013), increases sensitivity to implicit and unattended emotional face cues (Leknes et al, 2013), and enhances discrimination of masked facial emotions (Schulze et al, 2011). Together, these results suggest that oxytocin influences facial emotion processing at very early stages of stimulus perception.
The neural mechanisms underlying these early oxytocin effects on facial emotion processing have not yet been investigated. However, effects of intranasal oxytocin on both reflexive eye gazing as well as emotion decoding from facial expressions seem associated with altered amygdala reactivity (Bertsch et al, 2013; Gamer et al, 2010). The amygdala responds to subliminal emotional signals and is thought to be part of an alarm or vigilance system that rapidly evaluates the biological significance of a stimulus (Adolphs, 2008; Liddell et al, 2005; Pessoa and Adolphs, 2010; Tamietto and de Gelder, 2010). For example, masked fearful faces evoke amygdala responses in the absence of visual awareness (Liddell et al, 2005; Whalen et al, 1998). Therefore, the amygdala represents a main target site for oxytocin effects at initial stages of emotion perception.
Against this background, we hypothesized that oxytocin modulates brain activity within circuits for rapid emotion processing, particularly the amygdala and associated visual areas such as the fusiform gyrus, under conditions of limited visual awareness. We tested these predictions using a backward-masking task with positive (happy) and negative (angry) facial emotions while assessing regional brain responses by means of functional MRI in healthy men. Two different presentation conditions were used, both covering early stages along the continuum of visual awareness. In order to disentangle amygdala-based effects of oxytocin on facial emotion processing and eye gazing behavior (Gamer et al, 2010), we controlled for attention to the eye or the mouth region, respectively. As oxytocin was found to reduce attentional orienting to angry eyes (Bertsch et al, 2013; Domes et al, 2013c) and to decrease amygdala reactivity to social threat in men (Kirsch et al, 2005; Petrovic et al, 2008), we expected oxytocin to preferentially reduce amygdala responses for masked cues of threat from the eye region.
MATERIALS AND METHODS
Participants and Experimental Procedure
A total of 49 healthy men with a mean age of 23.64 years (range: 20–31; SD=2.81) were recruited via on-campus announcements at the University of Freiburg. All subjects were right-handed and had normal or corrected-to-normal vision. Subjects were excluded from participation if they reported any current medical illnesses, were positively screened for current or past psychiatric disorders by the Structured Clinical Interview for DSM-IV (Wittchen et al, 1997), smoking, regular medication, drug intake, or MRI contraindications. All participants gave their written informed consent prior to participation and were instructed to abstain from caffeine or alcohol intake on the day of testing.
On the testing day, participants were randomly assigned to receive a single dose of intranasal oxytocin or a placebo in a double-blind manner. Under supervision of the investigator, participants self-administered three puffs of oxytocin (Syntocinon-Spray, Novartis, Switzerland; each puff with 4 IU oxytocin; total dose of 24 IU oxytocin) or placebo spray (containing all ingredients of the verum except for the peptide) to each nostril. Potential substance effects on mood, wakefulness, and calmness were assessed via a multidimensional questionnaire (Steyer et al, 1997) immediately before nasal spray inhalation and before MRI scanning. Functional imaging sessions and the facial emotion detection task started approximately 45 min after substance administration. In total, the experiment lasted approximately 2 h, with 45 min spent on MRI scanning. The study protocol was in accordance with the Declaration of Helsinki and was approved by the local ethics commission.
Facial Emotion Detection Task
Photographs of ten individuals (five male, five female) displaying an angry, happy, or neutral expression were selected from the NimStim Face Stimulus Set (Tottenham et al, 2009). To avoid any perceptual advantage induced by the visibility of teeth (particularly in the happy face condition), all facial stimuli had closed mouths. The hair and neck region of each face was digitally removed and pictures were converted to gray scale. Stimulus presentation and assessment of button presses were controlled by Presentation 14.0 (Neurobehavioral Systems, Albany, CA). Pictures were projected on a screen that was rendered visible via a head-mounted mirror. The experimental task consisted of four runs, two of which were designed to measure detection of angry or happy faces, respectively. Runs were balanced in order and lasted 7 min each. At the beginning of each run, the target emotion was announced, followed by 80 trials with a temporal interval jittered between 1000 and 2000 ms.
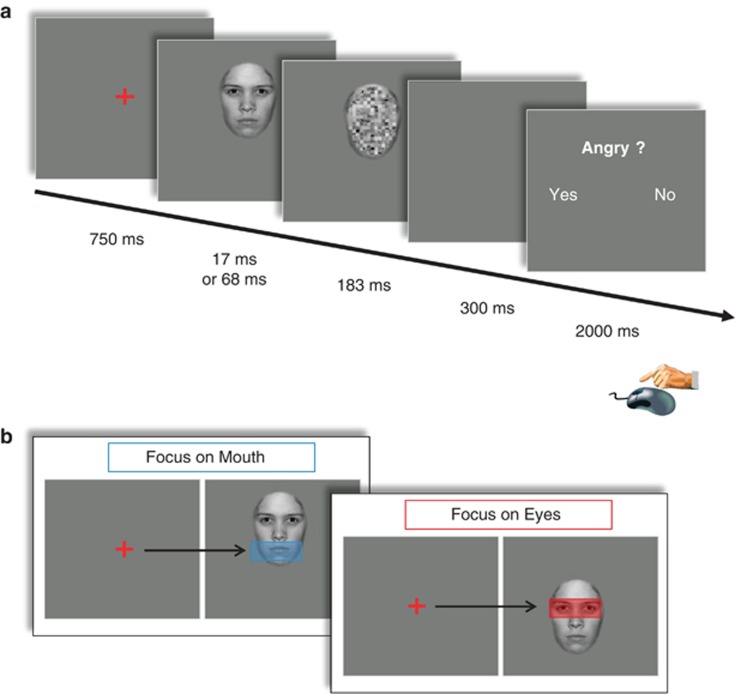
Following a fixation cross (750 ms), a target stimulus was displayed that either depicted the block-specific target emotion (angry or happy) or a neutral expression (Figure 1a). To control for the initial fixation, half of the target stimuli were unpredictably shifted up or downwards so that either the eye or the mouth region appeared at the position of preceding fixation cross (Figure 1b). Visual awareness for the masked faces was manipulated by using a 17 or 68 ms stimulus duration in half of the trials. The target stimulus was immediately followed by a masking stimulus (183 ms) that consisted of a scrambled version of the individual target stimulus. This masking procedure was chosen instead of the more common technique of a neutral face mask in order to avoid confounding effects of oxytocin administration on processing of the masking stimulus. Subsequent to each target-mask pair, participants indicated the presence of the target emotion by pressing one of two buttons within a 2-s time window.
Figure 1.
(a) Structure of experimental trials: both within anger and happiness detection runs, each trial started with a fixation cross, followed by the target face stimulus (emotional or neutral), and a masking stimulus (scrambled face). Participants then indicated the presence/absence of the target emotion via button press. Duration of target stimulus exposure was either set to 17 or 68 ms. (b) Experimental manipulation of initial fixation: following display of a fixation cross, the target stimulus was shifted upwards or downwards, thereby setting the focus of attention on the mouth or eye region, respectively.
Assessment and Analysis of Behavioral Data
Detection indices were calculated following signal detection theory by subtracting standardized values of hit and false alarms rates for each condition and subject [d′=Zhits−Zfalse alarms]. These indices were subjected to a mixed model ANOVA with substance (oxytocin, placebo) as a group factor, and emotion (angry, happy), stimulus duration (17, 68 ms), and attentional focus (eyes, mouth) as within-subject factors. In case of nonsphericity of covariances, Greenhouse–Geisser corrections were applied. Significant interactions were further analyzed using Student's t-tests. Analyses were performed using IBM SPSS Statistics version 21 at a significance level of p<0.05.
Assessment and Analysis of Imaging Data
Imaging data were collected on a 3-T Siemens Trio scanner at the University Medical Center, Freiburg. Motion and distortion artifacts were automatically eliminated during scanning using in-house algorithms based on point spread functions (Zaitsev et al, 2004). In addition, an inflatable cap was used to stabilize the head, and participants were instructed not to move during scanning. After positioning in the scanner, a total of 604 functional images (151 per run), each comprising 42 axial slices were assessed by means of an EPI sequence (voxel size: 3 × 3 × 3 mm3, TR=2620 ms, TE=30 ms, FA=90°, FOV=192 × 192 mm, matrix=64 × 64, interleaved acquisition, no gap). After the experiment, a single T1-weighted structural image was obtained using a MPRAGE sequence (voxel size: 1 × 1 × 1 mm3, TR=2200 ms, TE=4.11 ms, FA=12°, FOV=256 × 256 mm, Matrix=256 × 256, 160 slices).
Data preprocessing and analyses were performed in SPM8 (http://www.fil.ion.ucl.ac.uk/spm). The first five functional volumes were discarded to circumvent potential saturation effects. For data preprocessing, functional images were spatially realigned and co-registered with the structural image. Images were then normalized to a standard brain template in MNI space on the basis of tissue segmentation parameters. Finally, normalized images were spatially smoothed with a Gaussian kernel of 8 mm full-width at half maximum. On the single subject level, the onset of each trial was convolved with a canonical hemodynamic response function and experimental conditions were modeled within a general-linear model. Each run was modeled separately with session-specific movement parameters from spatial realignment to account for any residual movement artifacts. Slow frequency temporal drifts were removed from BOLD time series by means of a high-pass filter with a cut-off period of 128 s.
ROI-Analysis of Amygdala Activity
On the group level, region-of-interest (ROI) analyses within the left and right amygdala were conducted based on anatomical masks from the WFU PickAtlas (Tzourio-Mazoyer et al, 2002). We extracted the mean percent signal change within these masks for each experimental condition using rfxplot (http://rfxplot.sourceforge.net; Gläscher, 2009). The percent signal change induced by emotional as compared to neutral faces was then compared by calculating a difference score for each individual and each condition. Significant interactions were further explored by means of pairwise testing for independent samples.
Whole Brain Analysis
To account for additional oxytocin effects beyond the amygdala, we performed exploratory whole brain analyses at an uncorrected significance threshold of p<0.001, and a minimum cluster size of 10 voxels (critical T-value of 3.14; voxel-wise testing). In detail, contrast images reflecting individual reactivity to emotional as compared with neutral faces derived from single subject analyses were entered into two separate factorial models (one for angry and one for happy faces) with the factors substance, stimulus duration, and attentional focus.
Psychophysiological Interaction Analysis
The whole brain analyses revealed a region within the left fusiform gyrus whose activity was significantly modulated by oxytocin administration, specifically during processing eyes of the masked angry faces. Based on this finding, we conducted a psychophysiological interaction (PPI) analysis as implemented in SPM (Friston et al, 1997) that tested for oxytocin-induced modulations of left amygdala connectivity with the left fusiform gyrus when processing angry as compared with neutral eyes (for details on the analysis, see Supplementary Results). For all analyses, coordinates are reported in MNI space (Evans et al, 1993).
RESULTS
Functional imaging data of three participants were discarded due to severe motion artifacts (oxytocin: n=1, placebo: n=1), or anatomical abnormalities (placebo: n=1). Among the remaining participants, behavioral data of three individuals were missing due to technical problems (oxytocin: n=2, placebo: n=1) leaving a total sample of 46 subjects for fMRI analyses and 43 subjects for behavioral analyses. Administration of oxytocin did not differentially influence changes in subjectively rated alertness (F1,43=1.004, p=0.322), mood (F1,43=0.454, p=0.504), or calmness (F1,43=0.053, p=0.818) within these subjects.
ROI Analyses on Amygdala Reactivity
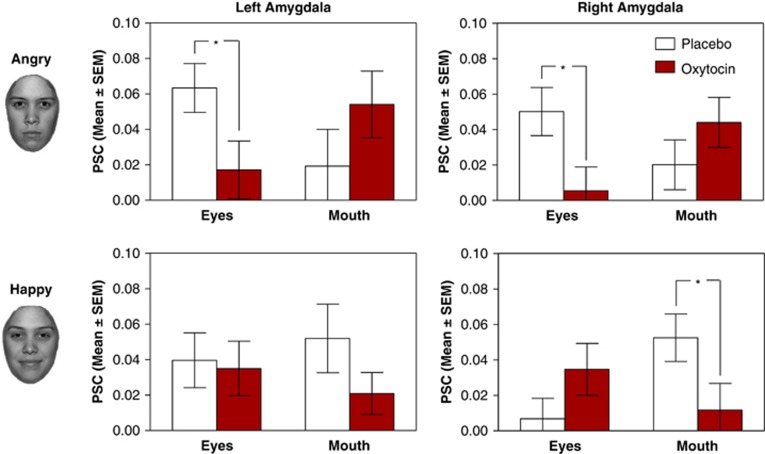
Initial analyses of variance for both facial emotions revealed that bilateral amygdala responses were generally enhanced for emotional as compared with neutral faces irrespective of substance group (see Supplementary Table S1). This effect was observed for both angry (left amygdala: F1,44=21.663, p<0.001; right amygdala: F1,44=15.922, p<0.001), and happy expressions (left amygdala: F1,44=25.277, p<0.001; right amygdala: F1,44=16.395, p<0.001). Difference scores between masked emotional and neutral faces were calculated as a measure of emotional reactivity by subtracting the mean values from the anatomical ROI for each subject and condition (eg, angry eyes 68 ms—neutral eyes 68 ms). These difference scores were then entered into a subsequent analysis, which yielded a single significant effect, namely an interaction of attentional focus, emotional valence, and substance group in both the left and right amygdala (left: F1,44=4.661, p=0.036; right: F1,44=12.944, p=0.001; Figure 2 and Supplementary Table S2): Oxytocin potently reduced left and right amygdala responses when subjects attended to the eye region of masked angry faces. Conversely, oxytocin dampened amygdala reactivity when participants focused on the mouth as compared to the eye region of masked happy faces. This interaction did not depend on visual awareness as indicated by the absence of a significant four-way interaction with stimulus duration. However, exploratory analyses within the two stimulus conditions revealed that the interaction was stronger in the short stimulus condition, whereas not reaching significance in the long stimulus condition (17 ms: F1,44=4.161, p=0.047; 68 ms: F1,44=1.044, p=0.312—see Supplementary Figures 1 and 2).
Figure 2.
Reactivity of the left and the right amygdala to masked angry (upper graphs) and happy (lower graphs) faces. Bar graphs depict the percent signal change within the left and right amygdala ROI as a function of emotional valence, attentional focus, and drug condition. For angry faces, oxytocin reduced amygdala responses when attending to the eye region. For happy faces, oxytocin decreased amygdala responses when attending to the mouth region. Error bars represent the SEM.
Whole Brain Analyses
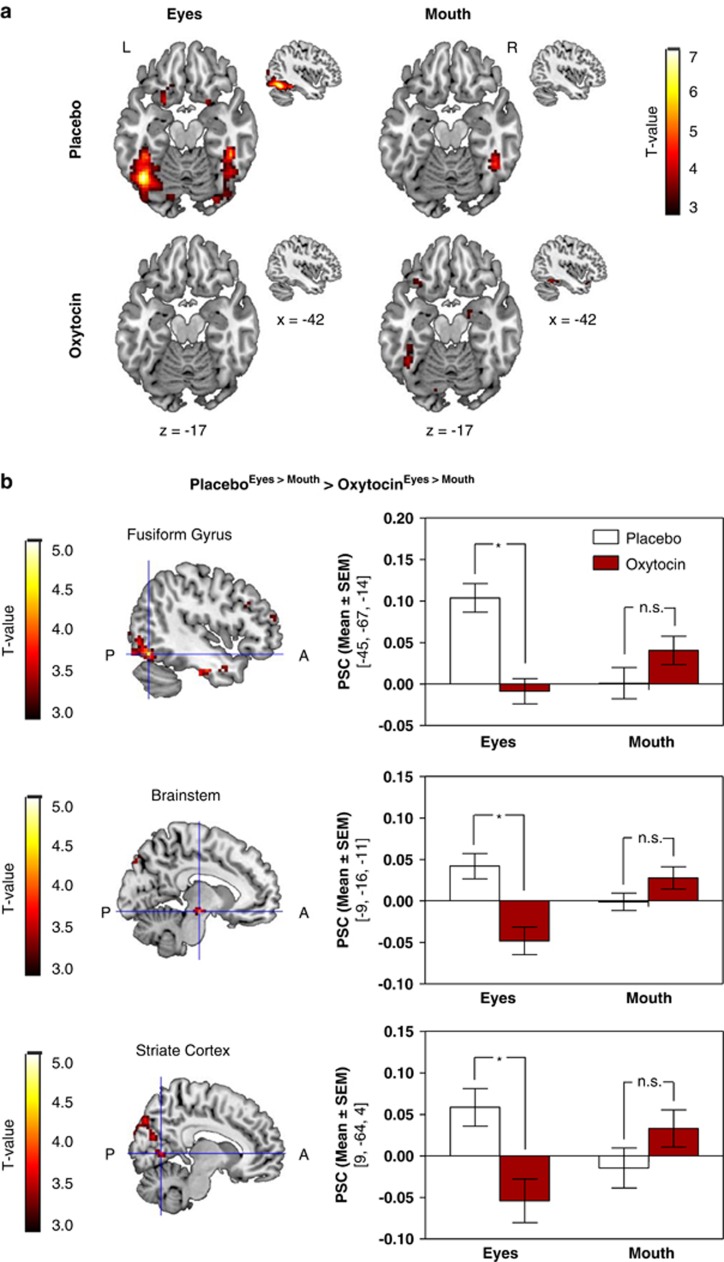
Using whole brain analysis, we first focused on neural responses to angry as compared with neutral faces. Under placebo conditions, attending to the eyes of masked angry faces evoked strong responses within the left fusiform gyrus (x, y, and z: −42,−61,−17; Z=6.76; k=1108; puncorr<0.001) as well as in right tempo-occipital cortex regions (x, y, and z: 45,−40,−17; Z=5.41; k=633; puncorr<0.001). Within the left fusiform gyrus, this enhanced reactivity to anger cues from the eye region was significantly reduced after oxytocin administration (x, y, and z: −42,−82,−14; Z=4.69; k=330; puncorr<0.001). Similar modulations by oxytocin were observed within brain stem regions and the striate cortex. A comprehensive list of activated clusters is provided in Supplementary Table S3.
We then tested for differential oxytocin effects on brain responses to threat cues from the eye as compared with the mouth region. This analysis confirmed that oxytocin administration specifically reduced the response of the fusiform gyrus to anger cues from the eye region (x, y, and z: −45,−67,−14; Z=4.48; k=315; puncorr<0.001; Figure 3). Similarly, intranasal oxytocin attenuated reactivity to masked threat from the eyes as compared to the mouth within brain stem regions (x, y, and z: −9,−16,−11; Z=3.92; k=13; puncorr<0.001), and the striate cortex (x, y, and z: +9, −64, +4; Z=3.65; k=23; puncorr<0.001). Additional clusters emerged within the right fusiform gyrus, the left inferior temporal cortex, and the medial orbitofrontal cortex (see Supplementary Table S4). Notably, oxytocin administration did not reduce activity within visual cortex and brain stem areas when attending to the eyes of happy faces, suggesting that the modulation was specific for masked cues of threat (see Supplementary Tables S5 and S6). However, oxytocin administration decreased responses to the mouth region of happy faces within the medial superior frontal gyrus.
Figure 3.
Oxytocin effects on brain activity when attending to the eye as compared to the mouth region of masked angry faces. (a) Attending to the eye region strongly increased activity within the left fusiform gyrus under placebo, but not following oxytocin administration. A similar pattern was absent when the focus of attention was set on the mouth region. (b) Oxytocin reduced BOLD reactivity to threat cues from the eyes within the left fusiform gyrus (x, y, and z: −45,−67,−14; Z=4.48; k=315), brain stem regions (x, y, and z: −9,−16,−11; Z=3.92; k=13), and the striate cortex (x, y, and z: 9,−64,4; Z=3.65; k=23). Statistical parametric maps are displayed with a threshold of puncorr<0.001. Right-sided panels indicate the percent signal change within peak voxels of significant clusters. Error bars represent the SEM.
To explore the modulatory influences of oxytocin on threat processing under different conditions of visual awareness, we subsequently performed separate analyses for the short and long stimulus condition (see Supplementary Tables S7 and S8). Whereas the oxytocin effect within the left fusiform gyrus was present for both stimulus durations, the cluster of significantly activated voxels was located more anterior in the long stimulus condition. For the short stimulus condition, oxytocin additionally attenuated reactivity to the eyes of masked angry faces within several visual cortex areas, comprising inferior occipital regions and temporal parts of the ventral stream, as well as within brain stem regions. Under conditions of longer stimulus exposure, this effect was observed within the mid-temporal gyrus, the superior colliculi, and the striate cortex.
PPI Analysis
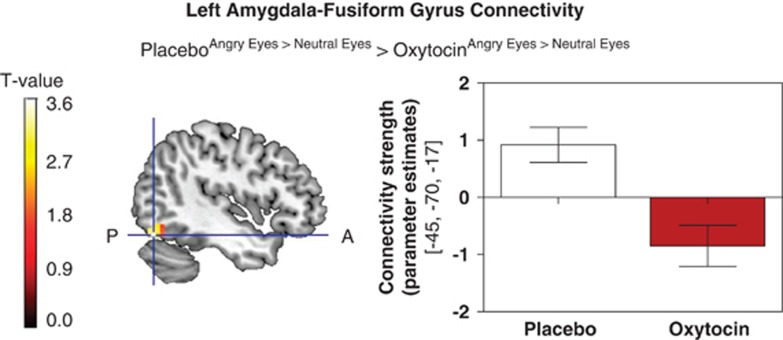
Based on the analogous effects of oxytocin within the amygdala and the fusiform gyrus when attending to the eye region of masked angry faces, we next tested for oxytocin effects on the functional coupling between both regions. Under placebo, attending to the eyes of angry as compared with neutral faces significantly increased connectivity of the left amygdala with the left fusiform gyrus. This effect was completely reversed under oxytocin as mirrored by a negative connectivity between these regions (x, y, and z: −45,−70,−17; Z=3.46; pSVC=0.007—Figure 4). Hence, the threat-induced increase in functional coupling between the amygdala and the fusiform gyrus observed under placebo was significantly reduced after oxytocin administration.
Figure 4.
Oxytocin treatment significantly reduced functional connectivity between the left amygdala and the left fusiform gyrus when attending to the eyes of angry as compared with neutral faces (x, y, and z: −45,−70,−17, pSVC=0.007, Z=3.74, k=28). Error bars represent the SEM.
Emotion Detection Performance and Response Latencies
Analyses of variance on behavioral measures revealed that oxytocin administration had no overall effect on emotion detection performance (d′ score) and response latencies (for detaile statistical results, see Supplementary Results and Supplementary Tables S9–S12).
DISCUSSION
We showed that intranasally administered oxytocin modulates neural responses to masked emotional cues within the amygdala, visual cortex areas, and brain stem regions based on the focus of attention. Under placebo, emotional cues elicited amygdala responses even when visual awareness for these cues were largely restricted by means of backward-masking (Liddell et al, 2005; Whalen et al, 1998). In addition, amygdala activation was modulated by attention to relevant facial features with increased reactivity to anger cues when attending to the eyes, and increased reactivity to happy faces when attending to the mouth. As the eyes are known as the most relevant feature for facial threat detection, whereas the mouth represents the most ‘diagnostic' feature for happy faces (eg, Eisenbarth and Alpers, 2011; Scheller et al, 2012; Smith et al, 2005), our results support the assumption that the amygdala rapidly encodes the biological relevance of emotional stimuli (Pessoa and Adolphs, 2010). A single dose of intranasal oxytocin reduced amygdala responses when attending to these salient facial features for masked presentations of angry eyes and happy mouths. This dampening effect might reflect a very early modulation of the neural representation of emotional salience.
Our results seem to contradict the positive effects of oxytocin on social salience processing as reported by previous studies (Gamer et al, 2010; Gordon et al, 2013). However, these differences might arise from differences in the experimental paradigms and the populations under study. For example, Gordon et al (2013) investigated oxytocin effects on neural reactivity to social and nonsocial stimuli in autistic children, whereas we tested for effects on emotional reactivity in healthy men. Gamer et al (2010) focused on a modulation of amygdala-based attention shifts to the eye region by oxytocin using relatively long stimulus presentations. Contrary to both studies, our study targeted earlier stages of emotion processing using very brief and masked stimulus presentations that rendered shifts of visual attention highly improbable. Therefore, our results extend previous studies by showing that oxytocin effects on emotional reactivity of the amygdala arise at very early perceptual stages, and critically depend on initial attention to specific facial features. Whereas exploratory analyses revealed a significant decrease in amygdala reactivity to masked presentation of angry eyes and happy mouths under oxytocin in the short presentation condition only, there was no interaction between substance administration and stimulus duration. Therefore, the seeming discrepancy between both presentation conditions likely represents a phenomenon of statistical threshold setting leading to a significant result in only the short presentation condition even though the effect was clearly observed in the long presentation condition as well.
Besides these effects within the amygdala, oxytocin administration reduced activation within visual cortex and brain stem areas as well as functional coupling between the left amygdala and the left fusiform gyrus for masked threat from the eye region. Whereas the brain stem is assumed as part of an alert system that rapidly detects subliminal cues of threat and initiates adequate motoric responses, such as fight/flight behavior (Liddell et al, 2005), functional connectivity between the amygdala and the fusiform gyrus shows associations with perceived threat from facial expressions (Miyahara et al, 2013). Our findings therefore suggest that oxytocin may reduce social threat perception from the eye region under conditions of limited awareness. This interpretation is in line with previous evidence for oxytocin effects on social threat processing under unrestricted viewing conditions. For example, oxytocin was found to decrease activity within the amygdala and the fusiform gyrus for aversively conditioned faces (Petrovic et al, 2008), and to reduce amygdala connectivity with brain stem regions (Kirsch et al, 2005) as well as self-perceived arousal for social as compared with nonsocial threat (Norman et al, 2011). A decrease in early arousal by threat signals from the eyes may also promote attentional neglect of these cues. For example, a recent study showed that the enhancing effect of oxytocin on eye gazing behavior vanishes if faces gradually adopt an angry expression (Domes et al, 2013c).
Notably, as oxytocin administration had no influence on actual detection of masked emotions, its effects on brain activity were not attributable to differences in performance, ie, target detection. In a previous study, oxytocin administration enhanced recognition of masked emotional faces in a backward-masking task (Schulze et al, 2011). In contrast, we had assessed mere emotion detection which referred to vigilance for a predefined emotional cue and did not require discrimination between emotional expressions. Therefore, behavioral performance reflected visibility of salient emotional signals rather than emotion recognition, which are thought to be associated with different stages of emotion perception (Liddell et al, 2004; Streit et al, 2003). This is also in accordance with results from a visual search task showing that intranasal oxytocin does not affect mere detection of angry and happy faces (Guastella et al, 2009). Importantly, our results do not allow for inferences on oxytocin effects in the context of unconscious emotion processing. Instead of presenting stimuli in complete absence of visual awareness, we used a dimensional approach using two different presentation conditions along the awareness continuum. Future studies should test whether oxytocin modulates neural correlates of facial emotion perception during unconscious stimulus processing, eg, by using individual detection thresholds. In this context, it seems important to address the question of whether oxytocin may alter individual detection thresholds themselves.
To our knowledge, this is the first study demonstrating the influences of intranasal oxytocin on neural correlates of emotion processing at a very early perceptual stage. Our results suggest that oxytocin may attenuate early arousal by threat signals from the eye region, which may increase the likelihood of engaging in social interactions. Future studies should therefore investigate whether oxytocin may normalize threat sensitivity in clinical anxiety by modulating neural correlates of early threat processing. In addition, the causality within communication pathways between the amygdala, the ventral visual system, and the brain stem and its potential modulation by oxytocin should be explored at different stages of emotion perception. Together, these attempts may increase our understanding of the temporal dynamics underlying oxytocin effects on the cognitive processing of social signals.
FUNDING AND DISCLOSURE
This study was supported by grants from the German Research Foundation to G.D. and M.H. (DFG, Do1312/2-1) and to M.H. (DFG, He 5310/1-1). The authors declare no conflict of interest.
Acknowledgments
We thank Pia Hollerbach and Fabian Dvořák for assistance with data collection. In addition, we are thankful to Valentina Colonnello for valuable discussion on the manuscript.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Adolphs R. Fear, faces, and the human amygdala. Curr Opin Neurobiol. 2008;18:166–172. doi: 10.1016/j.conb.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R, Gosselin F, Buchanan TW, Tranel D, Schyns P, Damasio AR. A mechanism for impaired fear recognition after amygdala damage. Nature. 2005;433:68–72. doi: 10.1038/nature03086. [DOI] [PubMed] [Google Scholar]
- Bate S, Cook SJ, Duchaine B, Tree JJ, Burns EJ, Hodgson TL. Intranasal inhalation of oxytocin improves face processing in developmental prosopagnosia. Cortex. 2014;50:55–63. doi: 10.1016/j.cortex.2013.08.006. [DOI] [PubMed] [Google Scholar]
- Bertsch K, Gamer M, Schmidt B, Schmidinger I, Walther S, Kästel T, et al. Oxytocin and reduction of social threat hypersensitivity in women with borderline personality disorder. Am J Psychiatry. 2013;170:1169–1177. doi: 10.1176/appi.ajp.2013.13020263. [DOI] [PubMed] [Google Scholar]
- Bethlehem RAI, Honk J, van Auyeung B, Baron-Cohen S. Oxytocin, brain physiology, and functional connectivity: a review of intranasal oxytocin fMRI studies. Psychoneuroendocrinology. 2013;38:962–974. doi: 10.1016/j.psyneuen.2012.10.011. [DOI] [PubMed] [Google Scholar]
- Domes G, Heinrichs M, Gläscher J, Büchel C, Braus DF, Herpertz SC. Oxytocin attenuates amygdala responses to emotional faces regardless of valence. Biol Psychiatry. 2007;62:1187–1190. doi: 10.1016/j.biopsych.2007.03.025. [DOI] [PubMed] [Google Scholar]
- Domes G, Heinrichs M, Kumbier E, Grossmann A, Hauenstein K, Herpertz SC. Effects of intranasal oxytocin on the neural basis of face processing in autism spectrum disorder. Biol Psychiatry. 2013;74:164–171. doi: 10.1016/j.biopsych.2013.02.007. [DOI] [PubMed] [Google Scholar]
- Domes G, Kumbier E, Heinrichs M, Herpertz SC. Oxytocin promotes facial emotion recognition and amygdala reactivity in adults with Asperger syndrome. Neuropsychopharmacology. 2014;39:698–706. doi: 10.1038/npp.2013.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domes G, Sibold M, Schulze L, Lischke A, Herpertz SC, Heinrichs M. Intranasal oxytocin increases covert attention to positive social cues. Psychol Med. 2013;43:1747–1753. doi: 10.1017/S0033291712002565. [DOI] [PubMed] [Google Scholar]
- Domes G, Steiner A, Porges SW, Heinrichs M. Oxytocin differentially modulates eye gaze to naturalistic social signals of happiness and anger. Psychoneuroendocrinology. 2013c;38:1198–1202. doi: 10.1016/j.psyneuen.2012.10.002. [DOI] [PubMed] [Google Scholar]
- Eisenbarth H, Alpers GW. Happy mouth and sad eyes: scanning emotional facial expressions. Emotion. 2011;11:860–865. doi: 10.1037/a0022758. [DOI] [PubMed] [Google Scholar]
- Ellenbogen MA, Linnen AM, Grumet R, Cardoso C, Joober R. The acute effects of intranasal oxytocin on automatic and effortful attentional shifting to emotional faces. Psychophysiology. 2012;49:128–137. doi: 10.1111/j.1469-8986.2011.01278.x. [DOI] [PubMed] [Google Scholar]
- Evans AC, Collins DL, Mills SR, Brown ED, Kelly RL, Peters TM. 3D statistical neuroanatomical models from 305 MRI volumes. Nuclear Sci Symp Med Imaging Confc. 1993;3:1813–1817. [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. NeuroImage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Gamer M, Zurowski B, Buchel C. Different amygdala subregions mediate valence-related and attentional effects of oxytocin in humans. Proc Natl Acad Sci USA. 2010;107:9400–9405. doi: 10.1073/pnas.1000985107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gläscher J. Visualization of group inference data in functional neuroimaging. Neuroinformatics. 2009;7:73–82. doi: 10.1007/s12021-008-9042-x. [DOI] [PubMed] [Google Scholar]
- Gordon I, Wyk BCV, Bennett RH, Cordeaux C, Lucas MV, Eilbott JA, et al. Oxytocin enhances brain function in children with autism. Proc Natl Acad Sci USA. 2013;110:20953–20958. doi: 10.1073/pnas.1312857110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guastella AJ, Carson DS, Dadds MR, Mitchell PB, Cox RE. Does oxytocin influence the early detection of angry and happy faces. Psychoneuroendocrinology. 2009;34:220–225. doi: 10.1016/j.psyneuen.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Heinrichs M, Dawans B, von Domes G. Oxytocin, vasopressin, and human social behavior. Front Neuroendocrinol. 2009;30:548–557. doi: 10.1016/j.yfrne.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Heinrichs M, Domes G. Neuropeptides and social behaviour: effects of oxytocin and vasopressin in humans. Prog Brain Res. 2008;170:337–350. doi: 10.1016/S0079-6123(08)00428-7. [DOI] [PubMed] [Google Scholar]
- Horley K, Williams LM, Gonsalvez C, Gordon E. Social phobics do not see eye to eye: a visual scanpath study of emotional expression processing. J Anxiety Disord. 2003;17:33–44. doi: 10.1016/s0887-6185(02)00180-9. [DOI] [PubMed] [Google Scholar]
- Hurlemann R, Patin A, Onur OA, Cohen MX, Baumgartner T, Metzler S, et al. Oxytocin enhances amygdala-dependent, socially reinforced learning and emotional empathy in humans. J Neurosci. 2010;30:4999–5007. doi: 10.1523/JNEUROSCI.5538-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanat M, Heinrichs M, Domes G.2013Oxytocin and the social brain: neural mechanisms and perspectives in human research Brain Res;e–pub ahead of print 8 November 2013;doi: 10.1016/j.brainres.2013.11.003 [DOI] [PubMed]
- Kirsch P, Esslinger C, Chen Q, Mier D, Lis S, Siddhanti S, et al. Oxytocin modulates neural circuitry for social cognition and fear in humans. J Neurosci. 2005;25:11489–11493. doi: 10.1523/JNEUROSCI.3984-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliemann D, Dziobek I, Hatri A, Steimke R, Heekeren HR. Atypical reflexive gaze patterns on emotional faces in autism spectrum disorders. J Neurosci. 2010;30:12281–12287. doi: 10.1523/JNEUROSCI.0688-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leknes S, Wessberg J, Ellingsen D-M, Chelnokova O, Olausson H, Laeng B. Oxytocin enhances pupil dilation and sensitivity to ‘hidden' emotional expressions. Soc Cogn Affect Neurosci. 2013;8:741–749. doi: 10.1093/scan/nss062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddell BJ, Brown KJ, Kemp AH, Barton MJ, Das P, Peduto A, et al. A direct brainstem–amygdala–cortical ‘alarm' system for subliminal signals of fear. NeuroImage. 2005;24:235–243. doi: 10.1016/j.neuroimage.2004.08.016. [DOI] [PubMed] [Google Scholar]
- Liddell BJ, Williams LM, Rathjen J, Shevrin H, Gordon E. A temporal dissociation of subliminal versus supraliminal fear perception: an event-related potential study. J Cogn Neurosci. 2004;16:479–486. doi: 10.1162/089892904322926809. [DOI] [PubMed] [Google Scholar]
- Lischke A, Berger C, Prehn K, Heinrichs M, Herpertz SC, Domes G. Intranasal oxytocin enhances emotion recognition from dynamic facial expressions and leaves eye-gaze unaffected. Psychoneuroendocrinology. 2012;37:475–481. doi: 10.1016/j.psyneuen.2011.07.015. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Domes G, Kirsch P, Heinrichs M. Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nat Rev Neurosci. 2011;12:524–538. doi: 10.1038/nrn3044. [DOI] [PubMed] [Google Scholar]
- Miyahara M, Harada T, Ruffman T, Sadato N, Iidaka T. Functional connectivity between amygdala and facial regions involved in recognition of facial threat. Soc Cogn Affect Neurosci. 2013;8:181–189. doi: 10.1093/scan/nsr085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman GJ, Cacioppo JT, Morris JS, Karelina K, Malarkey WB, DeVries AC, et al. Selective influences of oxytocin on the evaluative processing of social stimuli. J Psychopharmacol. 2011;25:1313–1319. doi: 10.1177/0269881110367452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L, Adolphs R. Emotion processing and the amygdala: from a ‘low road' to ‘many roads' of evaluating biological significance. Nat Rev Neurosci. 2010;11:773–783. doi: 10.1038/nrn2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovic P, Kalisch R, Singer T, Dolan RJ. Oxytocin attenuates affective evaluations of conditioned faces and amygdala activity. J Neurosci. 2008;28:6607–6615. doi: 10.1523/JNEUROSCI.4572-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prehn K, Kazzer P, Lischke A, Heinrichs M, Herpertz SC, Domes G. Effects of intranasal oxytocin on pupil dilation indicate increased salience of socioaffective stimuli. Psychophysiology. 2013;50:528–537. doi: 10.1111/psyp.12042. [DOI] [PubMed] [Google Scholar]
- Scheller E, Büchel C, Gamer M. Diagnostic features of emotional expressions are processed preferentially. PLoS ONE. 2012;7:e41792. doi: 10.1371/journal.pone.0041792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze L, Lischke A, Greif J, Herpertz SC, Heinrichs M, Domes G. Oxytocin increases recognition of masked emotional faces. Psychoneuroendocrinology. 2011;36:1378–1382. doi: 10.1016/j.psyneuen.2011.03.011. [DOI] [PubMed] [Google Scholar]
- Shahrestani S, Kemp AH, Guastella AJ. The impact of a single administration of intranasal oxytocin on the recognition of basic emotions in humans: a meta-analysis. Neuropsychopharmacology. 2013;38:1929–1936. doi: 10.1038/npp.2013.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ML, Cottrell GW, Gosselin F, Schyns PG. Transmitting and decoding facial expressions. Psychol Sci. 2005;16:184–189. doi: 10.1111/j.0956-7976.2005.00801.x. [DOI] [PubMed] [Google Scholar]
- Steyer R, Schwenkmezger P, Notz P, Eid M. Der Mehrdimensionale Befindlichkeitsfragebogen MDBF [Multidimensional Mood Questionnaire] Hogrefe: Göttingen, Germany; 1997. [Google Scholar]
- Streit M, Dammers J, Simsek-Kraues S, Brinkmeyer J, Wölwer W, Ioannides A. Time course of regional brain activations during facial emotion recognition in humans. Neurosci Lett. 2003;342:101–104. doi: 10.1016/s0304-3940(03)00274-x. [DOI] [PubMed] [Google Scholar]
- Tamietto M, de Gelder B. Neural bases of the non-conscious perception of emotional signals. Nat Rev Neurosci. 2010;11:697–709. doi: 10.1038/nrn2889. [DOI] [PubMed] [Google Scholar]
- Tollenaar MS, Chatzimanoli M, Wee NJA, van der, Putman P. Enhanced orienting of attention in response to emotional gaze cues after oxytocin administration in healthy young men. Psychoneuroendocrinology. 2013;38:1797–1802. doi: 10.1016/j.psyneuen.2013.02.018. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, et al. The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Res. 2009;168:242–249. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Rauch SL, Etcoff NL, McInerney SC, Lee MB, Jenike MA. Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. J Neurosci. 1998;18:411–418. doi: 10.1523/JNEUROSCI.18-01-00411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittchen H-U, Zaudif M, Fydrich T. Strukturiertes klinisches Interview für DSM-IV [Structured Clinical Interview for DSM-IV] Hogrefe: Göttingen; 1997. [Google Scholar]
- Zaitsev M, Hennig J, Speck O. Point spread function mapping with parallel imaging techniques and high acceleration factors: fast, robust, and flexible method for echo-planar imaging distortion correction. Magn Reson Med. 2004;52:1156–1166. doi: 10.1002/mrm.20261. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.