Abstract
The abuse of prescription opioids that are used for the treatment of chronic pain is a major public health concern, costing ∼$53.4 billion annually in lost wages, health-care costs, and criminal costs. Although opioids remain a first-line therapy for the treatment of severe chronic pain, practitioners remain cautious because of the potential for abuse and addiction. Opioids such as heroin are considered very rewarding and reinforcing, but direct and systematic comparisons of compulsive intake between commonly prescribed opioids and heroin in animal models have not yet been performed. In the present study, we evaluated the potential for compulsive-like drug seeking and taking, using intravenous self-administration of oxycodone, fentanyl, and buprenorphine in rats allowed long access sessions (12 h). We measured compulsive-like intake using an established escalation model and responding on a progressive ratio schedule of reinforcement. We compared the potential for compulsive-like self-administration of these prescription opioids and heroin, which has been previously established to induce increasing intake that models the transition to addiction in humans. We found that animals that self-administered oxycodone, fentanyl, or heroin, but not buprenorphine had similar profiles of escalation and increases in breakpoints. The use of extended access models of prescription opioid intake will help better understand the biological factors that underlie opioid dependence.
INTRODUCTION
A growing problem in the field of drug abuse is prescription opioid abuse and dependence, which has reached epidemic-like proportions. Overdose deaths that involve opioid pain relievers, also known as opioid analgesics, have increased and now exceed deaths that involve the use of heroin and cocaine combined (Centers for Disease Control and Prevention, 2011). A prominent contributor to this trend is the intake of oxycodone, which can be insufflated, injected, or smoked. As of 2012, nearly 14 million people aged 12 or older had used oxycodone for nonmedical reasons at least once during their lifetime (Substance Abuse and Mental Health Services Administration, 2008, 2012); the average age of patients enrolled in treatment programs for heroin users is significantly higher than for prescription opioid users. Therefore, the identification of opioids with increased efficacy for pain treatment and reduced potential to cause dependence is a critical goal for preclinical research.
Opioid addiction is a chronically relapsing disorder characterized by a compulsion to seek and take opioids, loss of control in limiting its intake, and emergence of a profound negative emotional state during withdrawal (Koob et al, 2013). Multiple sources of reinforcement have been identified in the course of the opioid addiction syndrome (American Psychiatric Association, 2013; World Health Organization, 1992), and animal models can reproduce many features of opioid dependence that resemble the human condition (Barbier et al, 2013; Vendruscolo et al, 2011). Animal models of self-administration include the escalation of self-administration of the drug and increased motivation for the drug under conditions of increased workload, defined here as increases in breakpoints under a progressive ratio (PR) schedule of reinforcement (Ahmed et al, 2000; Koob, 2009; Koob et al, 2004; Piazza et al, 2000). Rats allowed extended access to intravenous heroin self-administration rapidly escalate their drug intake and demonstrate signs of physical and motivational aspects of opioid dependence, including tolerance, physical withdrawal signs, increased drug intake, and increased motivation to take the drug in the face of adverse consequences (Ahmed et al, 2000; Bozarth and Wise, 1985; Chen et al, 2006; Negus, 2006; Vendruscolo et al, 2011).
The epidemic-like spread of the abuse of oxycodone and other opioids used to treat chronic pain indicates that these drugs have a high abuse potential. Opioids such as heroin and morphine have been shown to induce conditioned rewards and are reinforcing in animals, reflected by behavioral measures of conditioned place preference (Prus et al, 2009) and intravenous self-administration (Ko et al, 2002), respectively. The effects are dose dependent, particularly via the intravenous route of administration, in which the time to onset is immediate (Edwards and Koob, 2012). However, few studies have examined the differences in the potential of different opioid drugs to induce escalations in self-administration and increases in breakpoints. Evaluating the behavioral patterns of the self-administration of clinically relevant drugs, such as oxycodone, fentanyl, and buprenorphine, will provide an animal model with which to elucidate the neurobiological bases of their abuse potential.
In the present study, we used the intravenous self-administration paradigm with extended access to systematically compare the potential for escalation of self-administration and drug seeking and intake under conditions of increased cost of opioids that are commonly used for the treatment of chronic pain (ie, oxycodone, fentanyl, and buprenorphine). These drugs were compared with the illicit drug heroin, which as a μ-opioid receptor (MOR) agonist has been extensively studied in rodent models of self-administration. In the first experiment, we allowed separate groups of rats to self-administer several doses of oxycodone, fentanyl, and buprenorphine under both limited access (short access (ShA)) and extended access (long access (LgA)) conditions. In the second experiment, we examined the reinforcing strength of these drugs using a PR schedule of reinforcement.
MATERIALS AND METHODS
Subjects
Adult male Wistar rats (n=144 (6/group); Charles River, Raleigh, NC, USA), weighing 225–275 g at the beginning of the experiments, were housed in groups of 2–3 per cage in a temperature-controlled (22 °C) vivarium on a 12-h/12-h light/dark cycle (lights on at 1800 hours) with ad libitum access to food and water. The animals were allowed to acclimate to the animal facilities for at least 7 days before the surgery. All the procedures adhered to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of The Scripps Research Institute.
Drugs
Fentanyl citrate (F3886), oxycodone hydrochloride (O1378), and buprenorphine hydrochloride (B9275) were obtained from Sigma (St Louis, MO, USA). Fentanyl citrate and oxycodone hydrochloride were dissolved in 0.9% saline, and buprenorphine hydrochloride was dissolved in sterile water. Heroin was obtained from the United States National Institute on Drug Abuse and dissolved in 0.9% saline.
Surgery
The rats were anesthetized with isoflurane (1.5–2.5%) and prepared with chronic intravenous silastic catheters (Dow Corning, Midland, MI, USA) into the right jugular vein (Vendruscolo et al, 2011). The catheter was secured to the vein with suture thread and passed subcutaneously to exit dorsally on the animal's back. After surgery, the catheters were flushed daily with 0.2 ml of a sterile antibiotic solution that contained heparinized saline (30 USP units/ml) and the antibiotic cefazolin. The rats were allowed to recover for 7 days before behavioral testing.
Self-Administration Chambers
Self-administration sessions were conducted in standard operant chambers (Med Associates, St Albans, VT, USA). The chambers (30.5 cm × 24.1 cm × 29.2 cm) were located in a dimly lit room and individually enclosed in wooden cubicles fitted with a ventilation fan that also screened external noise. Each operant chamber had two opaque panels as the right and left walls and two clear Plexiglas panels as the front and back walls. The floor consisted of 6 mm diameter steel bars spaced 15 mm apart. Two retractable levers (2 × 4 × 0.3 cm) were mounted 7 cm above the grid floor on the right operant panel. A white light diode was mounted 8.5 cm above each lever. A 1.1-W miniature light bulb synchronized the vivarium's light/dark cycle illuminated the chamber. A spring-covered Tygon tube connected the animal's catheter through a fluid swivel to a syringe that contained heroin solution. The syringe was placed inside a syringe pump (Razel) that was placed outside and above the chamber. A food dispenser with a trap door (3 × 4 cm) positioned 4 cm above the grid floor, equidistant between the two levers, delivered food pellets (45 mg) upon nosepokes on a fixed ratio 3 (FR3) schedule of reinforcement. A nosepoke hole (1 cm diameter) equipped with an infrared beam activated water delivery (0.1 ml, FR1) into a drinking cup placed on the left side of the back wall, 5 cm above the grid floor. A computer controlled the delivery of fluids and presentation of visual stimuli, and recorded the behavioral data.
Self-Administration Procedures
Before each self-administration session the catheters were flushed with 0.2 ml of a sterile antibiotic solution that contained heparinized saline (30 USP units/ml) and the antibiotic cefazolin (160 mg/ml). Both the training and escalation phases followed an FR1 schedule of reinforcement in which every lever press was reinforced with an intravenous injection of the drug. Each infusion was followed by a 5-s timeout period during which lever presses did not result in an infusion. Animals were allowed to self-administer in 12-h sessions 5 days/week. During the 1- and 12-h sessions, animals were given ad libitum access to food and water that was delivered following nosepoke. Following each session, animals returned to their home cages.
Escalation of Opioids and PR Schedule
Appropriate doses of each opioid were based on previous studies in our laboratory and the literature (Martin and Ewan, 2008; Martin et al, 2007). The doses tested supported self-administration in ShA sessions. To examine the possible escalation of buprenorphine self-administration, we first tested the potential for rats that had previously been trained to self-administer heroin to substitute buprenorphine in 1-h sessions. In previous drug discrimination tests (eg, saline vs buprenorphine), morphine has been commonly used as a training drug to establish discrimination, which is then substituted with buprenorphine (Colpaert, 1978; Shannon et al, 1984). We found that lever pressing was stable following substitution of heroin with buprenorphine at the doses we examined in the subsequent studies. Heroin intake follows a U-shaped dose–response function (Negus, 2006), and other opioids have similar U-shaped curves (Beardsley et al, 2004). We then chose these doses for use in the extended access self-administration sessions.
The rats were only exposed to one dose of a drug for the duration of the experiment. The animals were trained to self-administer heroin, fentanyl, oxycodone, or buprenorphine in 1-h sessions. Following 10 days of training, when stable lever pressing was established (minimum of five lever presses for three consecutive days), the rats were allocated to 12-h LgA and 1-h ShA groups. The rats were given a day off after their final escalation session on FR1 and were then tested in a 6-h PR session as previously reported (Barbier et al, 2013). Each successive infusion required greater lever-pressing requirements, with the following PR breakpoint schedule: 1, 1, 2, 2, 2, 3, 3, 4, 4, 5, 5, 6, 6, 7, 7, 8, 9, 9, 10, 11, 11, 12, 13, 14, 14, and so on. In this test, the response requirement for the next reinforcer increased progressively and is a measure of the reinforcing strength of the drug. Breakpoints were determined with the last infusion taken in the 6-h self-administration session.
Statistical Analyses
All the data are expressed as means and SEM. For escalation functions, the data were analyzed using one-way repeated-measures analysis of variance (ANOVA). When appropriate, post hoc comparisons were performed using the Tukey multiple comparison test. Differences in the breakpoints were analyzed between LgA and ShA groups for each dose using Student's t-test. For all the tests, values of p<0.05 were considered statistically significant.
RESULTS
Escalation of Self-Administration
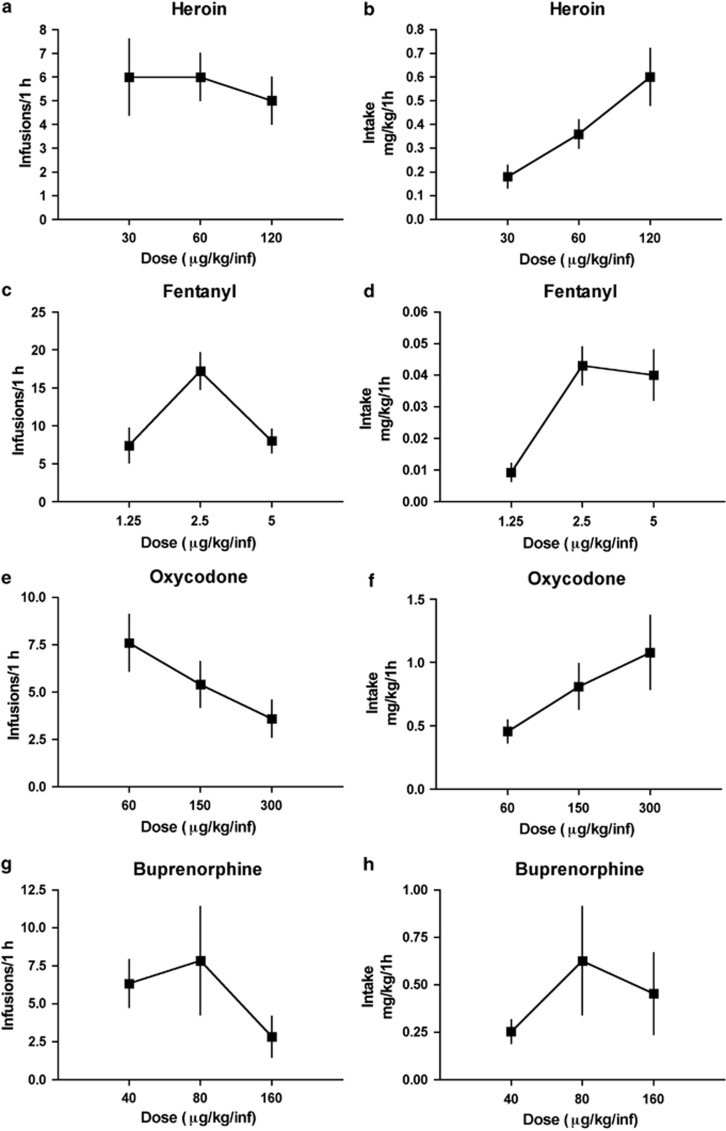
Figure 1 shows the dose–response function for infusions during pre-escalation (day 1) sessions of heroin, fentanyl, oxycodone, and buprenorphine self-administration. Three doses of each opioid were used to determine the doses of heroin, fentanyl, oxycodone, and buprenorphine (Figure 1a–h) that support self-administration. We tested doses of fentanyl and oxycodone that support self-administration at rates consistent with our established dose of heroin (60 μg/kg/inf). A dose–response function for number of infusions of heroin (Figure 1a), fentanyl (Figure 1c), oxycodone (Figure 1e), and buprenorphine (Figure 1g) was produced showing minimal relationship between responding for doses of each opioid before escalation of opioid intake. A dose–response function for resulting intake (mg/kg) of heroin (Figure 1b), fentanyl (Figure 1d), oxycodone (Figure 1f), and buprenorphine (Figure 1h) was also produced.
Figure 1.
Three doses of each opioid were used to determine the doses of heroin, fentanyl, oxycodone, and buprenorphine (a–h) that support self-administration. A dose–response function for infusions of heroin (a), fentanyl (c), oxycodone (e), and buprenorphine (g) was produced. A dose–response function for resulting intake of heroin (b), fentanyl (d), oxycodone (f), and buprenorphine (h) was also produced.
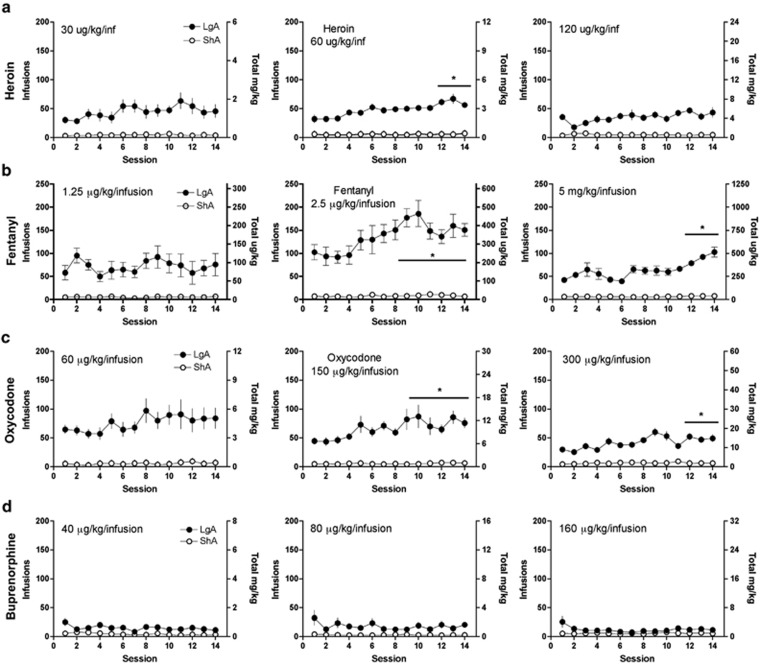
Subsequent repeated-measures ANOVA of the escalation phase data (Figure 2a–d) showed that the rats significantly escalated the self-administration of heroin at a dose of 60 μg/kg/inf (F4,52=5.343, p<0.0001; Figure 2a), fentanyl at doses of 2.5 μg/kg/inf (F4,52=19.2, p<0.0001) and 5 μg/kg/inf (F5,65=7.373, p<0.0001; Figure 2b), and oxycodone at doses of 150 μg/kg/inf (F5,60=3.325, p=0.001) and 300 μg/kg/inf (F6,72=4.517, p<0.0001; Figure 2c). Rats that were allowed to self-administer buprenorphine did not show an increase in lever pressing with extended access at any dose tested (Figure 2d), indicating that buprenorphine did not produce escalation of intake.
Figure 2.
Rats were allowed to self-administer heroin (a), fentanyl (b), oxycodone (c), and buprenorphine (d) over the course of 14 sessions at low, intermediate, and high doses during short (ShA) or long (LgA) access conditions to evaluate the escalation of drug intake.
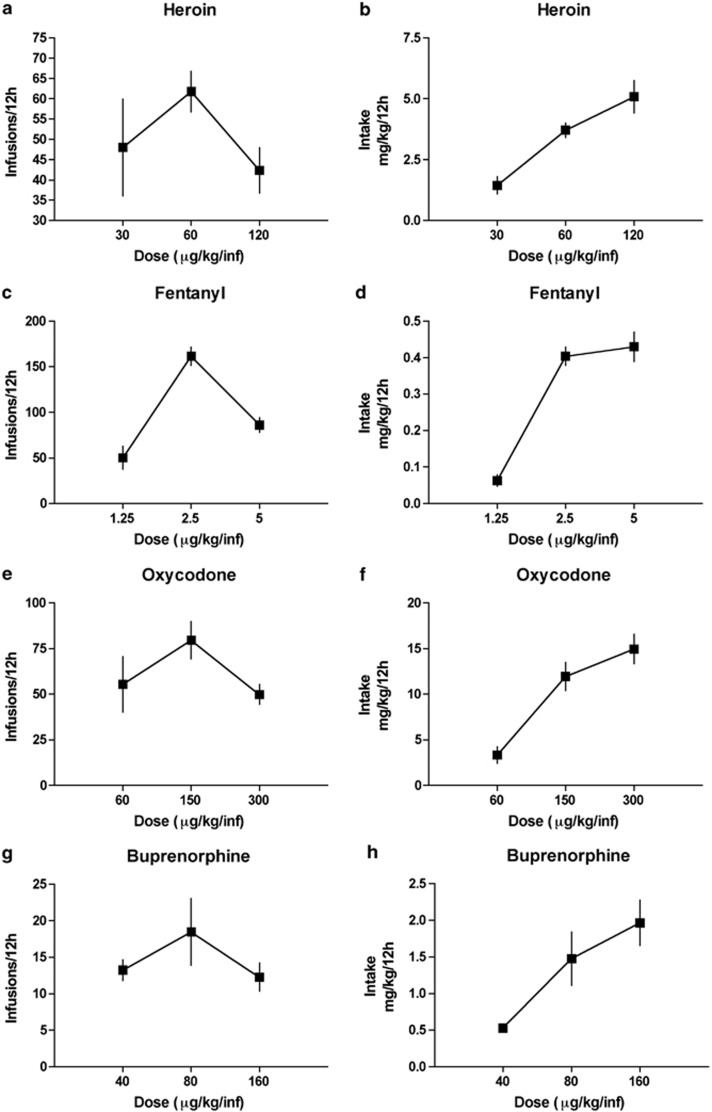
Dose Response
Responding during the last 3 days of the LgA sessions was averaged, and total responding (Figure 3a, c, e, g) and total intake (mg/kg) (Figure 3b, d, f, h) were recorded. All of the opioids tested produced an inverted U-shaped curve during the last 3 days of self-administration. Animals reduced responding at higher doses, consistent with a titration of opioid dosing to maintain similar brain levels.
Figure 3.
The last 3 days of self-administration on the FR1 schedule were averaged, and dose–response curves for infusions of heroin (a), fentanyl (c), oxycodone (e), and buprenorphine (g) were generated over the course of a 12-h session. Dose–intake curves for heroin (b), fentanyl (d), oxycodone (f), and buprenorphine (h) were also generated over the course of a 12-h session.
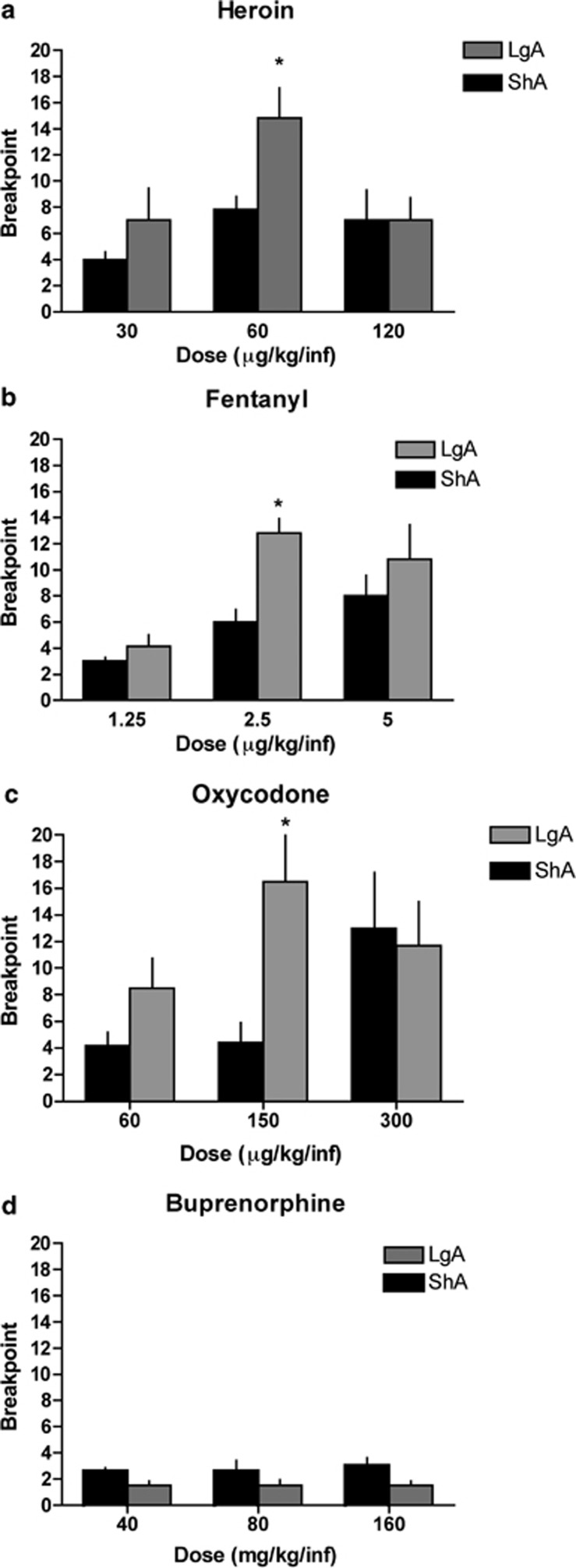
Progressive Ratio
Following the escalation period, the rats were subjected to a PR schedule of reinforcement, and the breakpoints for the last infusion over a 6-h session were recorded. Significant differences were found between the LgA and ShA groups only at the intermediate doses of heroin (p=0.019; Figure 4a), fentanyl (p=0.0008; Figure 4b), and oxycodone (p=0.016; Figure 4c). No significant differences were found between the LgA and ShA groups at the low and high doses of these opioids. The breakpoints for buprenorphine (Figure 4d) were not significantly different between the LgA and ShA groups at any dose tested.
Figure 4.
Rats were allowed to self-administer either buprenorphine over the course of 14 sessions to evaluate the escalation of drug intake. Following the last day of self-administration on a fixed ratio 1 (FR1) schedule, the animals were tested on a progressive ratio schedule to evaluate their motivation for heroin (a), fentanyl (b), oxycodone (c), and buprenorphine (d).
DISCUSSION
The present results show that, similar to heroin, both fentanyl and oxycodone also support self-administration behaviors, reflected by the escalation of lever pressing over time and increases in PR breakpoints in rats allowed extended drug access. Buprenorphine did not produce such effects. As expected, rats that were allowed limited drug access displayed low and stable lever pressing over time for all the four drugs. Animals that self-administered fentanyl, oxycodone, and heroin significantly escalated their intake over the course of the experiment for at least one dose tested. In addition, following escalation, a clear inverted U-shaped dose–effect function was demonstrated for fentanyl, oxycodone, and heroin, suggesting that the animals were titrating their doses. These findings indicate that, similar to heroin, oxycodone and fentanyl, but not buprenorphine, have the potential for escalation of self-administration. Our second measure, increases in breakpoints in a PR test, indicates reward efficacy or motivational properties of the drug (Hodos, 1961). Increases in breakpoint on a PR schedule were observed at the middle dose of the dose–effect function following escalation for fentanyl, oxycodone, and heroin, but not buprenorphine. LgA rats showed increased motivation for intermediate doses of heroin, oxycodone, and fentanyl compared with ShA rats. No group differences for buprenorphine were found. The overall low response for buprenorphine may explain the lack of differences between ShA and LgA rats. In agreement, previous data have demonstrated that PR breakpoints do not change by dose when self-administered by macaque monkeys (Mello et al, 1988). At high doses for each drug, no significant difference in PR responding was observed between ShA and LgA rats, suggesting that increasing the dose of opioid, even in limited access conditions, can alter the reinforcing efficacy of the drug, as demonstrated in earlier work (Koob and Le Moal, 2006; Roberts et al, 1989).
Heroin, fentanyl, oxycodone, and buprenorphine have few differences in time to onset when administered intravenously. However, each of these drugs shows differential binding affinities, efficacy and opioid subtype preference. A comparison of the binding affinity for MOR may be an important factor of their reinforcing effect. Whereas heroin, fentanyl, and oxycodone all have similar binding affinities (1–25 nM) (Frances et al, 1992; Volpe et al, 2011) buprenorphine has a much higher affinity for MOR of 0.21 nM, indicating that time to offset from the receptor for buprenorphine may be of a longer duration than that of the other ligands (Volpe et al, 2011).
In addition to the effects at MOR, buprenorphine also acts as a partial agonist at the MOR, nociceptin (ORL-1; Ki=285 nM), and kappa-opioid (Ki=0.91 nM) receptors and as an antagonist at kappa-opioid receptors (Ki=0.11 nM) (Huang et al, 2001). Activation of ORL-1 has been shown to have opposite effects on the mesolimbic dopamine receptors than MOR activation by decreasing extracellular dopamine levels in the nucleus accumbens (Margolis et al, 2003; Murphy et al, 1996). In addition, its effects at the kappa-opioid receptor are differential. At low doses buprenorphine acts as an antagonist at the kappa-receptor (Kajiwara et al, 1986). Antagonism of the kappa-receptor has previously been shown to inhibit self-administration of cocaine and other drugs of abuse (Cashman and Azar, 2014; Mello and Negus, 2007; Wee et al, 2009). At high doses, buprenorphine has weak agonist effects that might render buprenorphine aversive (Kajiwara et al, 1986; Sante et al, 2000) and similar to its effects at ORL-1, activation of kappa-receptors directly inhibits dopaminergic neurons (Margolis et al, 2003). That buprenorphine acts as a partial agonist at MOR, kappa-opioid receptor and ORL-1 and an antagonist at the kappa-opioid receptor may contribute to decreased self-administration of buprenorphine.
It has been hypothesized that tolerance to reward is a primary factor in the escalation of drug intake. Many studies evaluating cocaine intake over time show that, in fact, tolerance to the rewarding effects of the drug correlates with increased intake throughout the course of the experiment (Ahmed et al, 2002). In addition, cocaine has been shown to decrease cocaine-induced dopamine uptake inhibition, and reduced cocaine-induced dopamine overflow (Calipari et al, 2014). Because cocaine acts at the dopamine transporter it is possible that this effect of reward tolerance is specific to escalation of cocaine intake. Furthermore, it has been shown that animals that self-administer opioids are likely to self-administer above reward-threshold doses than animals that self-administer stimulants (Zernig et al, 2007). This suggests that escalation of opioid self-administration may be due to different mechanisms.
In the present study we demonstrated that, similar to heroin, both oxycodone and fentanyl induced escalation of self-administration and increases in motivation, measured by increased breakpoints in a PR test, in rats with extended access to the drug. Buprenorphine self-administration resulted in no such effects. The use of long access sessions was a useful tool to investigate the biological mechanism of excessive intake of prescription opioids.
FUNDING AND DISCLOSURE
This research was financially supported by National Institutes of Health grants DA004043 from the National Institute on Drug Abuse and the Pearson Center for Alcoholism and Addiction Research. The authors declare no conflict of interest.
Acknowledgments
The authors thank Michael Arends for proofreading the manuscript.
References
- Ahmed SH, Kenny PJ, Koob GF, Markou A. Neurobiological evidence for hedonic allostasis associated with escalating cocaine use. Nat Neurosci. 2002;5:625–626. doi: 10.1038/nn872. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Walker JR, Koob GF. Persistent increase in the motivation to take heroin in rats with a history of drug escalation. Neuropsychopharmacology. 2000;22:413–421. doi: 10.1016/S0893-133X(99)00133-5. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association, Diagnostic and Statistical Manual of Mental Disorders5th edn,American Psychiatric Press: Washington, DC, USA; 2013 [Google Scholar]
- Barbier E, Vendruscolo LF, Schlosburg JE, Edwards S, Juergens N, Park PE, et al. The NK1 receptor antagonist l822429 reduces heroin reinforcement. Neuropsychopharmacology. 2013;38:976–984. doi: 10.1038/npp.2012.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beardsley PM, Aceto MD, Cook CD, Bowman ER, Newman JL, Harris LS. Discriminative stimulus, reinforcing, physical dependence, and antinociceptive effects of oxycodone in mice, rats, and rhesus monkeys. Exp Clin Psychopharmacol. 2004;12:163–172. doi: 10.1037/1064-1297.12.3.163. [DOI] [PubMed] [Google Scholar]
- Bozarth MA, Wise RA. Toxicity associated with long-term intravenous heroin and cocaine self-administration in the rat. JAMA. 1985;254:81–83. [PubMed] [Google Scholar]
- Calipari ES, Ferris MJ, Jones SR. Extended access of cocaine self-administration results in tolerance to the dopamine-elevating and locomotor-stimulating effects of cocaine. J Neurochem. 2014;128:224–232. doi: 10.1111/jnc.12452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashman JR, Azar MR. Potent inhibition of alcohol self-administration in alcohol-preferring rats by a kappa-opioid receptor antagonist. J Pharmacol Exp Ther. 2014;350:171–180. doi: 10.1124/jpet.114.214262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention Vital signs: overdoses of prescription opioid pain relievers-: United States, 1999–2008. Morb Mortal Wkly Rep. 2011;60:1487–1492. [PubMed] [Google Scholar]
- Chen SA, O'Dell LE, Hoefer ME, Greenwell TN, Zorrilla EP, Koob GF. Unlimited access to heroin self-administration: independent motivational markers of opiate dependence. Neuropsychopharmacology. 2006;31:2692–2707. doi: 10.1038/sj.npp.1301008. [DOI] [PubMed] [Google Scholar]
- Colpaert FC. Discriminative stimulus properties of narcotic analgesic drugs. Pharmacol Biochem Behav. 1978;9:863–887. doi: 10.1016/0091-3057(78)90370-2. [DOI] [PubMed] [Google Scholar]
- Edwards S, Koob GF. Experimental psychiatric illness and drug abuse models: from human to animal, an overview. Methods Mol Biol. 2012;829:31–48. doi: 10.1007/978-1-61779-458-2_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frances B, Gout R, Monsarrat B, Cros J, Zajac JM. Further evidence that morphine-6 beta-glucuronide is a more potent opioid agonist than morphine. J Pharmacol Exp Ther. 1992;262:25–31. [PubMed] [Google Scholar]
- Hodos W. Progressive ratio as a measure of reward strength. Science. 1961;134:943–944. doi: 10.1126/science.134.3483.943. [DOI] [PubMed] [Google Scholar]
- Huang P, Kehner GB, Cowan A, Liu-Chen LY. Comparison of pharmacological activities of buprenorphine and norbuprenorphine: norbuprenorphine is a potent opioid agonist. J Pharmacol Exp Ther. 2001;297:688–695. [PubMed] [Google Scholar]
- Kajiwara M, Aoki K, Ishii K, Numata H, Matsumiya T, Oka T. Agonist and antagonist actions of buprenorphine on three types of opioid receptor in isolated preparations. Japanese journal of pharmacology. 1986;40:95–101. doi: 10.1254/jjp.40.95. [DOI] [PubMed] [Google Scholar]
- Ko MC, Terner J, Hursh S, Woods JH, Winger G. Relative reinforcing effects of three opioids with different durations of action. J Pharmacol Exp Ther. 2002;301:698–704. doi: 10.1124/jpet.301.2.698. [DOI] [PubMed] [Google Scholar]
- Koob GF. Neurobiological substrates for the dark side of compulsivity in addiction. Neuropharmacology. 2009;56 (Suppl 1:18–31. doi: 10.1016/j.neuropharm.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Ahmed SH, Boutrel B, Chen SA, Kenny PJ, Markou A, et al. Neurobiological mechanisms in the transition from drug use to drug dependence. Neurosci Biobehav Rev. 2004;27:739–749. doi: 10.1016/j.neubiorev.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Koob GF, Buck CL, Cohen A, Edwards S, Park PE, Schlosburg JE, et al. Addiction as a stress surfeit disorder. Neuropharmacology. 2013;76b:370–382. doi: 10.1016/j.neuropharm.2013.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Neurobiology of Addiction. Academic Press: London, UK; 2006. [Google Scholar]
- Margolis EB, Hjelmstad GO, Bonci A, Fields HL. Kappa-opioid agonists directly inhibit midbrain dopaminergic neurons. J Neurosci. 2003;23:9981–9986. doi: 10.1523/JNEUROSCI.23-31-09981.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin TJ, Ewan E. Chronic pain alters drug self-administration: implications for addiction and pain mechanisms. Exp Clin Psychopharmacol. 2008;16:357–366. doi: 10.1037/a0013597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin TJ, Kim SA, Buechler NL, Porreca F, Eisenach JC. Opioid self-administration in the nerve-injured rat: relevance of antiallodynic effects to drug consumption and effects of intrathecal analgesics. Anesthesiology. 2007;106:312–322. doi: 10.1097/00000542-200702000-00020. [DOI] [PubMed] [Google Scholar]
- Mello NK, Lukas SE, Bree MP, Mendelson JH. Progressive ratio performance maintained by buprenorphine, heroin and methadone in Macaque monkeys. Drug Alcohol Depend. 1988;21:81–97. doi: 10.1016/0376-8716(88)90053-1. [DOI] [PubMed] [Google Scholar]
- Mello NK, Negus SS. Effects of d-amphetamine and buprenorphine combinations on speedball (cocaine+heroin) self-administration by rhesus monkeys. Neuropsychopharmacology. 2007;32:1985–1994. doi: 10.1038/sj.npp.1301319. [DOI] [PubMed] [Google Scholar]
- Murphy NP, Ly HT, Maidment NT. Intracerebroventricular orphanin FQ/nociceptin suppresses dopamine release in the nucleus accumbens of anaesthetized rats. Neuroscience. 1996;75:1–4. doi: 10.1016/0306-4522(96)00322-3. [DOI] [PubMed] [Google Scholar]
- Negus SS. Choice between heroin and food in nondependent and heroin-dependent rhesus monkeys: effects of naloxone, buprenorphine, and methadone. J Pharmacol Exp Ther. 2006;317:711–723. doi: 10.1124/jpet.105.095380. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deroche-Gamonent V, Rouge-Pont F, Le Moal M. Vertical shifts in self-administration dose-response functions predict a drug-vulnerable phenotype predisposed to addiction. J Neurosci. 2000;20:4226–4232. doi: 10.1523/JNEUROSCI.20-11-04226.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prus AJ, James JR, Rosecrans JA.2009Conditioned place preferenceIn: Buccafusco JJ, (ed).Methods of Behavior Analysis in Neuroscience2nd edn,CRC Press: Boca Raton, FL, USA; [PubMed] [Google Scholar]
- Roberts DC, Loh EA, Vickers G. Self-administration of cocaine on a progressive ratio schedule in rats: dose-response relationship and effect of haloperidol pretreatment. Psychopharmacology (Berl) 1989;97:535–538. doi: 10.1007/BF00439560. [DOI] [PubMed] [Google Scholar]
- Sante AB, Nobre MJ, Brandao ML. Place aversion induced by blockade of mu or activation of kappa opioid receptors in the dorsal periaqueductal gray matter. Behav Pharmacol. 2000;11:583–589. doi: 10.1097/00008877-200011000-00005. [DOI] [PubMed] [Google Scholar]
- Shannon HE, Cone EJ, Gorodetzky CW. Morphine-like discriminative stimulus effects of buprenorphine and demethoxybuprenorphine in rats: quantitative antagonism by naloxone. J Pharmacol Exp Ther. 1984;229:768–774. [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration Results from the 2007 National Survey on http://wwwdasis.samhsa.gov/webt/quicklink/US12.htm (2012). Drug Use and Health: National Findings (NSDUH Series H-34, DHHS publication no. SMA 08-4343). Office of Applied Statistics: Rockville, MD, USA, (2008
- Substance Abuse and Mental Health Services Administration Treatment Episode Data Set. Substance Abuse and Mental Health Services Administration ( ( http://www.oas.samhsa.gov/2k10/230/230PainRelvr2k10.htm ; accessed 22 May 2009). Office of Applied Studies: Rockville, MD, USA, (2012
- Vendruscolo LF, Schlosburg JE, Misra KK, Chen SA, Greenwell TN, Koob GF. Escalation patterns of varying periods of heroin access. Pharmacol Biochem Behav. 2011;98:570–574. doi: 10.1016/j.pbb.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe DA, McMahon Tobin GA, Mellon RD, Katki AG, Parker RJ, Colatsky T, et al. Uniform assessment and ranking of opioid mu receptor binding constants for selected opioid drugs. Regul Toxicol Pharmacol. 2011;59:385–390. doi: 10.1016/j.yrtph.2010.12.007. [DOI] [PubMed] [Google Scholar]
- Wee S, Orio L, Ghirmai S, Cashman JR, Koob GF. Inhibition of kappa opioid receptors attenuated increased cocaine intake in rats with extended access to cocaine. Psychopharmacology (Berl) 2009;205L:565–575. doi: 10.1007/s00213-009-1563-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. International Statistical Classification of Diseases and Related Health Problems, 10th revision. World Health Organization: Geneva, Switzerland; 1992. [Google Scholar]
- Zernig G, Ahmed SH, Cardinal RN, Morgan D, Acquas E, Foltin RW, et al. Explaining the escalation of drug use in substance dependence: models and appropriate animal laboratory tests. Pharmacology. 2007;80:65–119. doi: 10.1159/000103923. [DOI] [PubMed] [Google Scholar]