Abstract
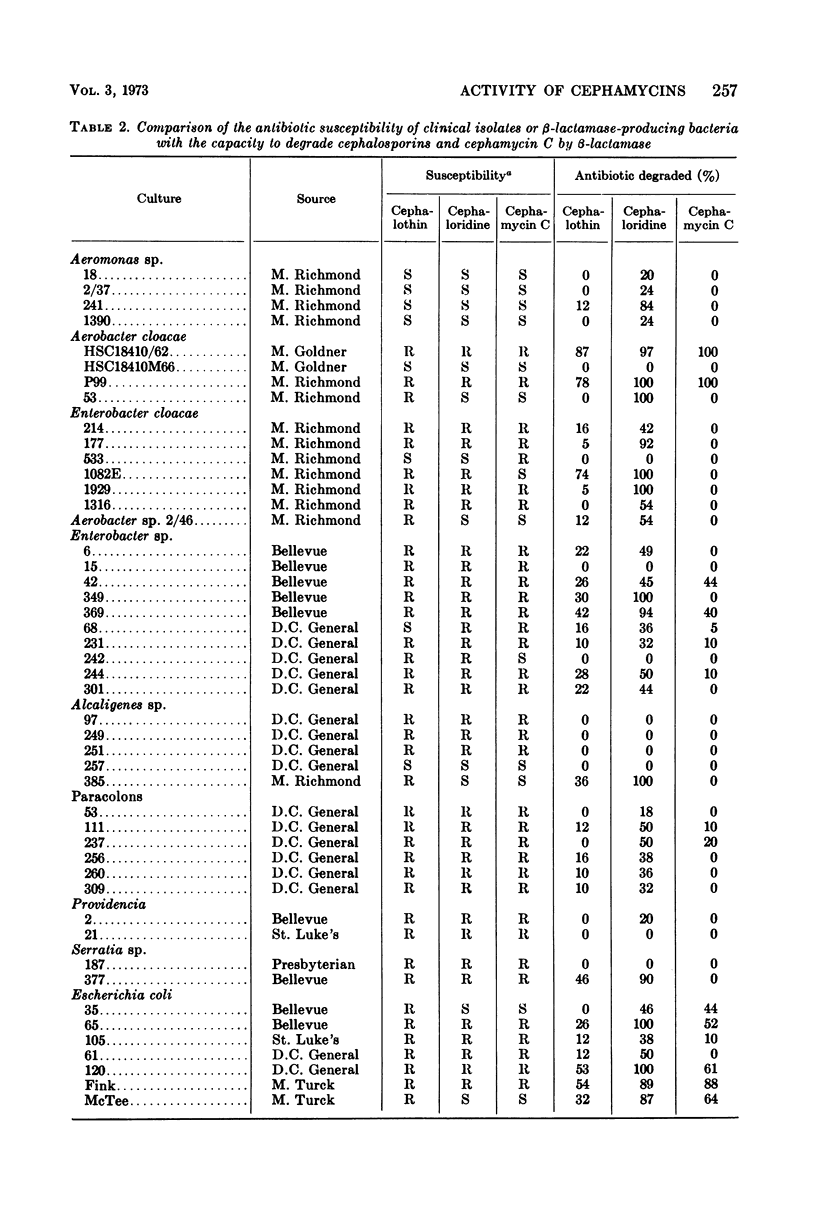
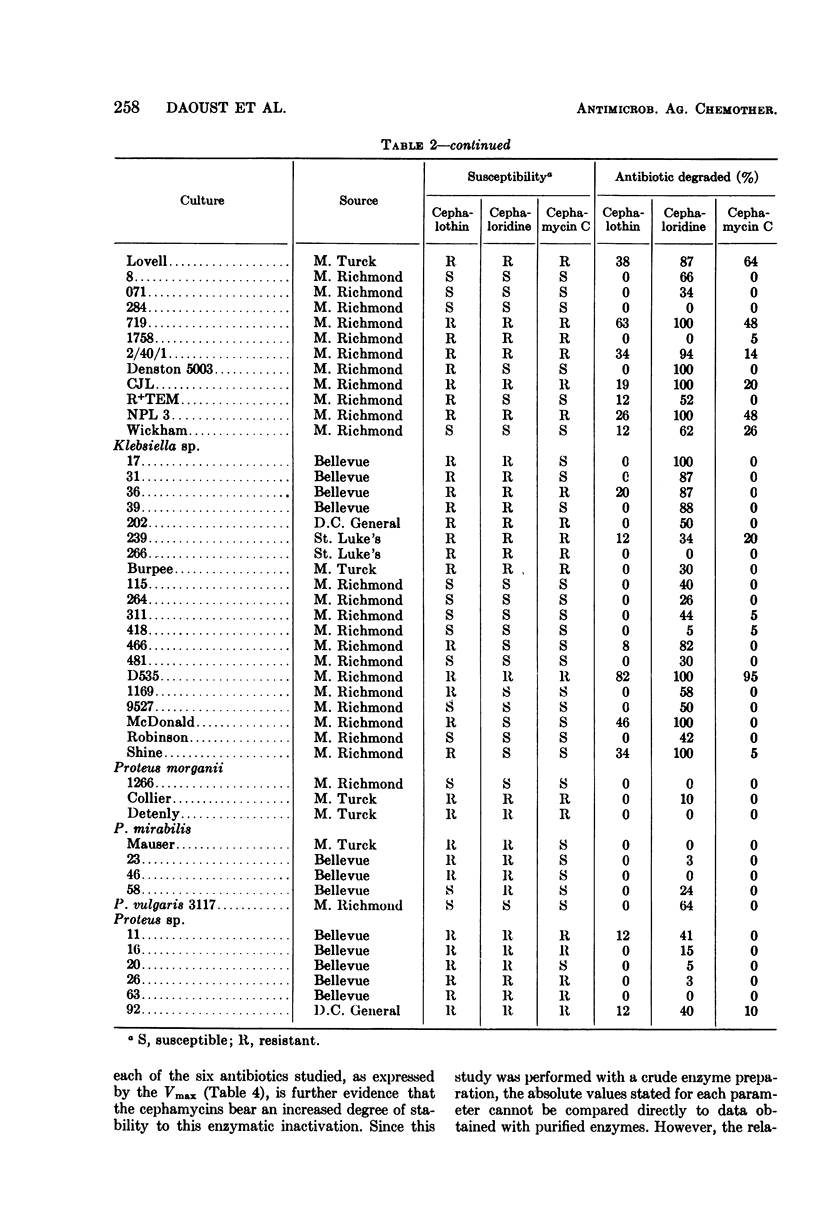
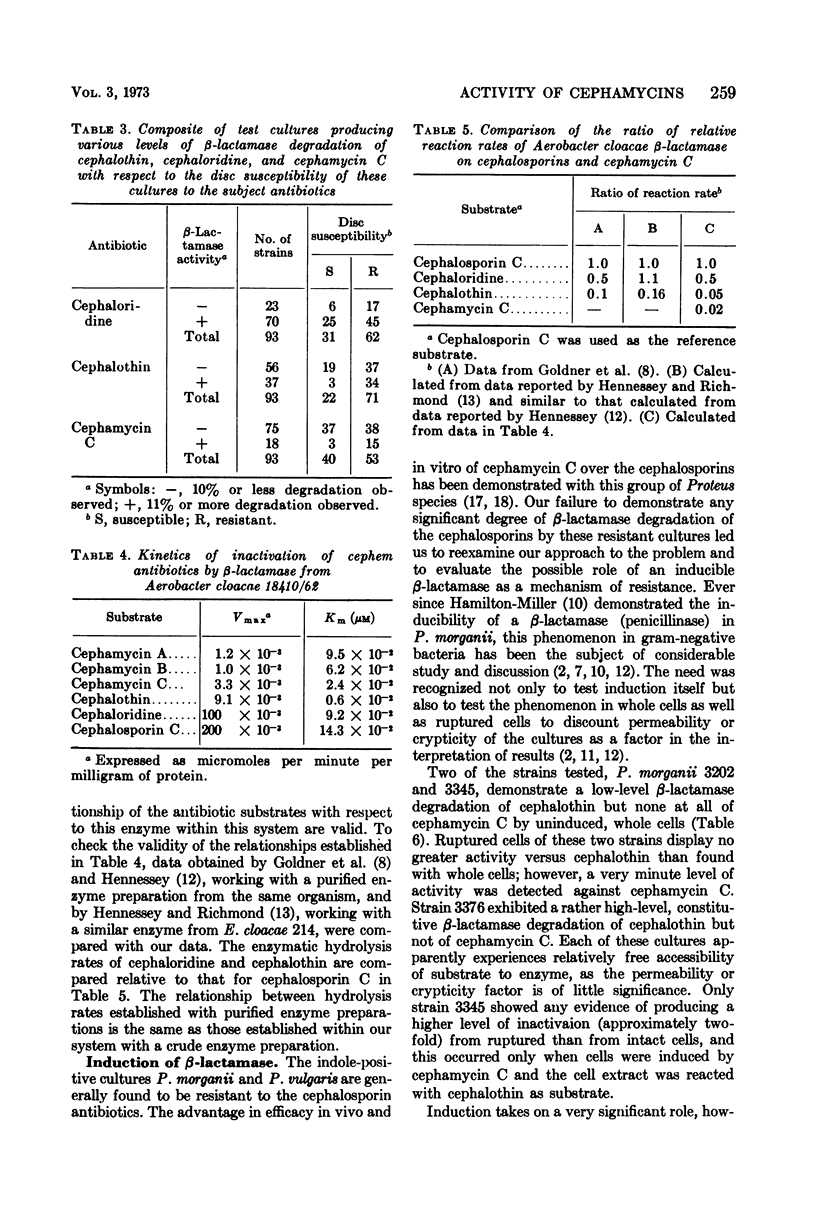
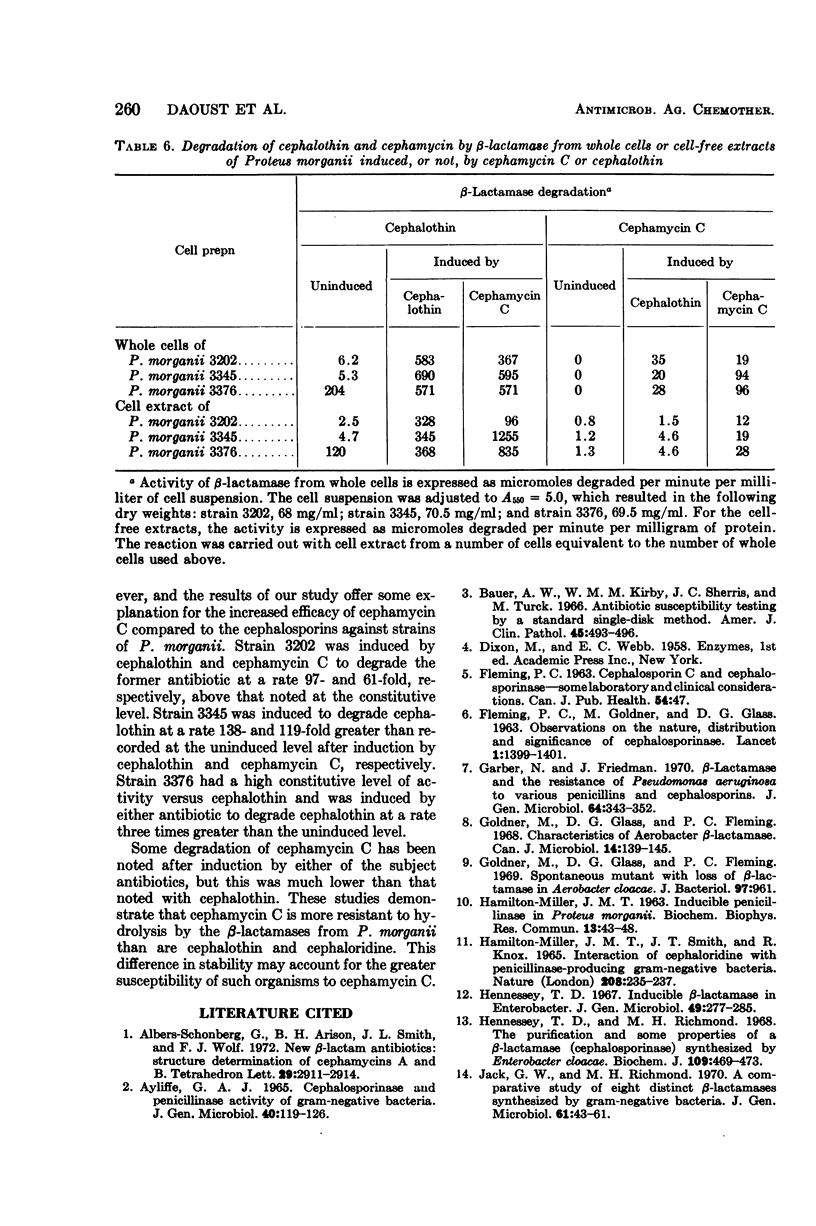
The susceptibility to some cephalosporin antibiotics and to cephamycin C, a member of a new family of β-lactam antibiotics, was evaluated for 466 cultures representing 11 different genera or species of gram-negative clinical isolates. The susceptibility of 39 gram-negative cultures known to produce β-lactamase was also determined. The β-lactamase activity of a representative group of the clinical isolates and the 39 enzyme producers was studied with the cephalosporins (cephalothin and cephaloridine) and cephamycin C as substrates and was related to the in vitro disc susceptibility to these same antibiotics. The significant resistance to β-lactamase displayed by the cephamycins is reflected in the kinetics of enzyme activity (Km and Vmax) that are reported for the cephalosporins and the cephamycins. Resistance to β-lactamase is probably one of the reasons that many cephalosporin-resistant cultures are susceptible to cephamycin C.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayliffe G. A. Cephalosporinase and penicillinase activity of Gram-negative bacteria. J Gen Microbiol. 1965 Jul;40(1):119–126. doi: 10.1099/00221287-40-1-119. [DOI] [PubMed] [Google Scholar]
- Bauer A. W., Kirby W. M., Sherris J. C., Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966 Apr;45(4):493–496. [PubMed] [Google Scholar]
- FLEMING P. C., GOLDNER M., GLASS D. G. Observations on the nature, distribution, and significance of cephalosporinase. Lancet. 1963 Jun 29;1(7296):1399–1401. doi: 10.1016/s0140-6736(63)92051-8. [DOI] [PubMed] [Google Scholar]
- Garber N., Friedman J. Beta-lactamase and the resistance of Pseudomonas aeruginosa to various penicillins and cephalosporins. J Gen Microbiol. 1970 Dec;64(3):343–352. doi: 10.1099/00221287-64-3-343. [DOI] [PubMed] [Google Scholar]
- Goldner M., Glass D. G., Fleming P. C. Characteristics of Aerobacter beta-lactamase. Can J Microbiol. 1968 Feb;14(2):139–145. doi: 10.1139/m68-023. [DOI] [PubMed] [Google Scholar]
- Goldner M., Glass D. G., Fleming P. C. Spontaneous mutant with loss of beta-lactamase in Aerobacter cloacae. J Bacteriol. 1969 Feb;97(2):961–961. doi: 10.1128/jb.97.2.961-.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAMILTON-MILLER J. M. INDUCIBLE PENICILLINASE IN PROTEUS MORGANI. Biochem Biophys Res Commun. 1963 Sep 10;13:43–48. doi: 10.1016/0006-291x(63)90159-1. [DOI] [PubMed] [Google Scholar]
- Hamilton-Miller J. M., Smith J. T., Knox R. Interaction of cephaloridine with penicillinase-producing gram-negative bacteria. Nature. 1965 Oct 16;208(5007):235–237. doi: 10.1038/208235a0. [DOI] [PubMed] [Google Scholar]
- Hennessey T. D. Inducible beta-lactamase in Enterobacter. J Gen Microbiol. 1967 Nov;49(2):277–285. doi: 10.1099/00221287-49-2-277. [DOI] [PubMed] [Google Scholar]
- Hennessey T. D., Richmond M. H. The purification and some properties of a beta-lactamase (cephalosporinase) synthesized by Enterobactercloacae. Biochem J. 1968 Sep;109(3):469–473. doi: 10.1042/bj1090469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack G. W., Richmond M. H. A comparative study of eight distinct beta-lactamases synthesized by gram-negative bacteria. J Gen Microbiol. 1970 Apr;61(1):43–61. doi: 10.1099/00221287-61-1-43. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Miller A. K., Celozzi E., Kong Y., Pelak B. A., Kropp H., Stapley E. O., Hendlin D. Cephamycins, a new family of beta-lactam antibiotics. IV. In vivo studies. Antimicrob Agents Chemother. 1972 Oct;2(4):287–290. doi: 10.1128/aac.2.4.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. K., Celozzi E., Pelak B. A., Stapley E. O., Hendlin D. Cephamycins, a new family of beta-lactam antibiotics. 3. In vitro studies. Antimicrob Agents Chemother. 1972 Oct;2(4):281–286. doi: 10.1128/aac.2.4.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller T. W., Goegelman R. T., Weston R. G., Putter I., Wolf F. J. Cephamycins, a new family of beta-lactam antibiotics. II. Isolation and chemical characterization. Antimicrob Agents Chemother. 1972 Sep;2(3):132–135. doi: 10.1128/aac.2.3.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOVICK R. P. Micro-iodometric assay for penicillinase. Biochem J. 1962 May;83:236–240. doi: 10.1042/bj0830236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERRET C. J. Iodometric assay of penicillinase. Nature. 1954 Nov 27;174(4439):1012–1013. doi: 10.1038/1741012a0. [DOI] [PubMed] [Google Scholar]
- Stapley E. O., Jackson M., Hernandez S., Zimmerman S. B., Currie S. A., Mochales S., Mata J. M., Woodruff H. B., Hendlin D. Cephamycins, a new family of beta-lactam antibiotics. I. Production by actinomycetes, including Streptomyces lactamdurans sp. n. Antimicrob Agents Chemother. 1972 Sep;2(3):122–131. doi: 10.1128/aac.2.3.122. [DOI] [PMC free article] [PubMed] [Google Scholar]



