Abstract
♦ Introduction:
Peritoneal dialysis (PD) patients are commonly required to transfer to hemodialysis (HD), however the literature describing the outcomes of such transfers is limited. The aim of our study was to describe the predictors of these transfers and their outcomes according to vascular access at the time of transfer.
♦ Methods:
A retrospective cohort study using registry data of all adult patients commencing PD as their initial renal replacement therapy in Australia or New Zealand between 2004 – 2010 was performed. Follow-up was until 31 December 2010. Logistic regression models were constructed to determine possible predictors of transfer within both 6 and 12 months of PD commencement. Cox analysis and competing risks regression were used to determine the predictors of survival and transplantation post-transfer.
♦ Results:
The analysis included 4,781 incident PD patients, of whom 1,699 transferred to HD during the study period. Logistic models did not identify any clinically useful predictors of transfer within 6 or 12 months (c-statistics 0.54 and 0.55 respectively). 67% of patients commenced HD with a central venous catheter (CVC). CVC use at transfer was associated with increased mortality (hazard ratio 1.37, 95% confidence interval (CI) 1.11 – 1.68, p = 0.003) and a borderline significant reduction in the incidence of transplantation (subhazard ratio 0.76, 95% CI 0.58 – 1.00, p = 0.05).
♦ Conclusions:
It is difficult to predict the transfer to HD for incident PD patients. PD patients who commence HD with a CVC have a higher risk of mortality and a lower likelihood of undergoing renal transplantation.
Keywords: Epidemiology, hemodialysis, peritoneal dialysis, vascular access
Peritoneal dialysis (PD) is utilized worldwide as a means of renal replacement therapy, and has been demonstrated to provide relative short-term survival benefits compared to hemodialysis (HD) (1). In Australia and New Zealand, 19 and 35% of prevalent dialysis patients at the end of 2010 were on PD, respectively (2). However PD technique failure is common, requiring patients to transfer to HD. In 2010, 20% of patients on PD in Australia and New Zealand permanently transferred to HD (2). Similar rates have been reported in other countries (3). Technique failure can occur for a number of reasons, the most common being peritonitis, inadequate dialysis, catheter-related problems or patient’s choice (4–6). Furthermore, the risk for transferring to HD is relatively high in the first 6 months of commencing PD (4,5).
A number of studies have explored risk factors for PD technique failure. These include older age, higher peritoneal membrane transport status, reduced peritoneal ultrafiltration, poor nutrition, diabetes mellitus and increased body mass index (4,6–15). However, these factors have not been reported consistently. In addition, there are few available data examining the outcomes (including mortality) of patients who transfer from PD to HD. It has been widely documented that catheter use in HD is associated with increased infectious complications and mortality (16–18). This has not been assessed in patients transferring to HD from PD.
The main aim of this study was to determine patient survival after transfer from PD to HD according to the vascular access utilized at the first HD session. We hypothesized that patients transferring from PD to HD with a catheter would have a similar excess mortality to that seen in incident HD patients commencing with a catheter. Our other aim was to determine the presence of any predictors of these transfers. In order to do this, we examined the general characteristics of patients who transferred to HD, assessed for any predictors of the change, and assessed the circumstances and outcomes of the HD transfer.
Methods
Study Population
Our study included all incident adult (age ≥ 18 years) patients in Australia and New Zealand who commenced PD as their first ever form of renal replacement therapy between January 1, 2004, and December 31, 2010. Follow-up was until the end of 2010.
Data Collection
Data were obtained from the Australia and New Zealand Dialysis and Transplant (ANZDATA) Registry which collects information on all patients receiving renal replacement therapy in Australia and New Zealand. Full details regarding the structure and method of information collection are reported elsewhere (2). The data collected and analyzed consisted of demographic details, underlying cause of renal disease, comorbidities (diabetes, coronary artery disease, cerebrovascular disease, peripheral vascular disease, chronic lung disease and smoking), reasons for transfer to HD, duration of PD, and vascular access used at the time of first HD. Information such as residual renal function and peritoneal membrane status was not available in half of the patients who transferred and therefore these variables were not included in our analysis.
We classified the documented reason for transfer to HD as either potentially predictable transfers or unpredictable transfers. Transfers secondary to inadequate dialysis, patient preference, being unable to manage self-care or planned transfers were classed as being potentially predictable.
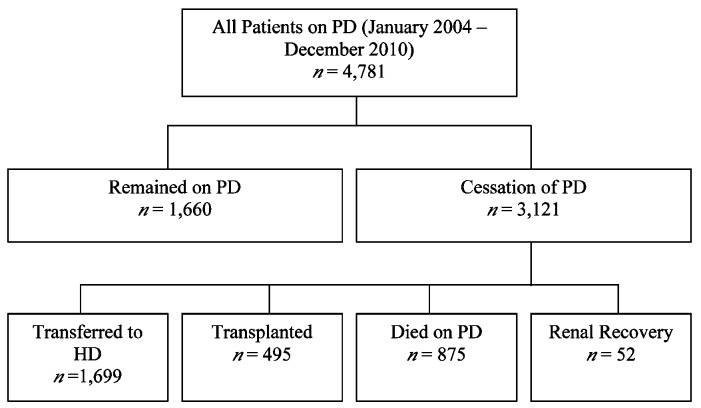
Patients were classified into those who continued PD until December 31, 2010, and those who ceased PD. Those who ceased PD were further classified into groups according to renal outcome (Figure 1). The date of transfer to HD was considered to be the date of first HD session irrespective of duration as reported to ANZDATA. Patients who were classified as having transferred to HD were then further assessed according to vascular access at first HD.
Figure 1 —
Flowchart of patients. Outcome of all patients who initially commenced peritoneal dialysis up until December 31, 2010.
Outcomes
Our study outcomes included: transfer from PD to HD at both 6 and 12 months post-commencement of PD; survival after transfer to HD; and renal transplantation after transfer to HD.
We classified all transfers from PD to HD, irrespective of duration of HD or whether they returned to PD, as a transfer in our analysis. This was felt to be important since the insertion of a central venous catheter can be associated with morbidity and mortality. In addition, identifying the importance of vascular access at transfer was a goal of our study and ANZDATA only collects this at the first ever HD and then at the end of each year.
Statistical Analysis
Results are expressed as mean ± standard deviation (SD) for continuous normally distributed data, as median and interquartile range for continuous non-normally distributed data, and as frequencies and percentages for categorical data. The cumulative incidence of each of transfer to HD, death, transplantation and renal recovery were estimated using a non-parametric, competing risk method (19).
Logistic regression was used to determine predictors of transfer at 6 and 12 months. This analysis included all patients and all transfers. The covariates initially included in the model were age, sex, race, body mass index (BMI), primary renal disease, diabetes, coronary artery disease, cerebrovascular disease, peripheral vascular disease, chronic lung disease and late referral. Late referral was defined as commencing dialysis within three months of first nephrology review. Factors associated with transfer to HD on univariable analysis (p value < 0.25) were included in a base multivariable model. Backward selection was then performed to remove nonsignificant covariates with p < 0.05 considered to be statistically significant. Age was included in the final model regardless of whether or not it was significant. Model calibration was tested using the Hosmer-Lemeshow goodness-of-fit test and discrimination using the c-statistic. A random-effects logistic regression clustered by initial center of dialysis was performed.
The probability of survival for any duration of follow-up post-transfer to HD was estimated using the Kaplan-Meier method. In addition, a Cox proportional hazards model was used to determine the effect of individual variables on the interest of mortality. This was again performed utilizing a backward stepwise elimination method to produce a final adjusted model. A Cox model stratified by initial center of dialysis was used to determine if there was any center-effect. Finally, a competing risks survival model was employed to determine the likelihood of transplantation post-transfer (20). Again, a similar method was employed in the construction of the final age-adjusted model. The proportional hazards assumption was assessed by examining plots of scaled Schoenfeld residuals and with time-varying covariates, and was found to have been met. Model discrimination was tested using Harrell’s C. The model fit was assessed using Cox-Snell residuals. All statistical analyses were conducted using Stata/IC 11.2 (College Station, TX, USA). P values < 0.05 were considered statistically significant.
Results
Baseline Characteristics
The study included 4,781 patients; baseline characteristics of the cohort are given in Table 1. Of these patients, 3,121 patients were found to have ceased PD – 1,699 transferred to HD, 495 were transplanted, 875 died whilst on PD and 52 had renal recovery (Figure 1). The proportion of patients ceasing PD over time and falling into one of these categories post-PD commencement is provided in Figure 2. The cumulative incidence at 1 year for each outcome was 19.1% (95% confidence interval (CI) 18.0 – 20.3%) for transferring to HD, 4.5% (CI 3.6 – 5.1%) for transplantation, and 6.5% (CI 5.8 – 7.2%) for death.
TABLE 1.
Baseline Characteristics of Patients on Peritoneal Dialysis (January 2004 – December 2010)
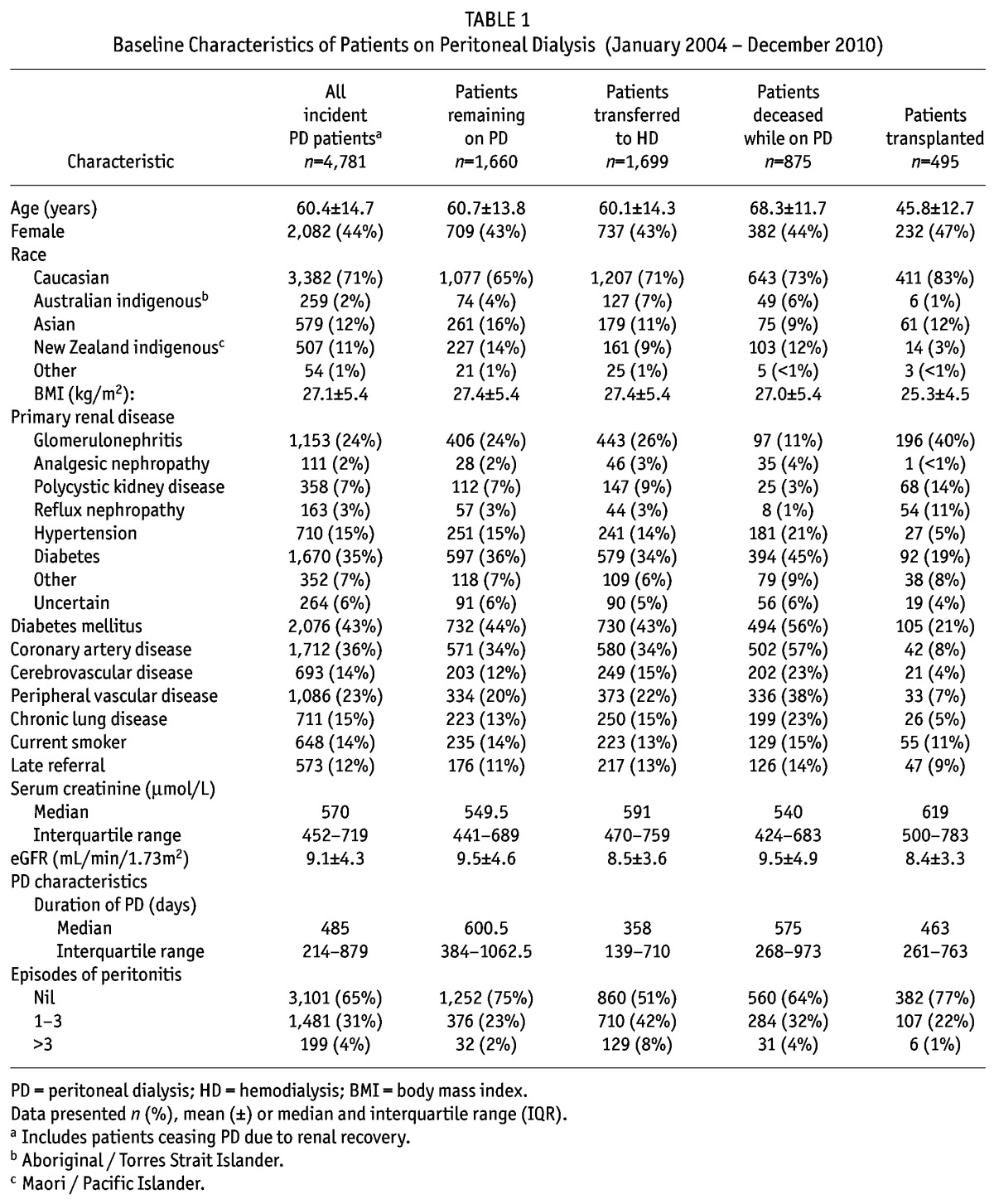
Figure 2 —
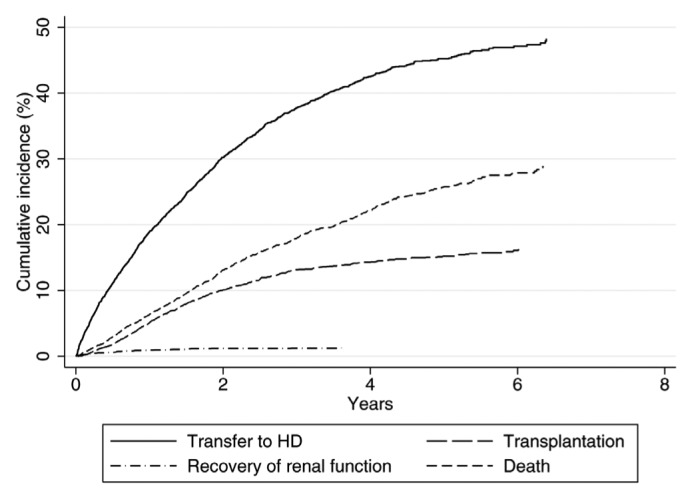
Cumulative incidence of peritoneal dialysis cessation for different reasons. Competing risks model of patients who initially commenced peritoneal dialysis between 2004-10, with time to first incidence. HD = hemodialysis.
The Circumstances of the Transfer
The most common reason for transfer was peritonitis, followed by inadequate dialysis, dialysate leak and being unable to manage self-care (Table 2). Overall, 893 patients (53% of all who transferred) commenced HD with a tunneled catheter, and 252 patients (15% of transfers) commenced with a temporary catheter. Only 554 patients (33% of transfers) commenced with an arteriovenous fistula or graft (AVF/AVG). Even when focusing on the potentially predictable transfers, only 50% of patients commenced with an AVF/AVG (Table 2).
TABLE 2.
Reasons for Transfer to Hemodialysis and Access Used at Time of Transfer
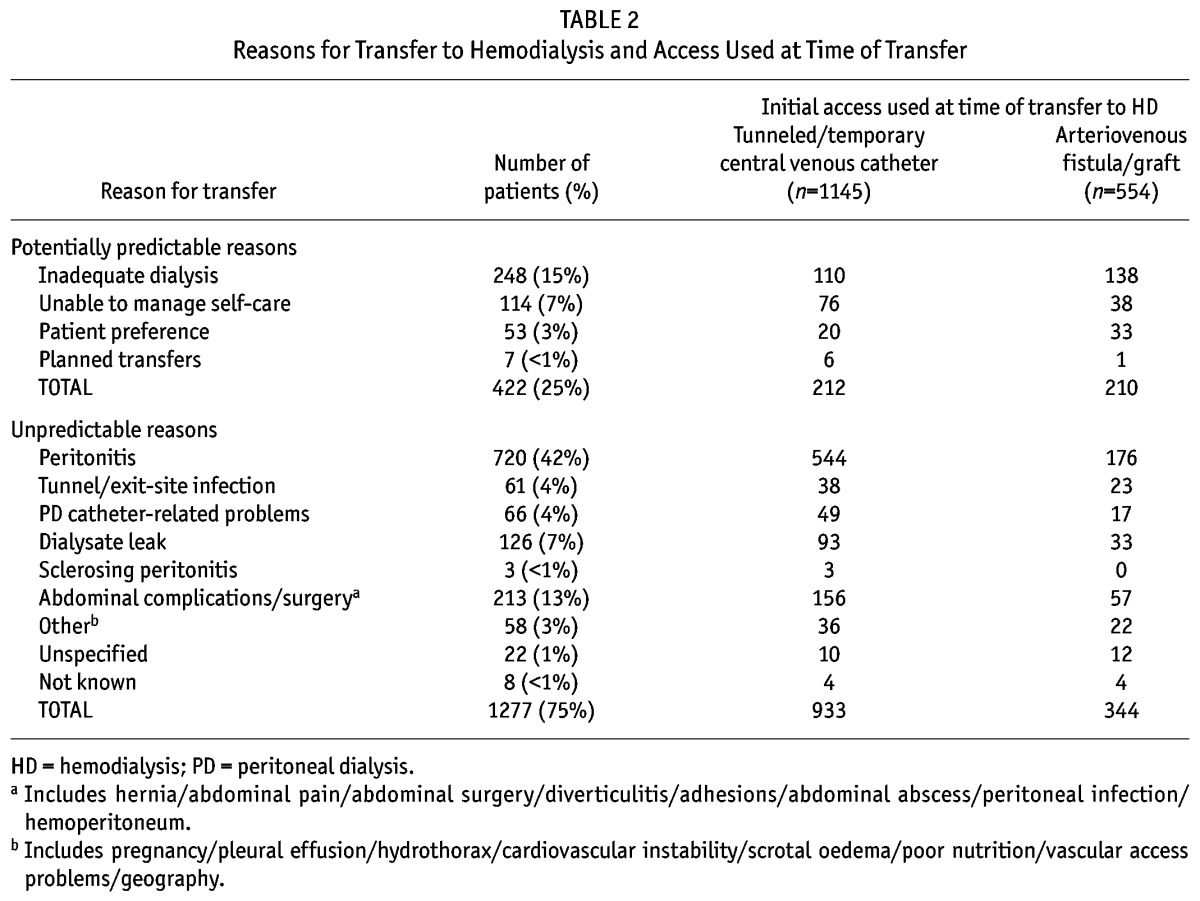
The majority of the transfers occurred at an in-hospital dialysis center (93% of all cases). This was also true for potentially predictable transfers where 87% occurred within hospital. For the patients who transferred, 30% of cases occurred within 6 months of commencing PD, and 51% within 12 months.
When assessing the initial center of dialysis, the percentage of patients who started HD with a catheter ranged from 0 to 100% within each center. This was the same for patients who started HD after a potentially predictable transfer. There was no correlation between each center’s vascular access performance in unpredictable transfers and potentially predictable transfers.
Predictors of Transfer at 6 and 12 Months After Commencing PD
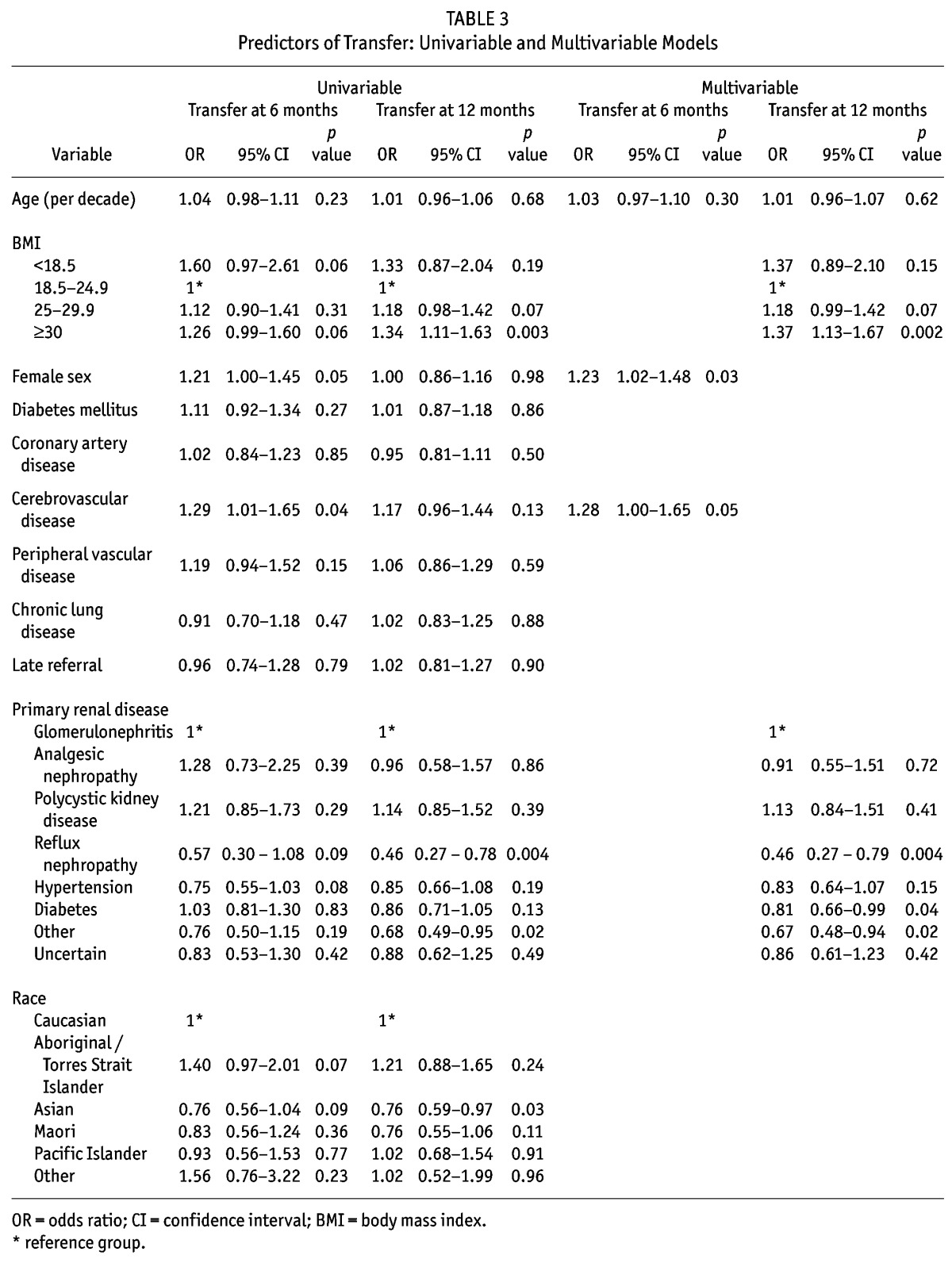
The results for predicting any transfer to HD within 6 and 12 months of commencing PD are provided in Table 3. 515 patients transferred to HD within 6 months and 864 had transferred within 12 months. In the final adjusted model for predicting transfer to HD within 6 months, female gender (odds ratio (OR) 1.23, 95% CI 1.02 – 1.48, p = 0.03) was found to be a statistically significant predictor of transfer, and a history of cerebrovascular disease (OR 1.28, 95% CI 1.00 – 1.65, p = 0.05) was found to be of borderline statistical significance. For transfer within 12 months, body mass index (BMI) (OR dependent on BMI class) and primary renal disease (OR dependent on underlying disease) were statistically significant. However, both models had a poor predictive ability indicating limited clinical significance (c-statistics 0.54 and 0.55, respectively). There was no clinically meaningful change in parameters for either the 6 or 12 month transfer model when clustering for initial center of dialysis.
TABLE 3.
Predictors of Transfer: Univariable and Multivariable Models
Patient Survival Post-Transfer to Hemodialysis
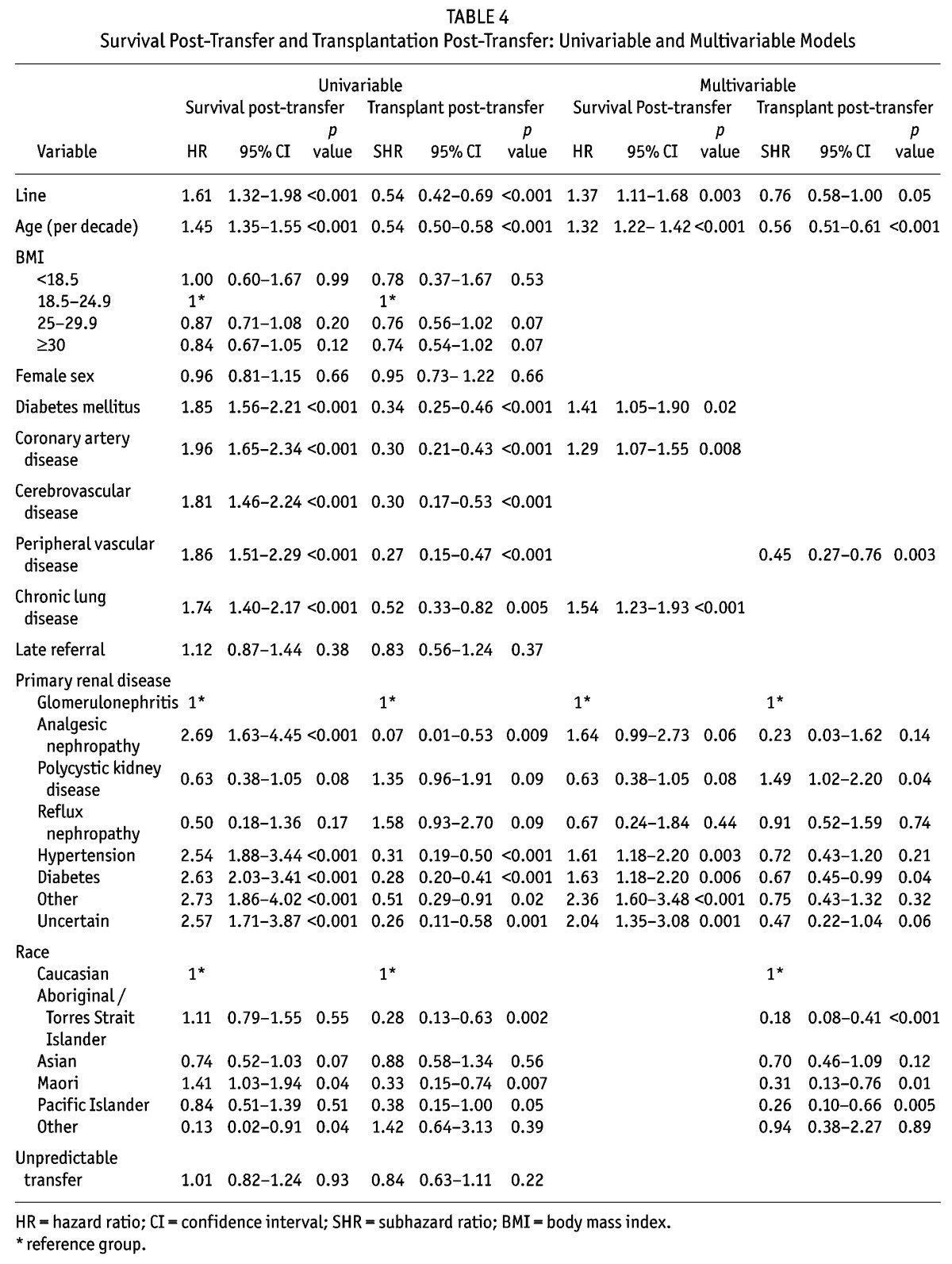
Patient survival post-transfer to HD was higher in those who commenced HD with an AVF/AVG than in those who commenced with a catheter (Figure 3). After adjusting for age and comorbidities, commencing HD with a catheter was associated with significantly increased mortality (hazard ratio (HR) 1.37, 95% CI 1.11 – 1.68, p = 0.003) (Table 4). Additionally, the mortality risk of a catheter at the time of transfer was greater in a potentially predictable transfer compared to an unpredictable transfer (HR 1.64, 95% CI 1.13 – 2.38, p = 0.01 vs HR 1.33, 95% CI 1.04 – 1.71, p = 0.03); however this interaction was not statistically significant (p = 0.36). A sensitivity analysis was performed in patients who transferred and remained on HD for 30 or more days. This confirmed the finding that transferring to HD with a catheter was associated with increased mortality. When stratifying by initial center of dialysis, catheter use continued to be associated with an increased mortality (HR 1.46, 95% CI 1.16 – 1.84, p = 0.001).
Figure 3 —
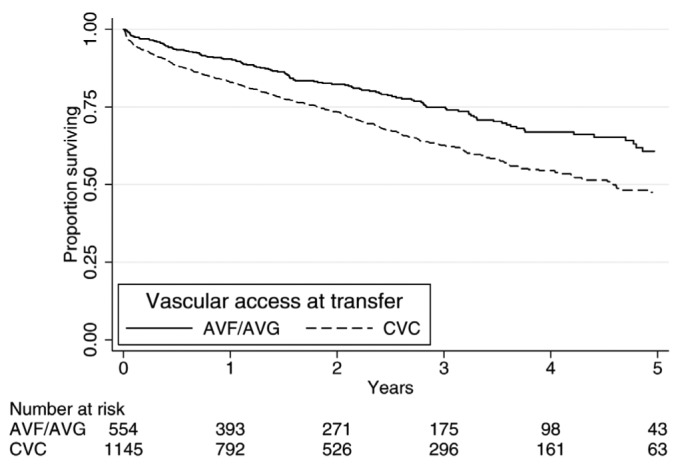
Post-transfer survival according to access at first hemodialysis. Survival after transfer including the numbers of patients up to 5 years post-transfer. AVF = arteriovenous fistula; AVG = arteriovenous graft; CVC = central venous catheter.
TABLE 4.
Survival Post-Transfer and Transplantation Post-Transfer: Univariable and Multivariable Models
Likelihood of Transplantation
In the univariable competing risks regression model (Table 4), commencing HD with a catheter was found to be associated with a lower likelihood of renal transplantation (subhazard ratio (SHR) 0.54, 95% CI 0.42 – 0.69, p < 0.01). However after adjusting for age, race, vascular disease and primary renal disease this effect was of borderline significance (SHR 0.76, 95% CI 0.58 – 1.00, p = 0.05) (Table 4).
Discussion
This study demonstrated that there were no clinically significant predictors of transfer from PD to HD in the Australian and New Zealand incident PD patient populations from baseline registry data. In addition, transferring to HD with a catheter is associated with reduced survival, independent of demographic characteristics, primary renal disease and comorbid illnesses. Furthermore, commencing HD with a catheter may also be associated with a lower incidence of transplantation.
In our analysis, our rate of transfer to HD was different to what has been reported by the ANZDATA Registry previously (2). The reason for this lies in our definition. As mentioned previously, we regarded all transfers to HD regardless of duration or a return to PD as a transfer. In contrast, the ANZDATA Registry only took into account permanent transfers to HD. Our reason for doing so was that we felt that the insertion of a central venous catheter adds to the patient’s burden of potential risks. As such, a temporary transfer that utilizes a functioning AVF does not expose the patient to the risks that are associated with having a catheter. Additionally, the Registry only collects information in regards to the vascular access used at a patient’s first hemodialysis session.
Our study found that of the patients who transferred, 30% did so within 6 months of commencing PD, and 51% within 12 months. This is consistent with previous studies that have demonstrated that the first 6 months of PD are a particularly vulnerable period (4,5). This is an important finding as it suggests that there may be a benefit for planning vascular access creation at the beginning of a patient’s dialysis journey. Unfortunately, which patients will require HD within 6 or 12 months of commencement of PD cannot be determined. The reasons for transfer explain to some extent why the transfer cannot be predicted. In our study, the main reason given for ceasing PD was peritonitis, consistent with what has been described in the literature (4,6,7). Other reasons for transfer that are difficult to predict clinically include dialysate leak, tunnel or exit-site infection, PD-catheter related problems, and abdominal complications or surgery.
Focusing on the predictors of transfer to HD within 6 months of commencing PD, we found that patient gender and a past history of cerebrovascular disease (borderline) were statistically significant in an age-adjusted multivariable model. However, the effect for both was not strong enough to affect a significant clinical endpoint. For transfer within 12 months of commencing PD, BMI and primary renal disease were the only statistically significant predictors. Again, their effect was not strong enough to affect a significant clinical endpoint. Additionally, it is important to note that the predictors found for both 6 and 12 months were not the same. This adds further evidence to the unpredictable nature of any transfer to HD.
In our study, increasing age was not found to be a predictor of transfer to HD at either 6 or 12 months. This is in keeping with what was found by Guo et al. in a study performed on PD patients in the United States (4). However, other studies have documented an increased risk for technique failure with increased age (8,11). In contrast to this, an Italian study performed by Maiorca demonstrated that technique failure was inversely related to age (10). This conflicting information clearly illustrates the complex nature of patients on dialysis, and demonstrates that the results of our study are not out of keeping with what is available in the literature. Additionally, the general characteristics of our patients were similar to those reported in other countries in regards to age and comorbidities (4,7,15,21).
In addition to age, other factors such as race, diabetes, coronary artery disease, peripheral vascular disease and late referral were not found to assist in determining which patients commenced on PD would require transfer to HD within 6 or 12 months.
In patients who transferred to HD, commencing HD with a catheter was associated with reduced survival. Even after adjusting for patient demographics, primary renal disease and comorbidities, the effect of commencing with a catheter was both statistically and clinically significant (HR 1.38, 95% CI 1.12 – 1.70, p = 0.002). This association has not been previously documented within the incident PD patient population; however, it is consistent with the findings of previous studies assessing the risks associated with catheter access in the incident HD population (16–18). Reasons proposed for this association include catheter-related complications, particularly infection, inadequate HD, and a higher prevalence of comorbidities in those who commence with a catheter. These issues are also relevant to our PD population who transfer to HD. Additionally, there may be selection bias in determining which patients transfer using a catheter. For instance, peritonitis is the leading cause for transfer, and cannot be predicted. As such, patients who transfer due to peritonitis are more likely to commence with a catheter, and at the same time, peritonitis has been found to be associated with reduced survival (22).
Commencing HD with a catheter was also found to be associated with a lower incidence of renal transplantation. This may reflect the fact that patients who commence with lines become too unwell for transplantation as a result of catheter-related complications. Alternatively, this may represent confounding as those who started HD with a line were older and had more comorbidities. Although our analyses adjusted for these factors, there may be additional confounders not recorded in ANZDATA.
In regards to the reasons for transfer, we found that a “potentially” predictable transfer was not associated with either increased survival or an increased likelihood of renal transplantation. However, only 50% of these transfers commenced with an AVF/AVG. This is an important issue that needs to be further addressed.
The demonstrated unpredictable nature of any transfer to HD, and the worse patient outcomes in patients unprepared for the transfer, emphasizes the crucial question of the timing of vascular access creation in patients on PD. A number of previous studies have attempted to address the issue of concurrent formation of an AVF and PD catheter insertion (23,24). The main conclusions from these studies have been that this practice was of no clinical benefit. However, these studies have been limited by being of small sample size, retrospective in nature, and performed over a decade ago. Furthermore, there may be a subgroup of patients (for example, those with a high life expectancy but who are unlikely to receive a kidney transplant) who would have the most chance of benefitting from early AVF formation. In light of the results of this study, this practice or a variation of this practice, such as vascular access creation after 12 months on PD, may now be appropriate. However, further research is required.
The main strength of our study lies in the fact that its cohort included all patients who commenced PD as their first form of renal replacement therapy in both Australia and New Zealand. As such we were able to capture a large number of patients, over a wide spectrum of demographic and socioeconomic situations. Additionally, we were able to follow the patients for up to six years, covering a large period of their dialysis history.
Our study has a number of limitations. Firstly, it is a retrospective cohort study using registry data. As such, it can only demonstrate the significant association between catheter use and increased mortality, and not demonstrate causation. Another limitation of the study is that we were not able to obtain further information on the patient’s PD characteristics. This includes peritoneal membrane transporter status and residual renal function, both of which have been found to influence PD technique failure previously (12). Finally, our findings may not be applicable to all PD populations worldwide. This is because the current dialysis practices in Australia and New Zealand may be different from other countries.
In conclusion, the transfer from PD to HD cannot be predicted, and those who commence HD with a line have a higher risk of mortality and lower likelihood of receiving renal transplantation. As a result, better preparation of patients for any transfer to HD in regards to vascular access is required. Further research is required in this area to determine how to achieve this.
Disclosure
The authors report no financial conflicts of interest.
Acknowledgments
The authors gratefully acknowledge the Australian and New Zealand renal units, patients and staff who contributed data to the Registry.
REFERENCES
- 1. McDonald SP, Marshall MR, Johnson DW, Polkinghorne KR. Relationship between dialysis modality and mortality. J Am Soc Nephrol 2009; 20:155–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Grace B, Hurst K, McDonald S. 34th Annual ANZDATA Report. Adelaide, South Australia: Australia and New Zealand Dialysis and Transplantation (ANZDATA) Registry; 2011. [Google Scholar]
- 3. Chiarelli G, Beaulieu M, Cozzolino M, Singh S, Kiaii M, Taylor P, et al. Vascular access planning in peritoneal dialysis patients. Perit Dial Int 2008; 28:585–90. [PubMed] [Google Scholar]
- 4. Guo A, Mujais S. Patient and technique survival on peritoneal dialysis in the United States: evaluation in large incident cohorts. Kidney Int Suppl 2003; 88:S3–12. [DOI] [PubMed] [Google Scholar]
- 5. Descoeudres B, Koller MT, Garzoni D, Wolff T, Steiger J, Schaub S, et al. Contribution of early failure to outcome on peritoneal dialysis. Perit Dial Int 2008; 28:259–67. [PubMed] [Google Scholar]
- 6. Mujais S, Story K. Peritoneal dialysis in the US: evaluation of outcomes in contemporary cohorts. Kidney Int Suppl 2006; 103:S21–6. [DOI] [PubMed] [Google Scholar]
- 7. Jager KJ, Merkus MP, Dekker FW, Boeschoten EW, Tijssen JG, Stevens P, et al. Mortality and technique failure in patients starting chronic peritoneal dialysis: results of The Netherlands Cooperative Study on the Adequacy of Dialysis. NECOSAD Study Group. Kidney Int 1999; 55:1476–85. [DOI] [PubMed] [Google Scholar]
- 8. Beddhu S, Zeidel ML, Saul M, Seddon P, Samore MH, Stoddard GJ, et al. The effects of comorbid conditions on the outcomes of patients undergoing peritoneal dialysis. Am J Med 2002; 112:696–701. [DOI] [PubMed] [Google Scholar]
- 9. Jansen MA, Termorshuizen F, Korevaar JC, Dekker FW, Boeschoten E, Krediet RT. Predictors of survival in anuric peritoneal dialysis patients. Kidney Int 2005; 68:1199–205. [DOI] [PubMed] [Google Scholar]
- 10. Maiorca R, Cancarini GC, Zubani R, Camerini C, Manili L, Brunori G, et al. CAPD viability: a long-term comparison with hemodialysis. Perit Dial Int 1996; 16:276–87. [PubMed] [Google Scholar]
- 11. Huisman RM, Nieuwenhuizen MG, Th de Charro F. Patient-related and centre-related factors influencing technique survival of peritoneal dialysis in The Netherlands. Nephrol Dial Transplant 2002; 17:1655–60. [DOI] [PubMed] [Google Scholar]
- 12. Rumpsfeld M, McDonald SP, Johnson DW. Higher peritoneal transport status is associated with higher mortality and technique failure in the Australian and New Zealand peritoneal dialysis patient populations. J Am Soc Nephrol 2006; 17:271–8. [DOI] [PubMed] [Google Scholar]
- 13. Cueto-Manzano AM, Quintana-Pina E, Correa-Rotter R. Long-term CAPD survival and analysis of mortality risk factors: 12-year experience of a single Mexican center. Perit Dial Int 2001; 21:148–53. [PubMed] [Google Scholar]
- 14. Snyder JJ, Foley RN, Gilbertson DT, Vonesh EF, Collins AJ. Body size and outcomes on peritoneal dialysis in the United States. Kidney Int 2003; 64:1838–44. [DOI] [PubMed] [Google Scholar]
- 15. Chidambaram M, Bargman JM, Quinn RR, Austin PC, Hux JE, Laupacis A. Patient and physician predictors of peritoneal dialysis technique failure: a population based, retrospective cohort study. Perit Dial Int 2011; 31:565–73. [DOI] [PubMed] [Google Scholar]
- 16. Polkinghorne KR, McDonald SP, Atkins RC, Kerr PG. Vascular access and all-cause mortality: a propensity score analysis. J Am Soc Nephrol 2004; 15:477–86. [DOI] [PubMed] [Google Scholar]
- 17. Dhingra RK, Young EW, Hulbert-Shearon TE, Leavey SF, Port FK. Type of vascular access and mortality in U.S. hemodialysis patients. Kidney Int 2001; 60:1443–51. [DOI] [PubMed] [Google Scholar]
- 18. Pastan S, Soucie JM, McClellan WM. Vascular access and increased risk of death among hemodialysis patients. Kidney Int 2002; 62:620–6. [DOI] [PubMed] [Google Scholar]
- 19. Kalbfleisch J, Prentice R. The statistical analysis of failure time data. New York, United States: Wiley; 1980. [Google Scholar]
- 20. Fine J, Gray R. A proportional hazards model for the subdistribution of a competing risk. J Amer Statist Assoc 1999; 94:496–509. [Google Scholar]
- 21. Li PK, Szeto CC. Success of the peritoneal dialysis programme in Hong Kong. Nephrol Dial Transplant 2008; 23:1475–78. [DOI] [PubMed] [Google Scholar]
- 22. Boudville N, Kemp A, Clayton P, Lim W, Badve SV, Hawley CM, et al. Recent peritonitis associates with mortality among patients treated with peritoneal dialysis. J Am Soc Nephrol 2012; 23:1398–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Beckingham IJ, O’Rourke JS, Bishop MC, Blamey RW. Are backup arteriovenous fistulae necessary for patients on continuous ambulatory peritoneal dialysis? Lancet 1993; 341:1384–6. [DOI] [PubMed] [Google Scholar]
- 24. Chui AK, Chiu EY, White EA, Yumiba T. An investigation into the practice of concurrent chronic ambulatory peritoneal dialysis catheter insertion and arteriovenous fistula formation in patients needing dialysis. Hong Kong Med J 2000; 6:312–5. [PubMed] [Google Scholar]