Abstract
♦ Objectives:
There is a lack of consensus on the risk factors for hernia formation, and the impact on peritoneal dialysis (PD) survival has seldom been studied.
♦ Methods:
This was a population-based study and all collected data were retrieved from the National Health Insurance Research Database of Taiwan. Patients who commenced PD between January 1998 and December 2006 were screened for inclusion. Multiple logistic regression and Cox proportional hazards models were applied to estimate the predictors for hernia formation and determine the predictors of PD withdrawal.
♦ Results:
A total of 6,928 PD patients were enrolled and followed until December 2009, with 631 hernia events and 391 hernioplasties being registered in 530 patients (7.7%). The incidence rate was 0.04 hernias/patient/year. Longer PD duration (per 1 month increase, hazard ratio (HR) 1.019) and history of mitral valve prolapse (MVP) (HR 1.584) were independent risk factors for hernia formation during PD, and female gender (HR 0.617) was a protective factor. On the other hand, there were 4,468 PD withdrawals, with cumulative incidence rates of 41% at 1 year, 66% at 3 years, and 82% at 5 years. Independent determinants for cumulative PD withdrawal included hernia formation during PD (HR 1.154), age (per 1 year increase, HR 1.014), larger dialysate volume (per 1 liter increase, HR 0.496), female gender (HR 0.763), heart failure (HR 1.092), hypertension (HR 1.207), myocardial infarction (HR 1.292), chronic obstructive pulmonary disease (COPD) (HR 1.227), cerebrovascular accident (CVA) (HR 1.364), and history of MVP (HR 0.712)
♦ Conclusions:
Prolonged PD duration was a risk factor for hernia formation, and female gender was protective. Hernia formation during PD therapy may increase the risk of PD withdrawal.
Keywords: Peritoneal dialysis, hernia, risk factor, survival
Abdominal hernias are relatively common non-infectious complications in peritoneal dialysis (PD) patients with reported prevalence rates ranging from 7% to 27.5% (1–5), and an incidence of 0.05 to 0.08 hernias per dialysis year (2,5,6). The risk factors for hernia formation include multiparous females, elderly males, polycystic kidney disease (PCKD), low body weight, previous hernia repair, history of more than three laparotomies, and midline incision for PD catheter placement (2,5,7,8). A larger dialysate diffusion volume is associated with increased intra-peritoneal pressure (IPP), and has been reported to increase the risk of hernia (9). However, some studies have not found a positive correlation between dialysate volume and hernia formation (2,10). Moreover, only a few studies have examined the influence of hernia formation on PD survival.
The aim of this study was to establish the incidence and the risk factors for hernia formation in patients receiving PD using the National Health Insurance Research Database (NHIRD) of Taiwan. In addition, we explored the impact of hernia formation on PD survival in a population-based incident cohort of PD patients.
Methods
The National Health Insurance program, which provides compulsory universal health insurance, was implemented in Taiwan in 1995. It currently enrolls up to 99% of the Taiwanese population and contracts with 97% of all medical providers. The National Health Insurance Research Database (NHIRD) contains comprehensive information on insured subjects, including identification number, gender, birth date, dates of admission and discharge, dates of clinical visits, ICD-9-CM (International Classification of Diseases, 9th Revision, Clinical Modification) diagnostic (up to five) and procedure codes (up to five), details of prescriptions, and amount of expenditure.
From the NHIRD, all patients who started receiving PD treatment between January 1998 and December 2006 were screened for inclusion. Patients were excluded if they had visited the PD clinic less than three times, did not have a recorded final register or dialysate prescription date in the NHIRD, or had a hernia or hernioplasty before the start of PD therapy. Eligible patients were divided into two groups: the hernia (patients with hernia or hernioplasty during PD) and no-hernia groups. The recruited patients were followed from the start of PD therapy until 31 December 2009.
Definitions
Start of PD Therapy: The date of the first PD clinic visit after the first peritoneal dialysate prescription was defined as the start of PD therapy.
End of PD Therapy: Withdrawal of PD therapy was defined as a final peritoneal dialysate prescription at least 30 days before the last follow-up date. The date of the last peritoneal dialysate prescription was defined as the end of PD therapy.
ICD Codes of Hernia, Hernioplasty and Other Comorbidities: From the NHIRD, we collected the date of birth, date of death, demographics, and comorbidities of the patients. The comorbidities included hypertension (ICD-9-CM 401-405), diabetes mellitus (DM, ICD-9-CM 250.1-9), heart failure (HF, ICD-9-CM 428.0-428.43, 428.9, 398.91), myocardial infarction (MI, ICD-9-CM 410, 412), chronic obstructive pulmonary disease (COPD, ICD-9-CM 490-496), cerebrovascular accident (CVA, ICD-9-CM 430-435), hernia (ICD-9-CM 550-553), hepatitis B virus carrier (HBV, ICD-9-CM 070.22, 070.23, 070.32, 070.33), hepatitis C virus carrier (HCV, ICD-9-CM 070.44, 070.54, 070.70, 070.71), mitral valve prolapse (MVP, ICD-9-CM 424.0), polycystic kidney disease (PCKD, ICD-9-CM 753.12, 753.13, 753.14), and peritonitis (ICD-9-CM 567.9, 996.68, 999.3). A diagnosis of systemic lupus erythematosus (SLE) was identified based on catastrophic illness certificates. The occurrence of hernioplasty was recorded according to operation codes.
Statistical Methods
The SAS statistical package, version 9.2 (SAS Institute, Inc., Cary, NC, USA), and SPSS version 18 (SPSS Inc., Chicago, IL, USA) were used for data analysis. Pearson’s chi-square test was used for categorical variables, demographic characteristics (gender) and comorbidities. Continuous variables, such as age, maximal dialysate volume, and PD duration were analyzed with two-sample t-tests. Multiple logistic regression was used to estimate the predictors for hernia formation or hernioplasty during PD based on age, gender, maximal dialysate volume, PD duration and comorbidities. Univariate Cox proportional hazards regression was applied to estimate the individual odds, including a time-dependent variable of hernia or hernioplasty during PD, for cumulative PD withdrawal. The significant variables (p < 0.05) in the univariate analysis were put into a final forward stepwise multivariate Cox model. The survival curves were generated using Kaplan-Meier survival analysis. All statistical assessments were two-sided and evaluated at the 0.05 level of significant difference.
Results
A total of 8,272 patients who commenced PD therapy between January 1998 and December 2006 were screened. We excluded those who had visited the PD clinic less than three times (n = 1,120), did not have a recorded final register date (n = 6), did not have a last dialysate prescription date (n = 4), or had a hernia or hernioplasty before the start of PD therapy (n = 214). Therefore, a total of 6,928 patients were enrolled in the study and were followed until December 2009 (Figure 1). The baseline characteristics of the patients were as follows: average age, 52 ± 16.9 (mean ± SD) years; females, 57.9% (n = 4,011); diabetic patients, 43.1% (n = 2,989); mean duration on PD therapy, 26.3 ± 22.5 months; average frequency of PD clinic visit, 30.84 ± 11.03 days/visit.
Figure 1 —

Algorithm of patient collection. PD = peritoneal dialysis.
A total of 631 hernia events and 391 hernioplasties were registered during PD in 530 patients (7.7% of the total population). The overall incidence rate was 0.04 hernias/patient/year. As shown in Figure 2, the types of hernia were inguinal in 306 cases (48.5%), femoral in 16 (2.5%), umbilical in 76 (12%), ventral in 140 (22.2%), diaphragmatic in 47 (7.4%), and others in 46 (7.3%). The mean time from the start of PD to the development of a hernia was 15.9 ± 17.2 months (range 0.3 – 105 months).
Figure 2 —

Distribution of abdominal hernias in 530 of 6,928 peritoneal dialysis patients from our survey.
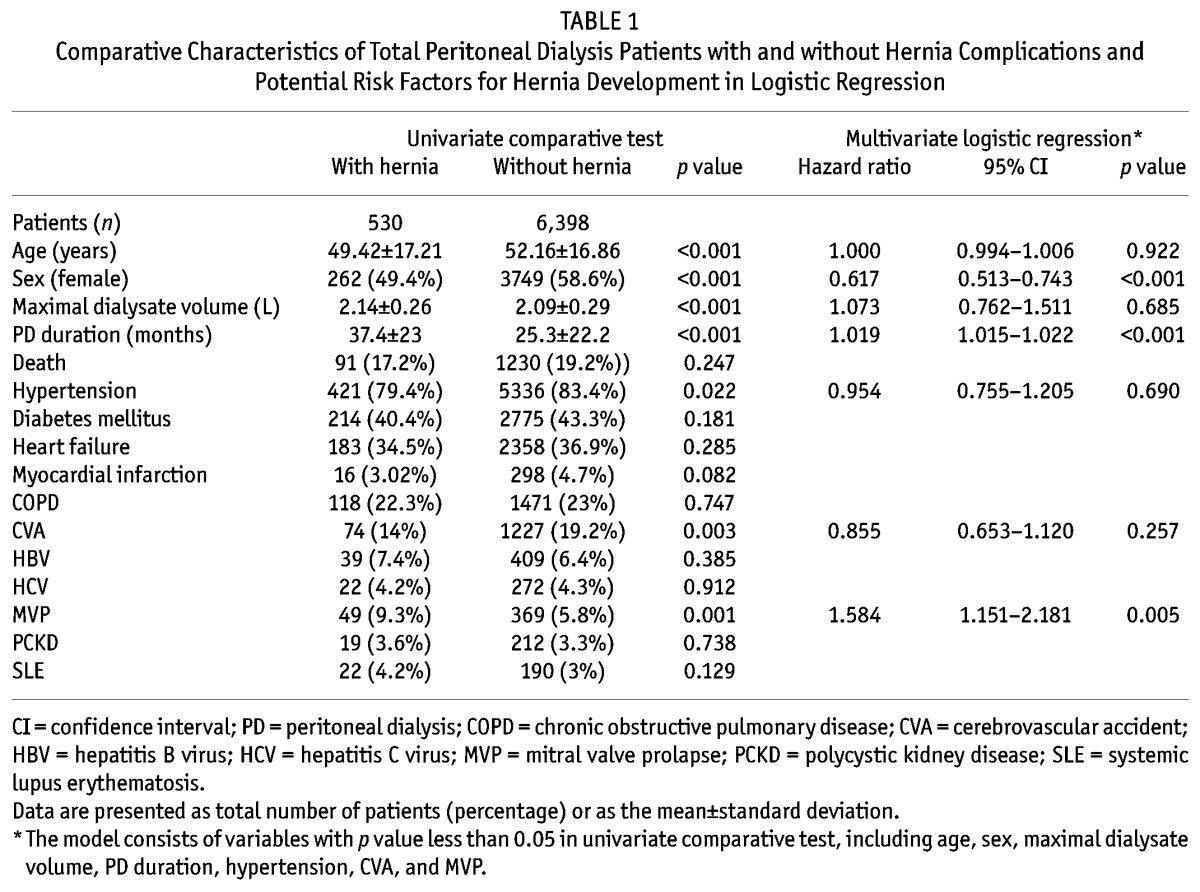
Table 1 summarizes the general characteristics of the patients with and without hernia. Compared to the patients without a hernia, the patients with a hernia were younger, used a larger volume of dialysate per exchange, were more likely to have a diagnosis of MVP, and underwent a longer duration of PD. Female patients and those with hypertension or CVA were less likely to develop a hernia during PD. There were no significant differences in other comorbidities including DM, HF, HBV, HCV, PCKD, SLE, and death between the two groups. Logistic regression analysis showed that a longer PD duration (per 1 month increase, hazard ratio (HR), 1.019; 95% confidence interval (CI), 1.015 – 1.022) and a diagnosis of MVP (HR, 1.584; 95% CI, 1.151 – 2.181) were independent risk factors for the development of a hernia during PD, whereas female gender (HR, 0.617; 95% CI, 0.513 – 0.743) was a protective factor. The other significant variables studied, including age, maximal dialysate volume, hypertension and CVA, were not determinants.
TABLE 1.
Comparative Characteristics of Total Peritoneal Dialysis Patients with and without Hernia Complications and Potential Risk Factors for Hernia Development in Logistic Regression
When stratified by gender, logistic regression analysis showed that a longer PD duration was an independent risk factor for hernia development in both male (HR, 1.02; 95% CI, 1.014 – 1.025) and female (HR, 1017; 95% CI, 1.013 – 1.022) patients. However, past histories of MVP (HR, 2.06; 95% CI, 1.276 – 3.328) or SLE (HR, 2.701; 95% CI, 1.142 – 6.389) were also independent risk factors for hernia development in males (Tables 2 and 3).
TABLE 2.
Comparative Characteristics of Male Peritoneal Dialysis Patients with and without Hernia Complications and Potential Risk Factors for Hernia Development in Logistic Regression

TABLE 3.
Comparative Characteristics of Female Peritoneal Dialysis Patients with and without Hernia Complications and Potential Risk Factors for Hernia Development in Logistic Regression

During the follow-up period (mean, 3.46 years), there were 1,321 deaths and 4,468 PD withdrawals. The overall 1-, 3- and 5-year survival rates were 87%, 78%, and 74%, respectively. The mortality rate was 12.6 per 100 patient-years. PD withdrawals were common, with cumulative incidence rates of 41% at 1 year, 66% at 3 years, and 82% at 5 years.
The cumulative PD survival was significantly decreased in patients with newly developed hernia during PD (Figure 3). We performed a Cox regression analysis to examine the independent determinants on cumulative PD survival (Table 4). The univariate model, including a time-dependent factor of hernia or hernioplasty during PD, showed that the factors associated with PD withdrawal were age, gender, maximal dialysate volume, and history of hypertension, DM, HF, MI, COPD, CVA, MVP, and hernia or hernioplasty during PD. All significant factors with p level less than 0.05 were than analyzed by the forward stepwise multivariate Cox regression. The results showed that the independent predictors included hernia or hernioplasty during PD (HR, 1.154; 95% CI, 1.032 – 1.289), age (per 1 year increase, HR, 1.014; 95% CI, 1.012 – 1.016), female gender (HR, 0.763; 95% CI, 0.718 – 0.811), maximal dialysate volume (per 1 liter increase, HR, 0.496; 95% CI, 0.446 – 0.55), histories of hypertension (HR, 1.207; 95% CI, 1.112 – 1.31), HF (HR, 1.092; 95% CI, 1.026 – 1.162), MI (HR, 1.292; 95% CI, 1.128 – 1.48), COPD (HR, 1.227; 95% CI, 1.139 – 1.32), CVA (HR, 1.364; 95% CI, 1.262 – 1.475), and MVP (HR, 0.712; 95% CI, 0.646 – 0.836). A history of DM was not significantly associated with the outcome.
Figure 3 —

Kaplan-Meier estimate of cumulated PD survival in patients with (dotted line) or without (solid line) hernias.
TABLE 4.
Hazard Ratios and 95% Confidence Intervals of Potential Risk Factors Associated with PD Withdrawal in Univariate Cox Regression and Multivariate Forward Stepwise Cox Regression

Discussion
This study used the Taiwan NHIRD to investigate the risk factors for hernia formation in PD patients and the impact on PD survival. We found that a longer duration on PD and a history of MVP were more likely and female gender was less likely to lead to hernia formation during PD therapy. In addition, hernia formation during PD therapy increased the risk of PD withdrawal.
In this study, there was a 7.7% prevalence rate and an incidence rate of 0.04 hernias/patient/year. Multiparous female was demonstrated to be one of the risk factors of hernia development in PD patients (11). In contrast, a more recent study with 1,864 patients from 75 randomly selected units in North America reported that female gender conferred an 80% reduction in the risk for the development of hernias (5). Our results also showed that female gender had a 38% reduction in risk of hernia formation (HR, 0.617; p < 0.001). However, the results may be biased by patient selection since multiparous women are possibly more commonly discouraged from choosing PD. Unfortunately, given that the status of childbirth is not recorded in the NHIRD, it is not possible to evaluate the effect of parity in this study.
When stratified by gender, longer stay in PD therapy remained a significant risk factor of hernia formation for both sexes (for every extra month, HR 1.02 in males; HR 1.017 in females). On the other hand, a comorbidity of MVP or SLE increased the risk of hernia only in males (HR 2.06 and 2.701, respectively). There are few studies discussing the relationship between MVP, SLE and abdominal hernia. Increased risk of MVP and abdominal hernia has been reported in patients with connective tissue disorders, including PCKD, benign joint hypermobility syndrome, Marfan syndrome, and Ehlers-Danlos Syndrome (12,13). However, in this study, MVP remained a significant risk factor whether PCKD was put into the regression model or not (data not shown). The possible linkage between MVP, SLE and abdominal hernia in male PD patients needs further evaluation.
PCKD has been shown to be an independent risk factor for hernias in dialysis patients in previous studies (2,5,14,15). Whereas increased IPP or other extracellular matrix production defects have been suggested as possible reasons, other authors have not found an increased incidence of hernias among patients with PCKD (16). In the current study, we were unable to demonstrate a significant difference in the incidence of PCKD between the patients with and without hernias (3.6% and 3.3%, respectively). Because patients with a hernia before PD therapy were excluded from this study, our results may provide evidence that further increases in IPP with dialysate infusion in PCKD patients may not increase the risk of hernia formation.
Pressure within the abdomen is increased in PD patients due to the presence of dialysis fluid in the peritoneal cavity. Although Dejardin et al. demonstrated a positive correlation between higher body mass index, larger dialysate volume and higher IPP (10), an association between the level of IPP and the risk of hernia in PD patients is still controversial. An increased IPP has been shown to correlate with the development of hernias in PD patients (9,17), yet other studies have not reported a correlation between IPP, dialysate volume and the risk of hernia formation (2,3,7,10). In our study, after the logistic regression analysis, a larger maximal dialysate volume was not significantly related to the risk of hernia formation, which further supports the idea that increased IPP alone may not increase the risk of hernia formation.
According to a recent study, survival on PD continues to improve with only modest changes in PD technique failure (18). The probability of patient survival at 5 years has been reported to range from 46.9 – 73.5% in different series (19–22). In this study, the overall 1-, 3- and 5-year survival rates were 87%, 78%, and 74%, respectively. However, patient withdrawal from PD therapy was quite frequent during follow-up, with cumulative PD withdrawal rates of 41% at 1 year, 66% at 3 years, and 82% at 5 years. After forward stepwise multivariate Cox regression analysis, hernia formation during PD therapy, older age at the start of PD therapy, and the comorbidities of hypertension, HF, MI, COPD, and CVA were found to be independently associated with an increased risk of cumulative PD withdrawal.
Only a few studies have explored the relationship between mechanical complications and technique survival in PD patients. Two observational studies revealed no negative influence of hernia formation on the dropout rates (23,24). However, hernia formation during PD therapy was associated with a 15% increased risk of PD withdrawal in our study (p = 0.012). It is well known that hernias can present as a tender lump, recurrent gram-negative peritonitis, as well as bowel obstruction and perforation if there is strangulation or incarceration of the bowel. Patients treated operatively usually have an excellent prognosis and are usually able to continue PD (1,25). Nevertheless, the development of a hernia during PD may limit the volume of dialysate prescription, thus leading to inadequate solute clearance. In addition, if the hernia recurs, the patient may be more prone to switch to hemodialysis.
Consistent with our results, advanced age, cardiovascular disease, HF, COPD, and CVA have been proven to be risk factors for death on PD or for switching to HD (19,26–30). A greater prevalence of comorbid diseases is likely to be responsible for the higher risk of mortality in the elderly. However, the findings in studies comparing technique failure between young and elderly PD patients remain inconsistent. A study using Australian and New Zealand Dialysis Registry data reported that while elderly patients had higher peritonitis-related and all-cause mortality, they had superior technique survival and similar peritonitis-free survival (23). Due to the limitations of registry data, further studies are needed to clarify these incongruous results.
The impact of gender on PD survival is still under debate. Although some studies showed no difference in technique failure (31,32), a recent study of 1,587 incident PD patients in the US revealed a 22% decreased risk of technique failure for females (31), which is consistent with our findings. We speculate that the survival advantage in female patients may be due to smaller body sizes, better compliance with standard exchange procedures, or more independence from others in maintaining their self-care than male patients. On the other hand, there is scant research discussing the relationship between dialysate volume and PD survival. In this study, a larger amount of maximal dialysate prescription was related to a lower risk of cumulative PD withdrawal. The larger dialysate volume per exchange may reflect either that PD was more tolerable in these patients, or that because one has a lower transporter character in peritoneal equilibration test (PET), larger dialysate volume was needed to maintain adequate dialysis. However, as the results of Kt/V and PET were not included in this registry data, it would be a major limitation for us to discuss this issue.
The impact of MVP on PD survival has never been discussed. Our results showed that patients with MVP had a 26.5% lower risk of cumulative PD withdrawal (p < 0.001). MVP syndrome has been associated with autonomic dysfunction, joint hypermobility syndrome, connective tissue disorders, anxiety and panic disorders in previous studies (33–36). Several loci on chromosomes 11, 13, and 16 have been found to be linked to MVP (36). Its beneficial effect on PD survival may be attributed to an overcautious personality or other associated anomalies in connective tissue. Further studies are needed to examine the exact relationship between MVP and PD survival.
DM has been demonstrated to be a risk factor for technique failure, death, and PD withdrawal in previous studies (19,22,26,27,37). Episodes of peritonitis and early-onset peritonitis have also been reported to be predictors for technique failure and mortality (38,39). In addition, it has been reported that, when compared to age- and gender-matched non-SLE non-diabetic patients, SLE patients undergoing PD have a poorer technique survival and higher mortality rate (40,41). However, in our study, DM, PD-related peritonitis, and SLE were not independent predictors for PD withdrawal in the multivariate Cox regression analysis. Further studies are required to determine whether the inconsistent results came from registration errors, racial differences, or other unknown reasons.
The strengths of the present study include its very large sample size and inclusiveness. We included all incident patients receiving PD in Taiwan during the study period, which greatly enhances the external validity of the findings. However, there are also several limitations to this study. The NHIRD does not record information such as methods of PD catheter insertion, patient height, body weight, body mass index, patient compliance, individual unit management protocols, PD training methods or times, laboratory values (such as C-reactive protein, hemoglobin, and serum albumin), adequacy of PD, residual renal function, severity of the comorbidities, visual acuity, cognitive function, nutritional status, education level, quality-of-life data, and whether PD was self-performed or assisted. Furthermore, insofar as the NHIRD is a voluntary registry and no external audit for data accuracy is performed, the possibility of coding or classification bias cannot be excluded.
Conclusion
Our results indicate that a prolonged PD duration and a history of MVP were risk factors for hernia formation, and that female gender was protective. Our results also revealed that hernia formation during PD therapy increased the risk of PD withdrawal. Further studies are needed to clarify the causative factors which may provide further treatment targets to help prevent withdrawal from PD.
Disclosures
The authors have no financial conflicts of interest in this study to declare.
REFERENCES
- 1. Garcia-Urena MA, Rodriguez CR, Vega Ruiz V, Carnero Hernandez FJ, Fernandez-Ruiz E, Vazquez Gallego JM, et al. Prevalence and management of hernias in peritoneal dialysis patients. Perit Dial Int 2006; 26(2):198–202. [PubMed] [Google Scholar]
- 2. Del Peso G, Bajo MA, Costero O, Hevia C, Gil F, Diaz C, et al. Risk factors for abdominal wall complications in peritoneal dialysis patients. Perit Dial Int 2003; 23(3):249–54. [PubMed] [Google Scholar]
- 3. Hussain SI, Bernardini J, Piraino B. The risk of hernia with large exchange volumes. Adv Perit Dial 1998; 14:105–7. [PubMed] [Google Scholar]
- 4. Suh H, Wadhwa NK, Cabralda T, Sokunbi D, Pinard B. Abdominal wall hernias in ESRD patients receiving peritoneal dialysis. Adv Perit Dial 1994; 10:85–8. [PubMed] [Google Scholar]
- 5. Van Dijk CM, Ledesma SG, Teitelbaum I. Patient characteristics associated with defects of the peritoneal cavity boundary. Perit Dial Int 2005; 25(4):367–73. [PubMed] [Google Scholar]
- 6. Mekki MO, Fedail HM, Ali EM, Abdelraheem MB, Al-Sanousi H, Elamin S, et al. Non-infectious complications of peritoneal dialysis among Sudanese patients: five years experience. Arab J Nephrol Transplant 2011; 4(1):27–30. [DOI] [PubMed] [Google Scholar]
- 7. Afthentopoulos IE, Panduranga Rao S, Mathews R, Oreopoulos DG. Hernia development in CAPD patients and the effect of 2.5 L dialysate volume in selected patients. Clin Nephrol 1998; 49(4):251–7. [PubMed] [Google Scholar]
- 8. Saha TC, Singh H. Noninfectious complications of peritoneal dialysis. South Med J 2007; 100(1):54–8. [DOI] [PubMed] [Google Scholar]
- 9. Aranda RA, Romao Junior JE, Kakehashi E, Domingos W, Sabbaga E, Marcondes M, et al. Intraperitoneal pressure and hernias in children on peritoneal dialysis. Pediatr Nephrol 2000; 14(1):22–4. [DOI] [PubMed] [Google Scholar]
- 10. Dejardin A, Robert A, Goffin E. Intraperitoneal pressure in PD patients: relationship to intraperitoneal volume, body size and PD-related complications. Nephrol Dial Transplant 2007; 22(5):1437–44. [DOI] [PubMed] [Google Scholar]
- 11. Rubin J, Raju S, Teal N, Hellems E, Bower JD. Abdominal hernia in patients undergoing continuous ambulatory peritoneal dialysis. Arch Intern Med 1982; 142(8):1453–5. [PubMed] [Google Scholar]
- 12. Skoumal M, Haberhauer G, Mayr H. Begleiterkrankungen bei primarer Gelenkhypermobilitat. [Concomitant diseases in primary joint hypermobility syndrome]. Med Klin (Munich) 2004; 99(10):585–90. [DOI] [PubMed] [Google Scholar]
- 13. Pirson Y. Extrarenal manifestations of autosomal dominant polycystic kidney disease. Adv Chronic Kidney Dis 2010; 17(2):173–80. [DOI] [PubMed] [Google Scholar]
- 14. Li L, Szeto CC, Kwan BC, Chow KM, Leung CB, Kam-Tao Li P. Peritoneal dialysis as the first-line renal replacement therapy in patients with autosomal dominant polycystic kidney disease. Am J Kidney Dis 2011; 57(6):903–7. [DOI] [PubMed] [Google Scholar]
- 15. Morris-Stiff G, Coles G, Moore R, Jurewicz A, Lord R. Abdominal wall hernia in autosomal dominant polycystic kidney disease. Br J Surg 1997; 84(5):615–7. [PubMed] [Google Scholar]
- 16. Hadimeri H, Johansson AC, Haraldsson B, Nyberg G. CAPD in patients with autosomal dominant polycystic kidney disease. Perit Dial Int 1998; 18(4):429–32. [PubMed] [Google Scholar]
- 17. Tokgoz B, Dogukan A, Guven M, Unluhizarci K, Oymak O, Utas C. Relationship between different body size indicators and hernia development in CAPD patients. Clin Nephrol 2003; 60(3):183–6. [DOI] [PubMed] [Google Scholar]
- 18. Perl J, Wald R, Bargman JM, Na Y, Jassal SV, Jain AK, et al. Changes in patient and technique survival over time among incident peritoneal dialysis patients in Canada. Clin J Am Soc Nephrol 2012; 7(7):1145–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chidambaram M, Bargman JM, Quinn RR, Austin PC, Hux JE, Laupacis A. Patient and physician predictors of peritoneal dialysis technique failure: a population based, retrospective cohort study. Perit Dial Int 2011; 31(5):565–73. [DOI] [PubMed] [Google Scholar]
- 20. Sipahioglu MH, Aybal A, Unal A, Tokgoz B, Oymak O, Utas C. Patient and technique survival and factors affecting mortality on peritoneal dialysis in Turkey: 12 years’ experience in a single center. Perit Dial Int 2008; 28(3):238–45. [PubMed] [Google Scholar]
- 21. Han SH, Lee JE, Kim DK, Moon SJ, Kim HW, Chang JH, et al. Long-term clinical outcomes of peritoneal dialysis patients: single center experience from Korea. Perit Dial Int 2008; 28 Suppl 3:S21–6. [PubMed] [Google Scholar]
- 22. Chien CC, Wang JJ, Sun YM, Sun DP, Sheu MJ, Weng SF, et al. Long-term survival and predictors for mortality among dialysis patients in an endemic area for chronic liver disease: a national cohort study in Taiwan. BMC Nephrol 2012; 13:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Prakash J, Singh LK, Shreeniwas S, Ghosh B, Singh TB. Non-infectious complications of continuous ambulatory peritoneal dialysis and their impact on technique survival. Indian J Nephrol 2011; 21(2):112–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lupo A, Tarchini R, Carcarini G, Catizone L, Cocchi R, De Vecchi A, et al. Long-term outcome in continuous ambulatory peritoneal dialysis: a 10-year-survey by the Italian Cooperative Peritoneal Dialysis Study Group. Am J Kidney Dis 1994; 24(5):826–37. [DOI] [PubMed] [Google Scholar]
- 25. Martinez-Mier G, Garcia-Almazan E, Reyes-Devesa HE, Garcia-Garcia V, Cano-Gutierrez S, Mora YFR, et al. Abdominal wall hernias in end-stage renal disease patients on peritoneal dialysis. Perit Dial Int 2008; 28(4):391–6. [PubMed] [Google Scholar]
- 26. Kolesnyk I, Dekker FW, Boeschoten EW, Krediet RT. Time-dependent reasons for peritoneal dialysis technique failure and mortality. Perit Dial Int 2010; 30(2):170–7. [DOI] [PubMed] [Google Scholar]
- 27. Portoles J, Del Peso G, Fernandez-Reyes MJ, Bajo MA, Lopez-Sanchez P. Previous comorbidity and lack of patient free choice of technique predict early mortality in peritoneal dialysis. Perit Dial Int 2009; 29(2):150–7. [PubMed] [Google Scholar]
- 28. Wang AY, Wang M, Lam CW, Chan IH, Lui SF, Sanderson JE. Heart failure in long-term peritoneal dialysis patients: a 4-year prospective analysis. Clin J Am Soc Nephrol 2011; 6(4):805–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Verdalles U, Abad S, Aragoncillo I, Villaverde M, Jofre R, Verde E, et al. Factors predicting mortality in elderly patients on dialysis. Nephron Clin Pract 2010; 115(1):c28–34. [DOI] [PubMed] [Google Scholar]
- 30. Coronel F, Cigarran S, Herrero JA. Morbimortalidad en pacientes diabeticos en dialisis peritoneal. Experiencia de 25 anos en un solo centro. [Morbidity and mortality in diabetic patients on peritoneal dialysis. Twenty-five years of experience at a single centre]. Nefrologia 2010; 30(6):626–32. [DOI] [PubMed] [Google Scholar]
- 31. Sipahi S, Hur E, Demirtas S, Kocayigit I, Bozkurt D, Tamer A, et al. Body composition monitor measurement technique for the detection of volume status in peritoneal dialysis patients: the effect of abdominal fullness. Int Urol Nephrol 2011; 43(4):1195–9. [DOI] [PubMed] [Google Scholar]
- 32. Goh YH. Omental folding: a novel laparoscopic technique for salvaging peritoneal dialysis catheters. Perit Dial Int 2008; 28(6):626–31. [PubMed] [Google Scholar]
- 33. Garcia Campayo J, Asso E, Alda M, Andres EM, Sobradiel N. Association between joint hypermobility syndrome and panic disorder: a case-control study. Psychosomatics 2010; 51(1):55–61. [DOI] [PubMed] [Google Scholar]
- 34. Orhan AL, Sayar N, Nurkalem Z, Uslu N, Erdem I, Erdem EC, et al. Assessment of autonomic dysfunction and anxiety levels in patients with mitral valve prolapse. Turk Kardiyol Dern Ars 2009; 37(4):226–33. [PubMed] [Google Scholar]
- 35. Grahame R. Pain, distress and joint hyperlaxity. Joint, bone, spine 2000; 67(3):157–63. [PubMed] [Google Scholar]
- 36. Grau JB, Pirelli L, Yu PJ, Galloway AC, Ostrer H. The genetics of mitral valve prolapse. Clin Genet 2007; 72(4):288–95. [DOI] [PubMed] [Google Scholar]
- 37. Wong PN, Mak SK, Lo KY, Tong GM, Wong Y, Wong AK. Adverse prognostic indicators in continuous ambulatory peritoneal dialysis patients without obvious vascular or nutritional comorbidities. Perit Dial Int 2003; 23(Suppl 2):S109–15. [PubMed] [Google Scholar]
- 38. Munoz de Bustillo E, Borras F, Gomez-Roldan C, Perez-Contreras FJ, Olivares J, Garcia R, et al. Impact of peritonitis on long-term survival of peritoneal dialysis patients. Nefrologia 2011; 31(6):723–32. [DOI] [PubMed] [Google Scholar]
- 39. Fourtounas C, Savidaki E, Dousdabanis P, Hardalias A, Kalliakmani P, Papachristou E, et al. Peritonitis during the first year after commencement of peritoneal dialysis has an impact on technique survival and patient morbidity. Adv Perit Dial 2006; 22:50–4. [PubMed] [Google Scholar]
- 40. Huang JW, Hung KY, Yen CJ, Wu KD, Tsai TJ. Systemic lupus erythematosus and peritoneal dialysis: outcomes and infectious complications. Perit Dial Int 2001; 21(2):143–7. [PubMed] [Google Scholar]
- 41. Siu YP, Leung KT, Tong MK, Kwan TH, Mok CC. Clinical outcomes of systemic lupus erythematosus patients undergoing continuous ambulatory peritoneal dialysis. Nephrol Dial Transplant 2005; 20(12):2797–802. [DOI] [PubMed] [Google Scholar]


