Previous clinical studies have demonstrated that biocompatible peritoneal dialysis (PD) solutions reduce inflammatory responses in the peritoneal cavity, improve ultrafiltration (UF), and increase biochemical markers of peritoneal integrity, including cancer antigen 125 (CA125) (1–4). Moreover, recent reports showed that bio-compatible PD solutions improved clinical outcomes in PD patients (5,6), although a recent meta-analysis revealed controversial results (7). However, most studies have focused on short-term or incident PD patients, and little is known about the effect of bio-compatible PD solutions on long-term PD patients who are anuric and have been treated with conventional PD solutions for several years. The aim of this study was to elucidate the effects of a neutral-pH bicarbonate/lactate (B/L)-buffered PD solution on long-term PD patients previously treated with a conventional PD solution for more than 5 years.
Methods
This study was a single-center, prospective, randomized comparison of conventional PD solution and neutral-pH, B/L-buffered PD solution in long-term PD patients. Peritoneal dialysis patients who were stable and had previously been treated using a conventional PD solution (Dianeal, Baxter Healthcare Co., Singapore) for more than 5 years at Yonsei University Hospital were recruited for inclusion in this study. Patients were randomized into either the control group or B/L group at a ratio of 1:2 using computer generated random numbers. After randomization, the PD solution in the B/L group was changed to Physioneal (25 mmol/L bicarbonate, 15 mmol/L lactate, Baxter Healthcare Co., Singapore) while Dianeal was maintained in the control group. Patients were followed during a 1-year period. A total of 135 patients were initially recruited, of whom 45 were randomly allocated to the control group and 90 were randomly allocated to the B/L group. Twenty-one patients were excluded for various reasons (consent withdrawal, switch to hemodialysis or kidney transplantation, and patient death). A total of 114 patients completed the study. Most of the study patients were anuric, and only 9 patients (7 in the B/L group and 2 in the control group) maintained a 24-hour urine output of more than 100 mL at the beginning of the study. The study protocol was approved by the local Institutional Review Board (IRB No. 4-0086) and written informed consent was obtained from the patients. Peritoneal effluent CA125 (Immulite 2000, Los Angeles, CA, USA), interleukin-6 (IL-6, R&D Systems Inc, Minneapolis, MN, USA), and hyaluronan (HA, Echelon Biosciences Inc, Salt Lake City, UT, USA) levels were measured using commercially available kits. Statistical analyses were performed using the statistical package SPSS for Windows Ver. 20.0 (SPSS, Inc., Chicago, IL, USA).
Results
Baseline demographics
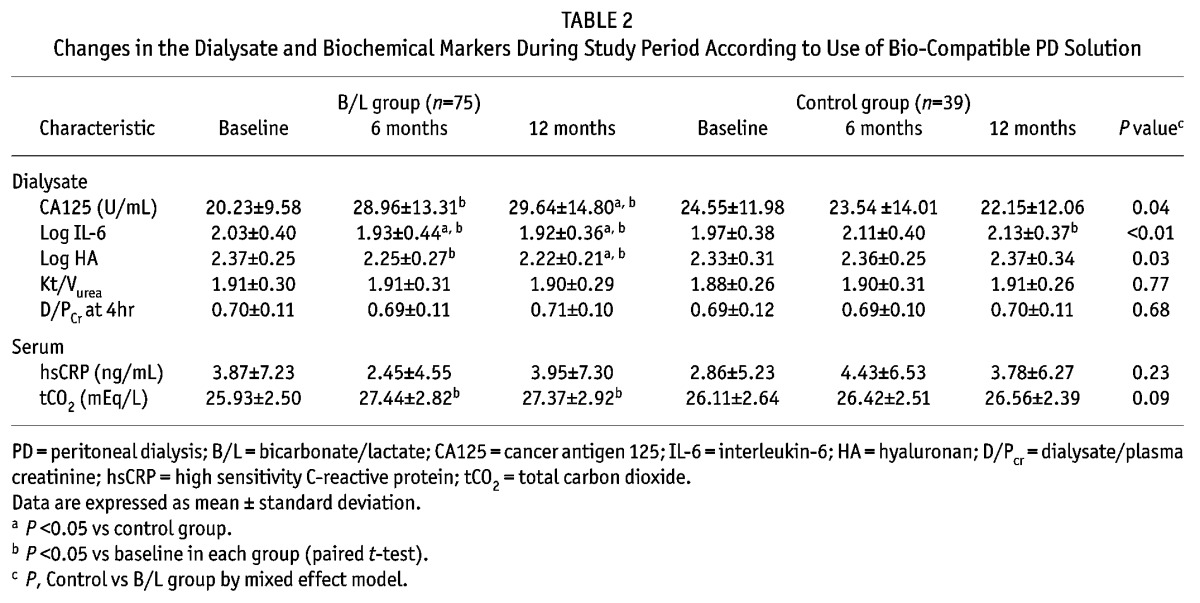
The clinical and demographic characteristics of the patients are shown in Table 1. There were no significant differences in age, gender, or body mass index (BMI) between the 2 groups. The total carbon dioxide (tCO2) levels did not change over time in the control group. However, tCO2 levels at 6 and 12 months in the B/L group increased significantly compared to baseline levels. Serum high sensitivity C-reactive protein (hsCRP) levels were similar among the groups during the entire study period (Table 2). No differences were seen in weekly Kt/V, 2-hour and 4-hour dialysate to plasma creatinine ratio (D/Pcr) between the control and B/L group at baseline. In both groups, weekly Kt/V, 2-hour and 4-hour D/Pcr did not change during the study period (Table 2).
TABLE 1.
Demographic and Clinical Characteristics of the Study Patientsa
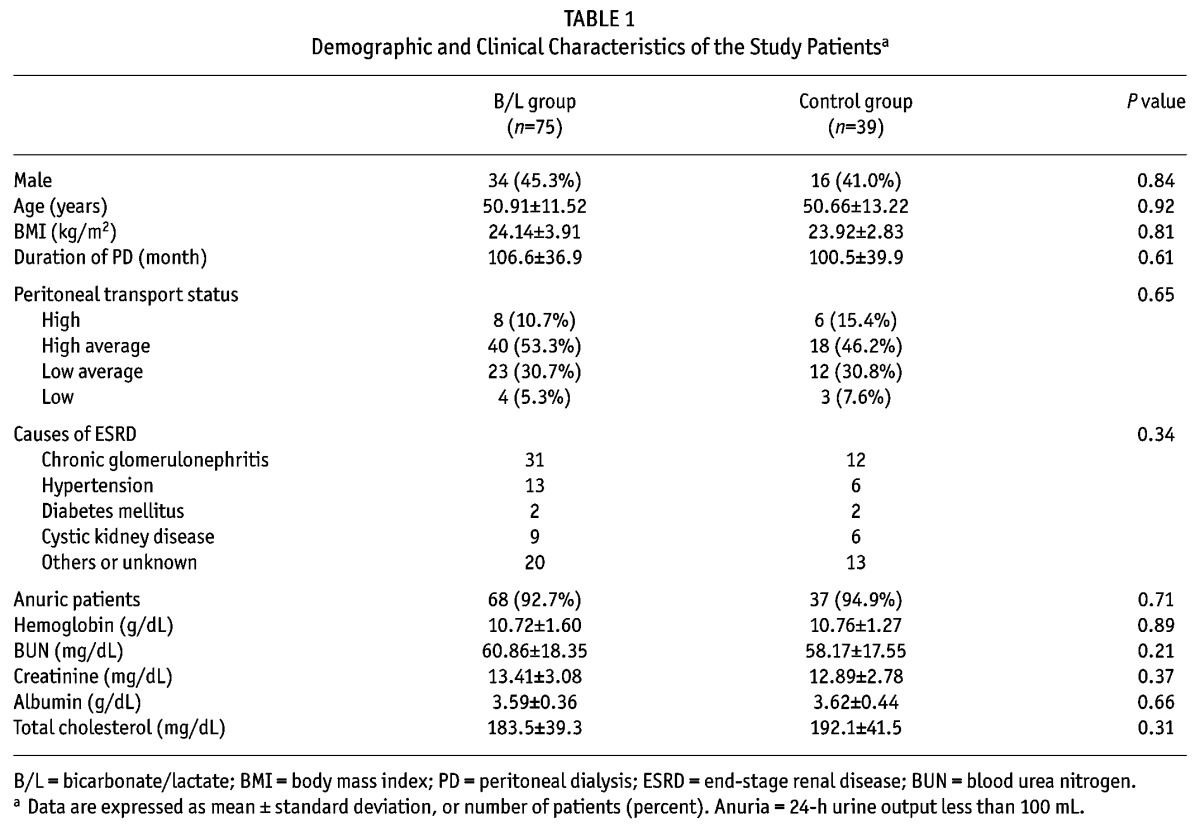
TABLE 2.
Changes in the Dialysate and Biochemical Markers During Study Period According to Use of Bio-Compatible PD Solution
Peritoneal effluent markers
At baseline, there were no significant differences in CA125, IL-6 and HA levels between the 2 groups. Cancer antigen 125 (CA125) levels increased serially in the B/L group, and did not change in the control group. The IL-6 and HA levels during the study period decreased steadily in the B/L group and increased serially in the control group (Table 2). When further analysis was performed using repeated measures ANOVA, there was a significant effect of B/L on the changes in CA125 (F(2.0, 210.0) = 15.3, p < 0.01), in IL-6 (F(2.0, 214.0) = 4.10, p < 0.01) and HA (F(2.0, 120.0) = 155.7, p < 0.01).
Discussion
In the B/L group of the present study, effluent CA125 levels were increased during follow-up periods; however, CA125 levels did not change in the control group. Previous studies reported that peritoneal CA125 is secreted from human mesothelial cells and is a marker of mesothelial integrity and viability (8). In addition, CA125 levels showed a negative trend over time during PD and were used to monitor the mesothelial integrity in PD patients (9). Several prospective studies showed that CA125 levels more than doubled compared to baseline levels after conversion to biocompatible PD solution in incidental or prevalent PD patients treated for a relatively short period of time (2,3). Our study demonstrated that CA125 levels increased by 56.9% and 63.2% at 6 and 12 months respectively in the B/L group. These findings suggest that conversion to biocompatible PD solution even in long-term continuous ambulatory peritoneal dialysis (CAPD) patients has beneficial effects on the restoration of mesothelial integrity, although these effects might be limited compared to short-term or incident PD groups.
It has been reported that dialysate IL-6 and HA levels in CAPD patients reflect intraperitoneal inflammation and are associated with structural and functional changes in the peritoneums of long-standing CAPD patients (10–12). In this study, dialysate IL-6 and HA decreased compared to baseline values in the B/L group. Advanced glycation endproducts (AGEs) are thought to contribute to local and systemic microinflammation in PD patients (13,14) and AGE accumulation in peritoneums treated with standard PD solution is more prominent than with low-glucose degradation product (GDP) solutions in an experimental study (15). Based on these observations, biocompatible solutions that contain low GDPs could reduce intraperitoneal inflammation in a B/L group.
The present study has several shortcomings. We did not measure the peritoneal UF and the follow-up duration was too short to reveal clinical outcomes. Future studies should be performed to compare the long-term effects of B/L on peritoneal transport characteristics, UF and the PD patient’s outcomes. In conclusion, this study suggests that the B/L solution might have had beneficial effects for long-standing prevalent CAPD patients, not only for incident and short-term CAPD patients as previously reported.
REFERENCES
- 1. Simonsen O, Sterner G, Carlsson O, Wieslander A, Rippe B. Improvement of peritoneal ultrafiltration with peritoneal dialysis solution buffered with bicarbonate/lactate mixture. Perit Dial Int 2006; 26:353–9. [PubMed] [Google Scholar]
- 2. Rippe B, Simonsen O, Heimburger O, Christensson A, Haraldsson B, Stelin G, et al. Long-term clinical effects of a peritoneal dialysis fluid with less glucose degradation products. Kidney Int 2001; 59:348–57. [DOI] [PubMed] [Google Scholar]
- 3. Jones S, Holmes CJ, Krediet RT, Mackenzie R, Faict D, Tranaeus A, et al. Bicarbonate/lactate-based peritoneal dialysis solution increases cancer antigen 125 and decreases hyaluronic acid levels. Kidney Int 2001; 59:1529–38. [DOI] [PubMed] [Google Scholar]
- 4. Mortier S, Faict D, Schalkwijk CG, Lameire NH, De Vriese AS. Long-term exposure to new peritoneal dialysis solutions: Effects on the peritoneal membrane. Kidney Int 2004; 66:1257–65. [DOI] [PubMed] [Google Scholar]
- 5. Johnson DW, Brown FG, Clarke M, Boudville N, Elias TJ, Foo MW, et al. Effects of biocompatible versus standard fluid on peritoneal dialysis outcomes. J Am Soc Nephrol 2012; 23:1097–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Han SH, Ahn SV, Yun JY, Tranaeus A, Han DS. Mortality and technique failure in peritoneal dialysis patients using advanced peritoneal dialysis solutions. Am J Kidney Dis 2009; 54:711–20. [DOI] [PubMed] [Google Scholar]
- 7. Cho Y, Johnson DW, Badve SV, Craig JC, Strippoli GF, Wiggins KJ. The impact of neutral-ph peritoneal dialysates with reduced glucose degradation products on clinical outcomes in peritoneal dialysis patients. Kidney Int 2013; 84(5):969–79. [DOI] [PubMed] [Google Scholar]
- 8. Visser CE, Brouwer-Steenbergen JJ, Betjes MG, Koomen GC, Beelen RH, Krediet RT. Cancer antigen 125: a bulk marker for the mesothelial mass in stable peritoneal dialysis patients. Nephrol Dial Transplant 1995; 10:64–9. [PubMed] [Google Scholar]
- 9. Ho-dac-Pannekeet MM, Hiralall JK, Struijk DG, Krediet RT. Markers of peritoneal mesothelial cells during treatment with peritoneal dialysis. Adv Perit Dial 1997; 13:17–22. [PubMed] [Google Scholar]
- 10. Yamagata K, Tomida C, Koyama A. Intraperitoneal hyaluronan production in stable continuous ambulatory peritoneal dialysis patients. Perit Dial Int 1999; 19:131–7. [PubMed] [Google Scholar]
- 11. Lai KN, Lai KB, Lam CW, Chan TM, Li FK, Leung JC. Changes of cytokine profiles during peritonitis in patients on continuous ambulatory peritoneal dialysis. Am J Kidney Dis 2000; 35:644–52. [DOI] [PubMed] [Google Scholar]
- 12. Yung S, Coles GA, Williams JD, Davies M. The source and possible significance of hyaluronan in the peritoneal cavity. Kidney Int 1994; 46:527–33. [DOI] [PubMed] [Google Scholar]
- 13. Peppa M, Uribarri J, Cai W, Lu M, Vlassara H. Glycoxidation and inflammation in renal failure patients. Am J Kidney Dis 2004; 43:690–5. [DOI] [PubMed] [Google Scholar]
- 14. Lieuw AFML, van Hinsbergh VW, Teerlink T, Barto R, Twisk J, Stehouwer CD, et al. Increased levels of n(epsilon)-(carboxymethyl)lysine and n(epsilon)-(carboxyethyl)lysine in type 1 diabetic patients with impaired renal function: correlation with markers of endothelial dysfunction. Nephrol Dial Transplant 2004; 19:631–6. [DOI] [PubMed] [Google Scholar]
- 15. Mortier S, Faict D, Lameire NH, De Vriese AS. Benefits of switching from a conventional to a low-GDP bicarbonate/lactate-buffered dialysis solution in a rat model. Kidney Int 2005; 67:1559–65. [DOI] [PubMed] [Google Scholar]


