Although rare, the incidence of nontuberculous mycobacteria (NTM) peritonitis in patients on peritoneal dialysis (PD) seems on the rise and is a diagnosis and treatment challenge (1). Song Y et al. summarized 57 cases from 41 papers and found that the mean time from diagnosis to initiation of appropriate treatment was 4 weeks (2). Among them, 8.8% had Mycobacterium abscessus infection. Early diagnosis of NTM peritonitis is very difficult since the symptoms and signs are indistinguishable from bacterial peritonitis and tuberculous peritonitis (1,2). Here we present 2 cases of PD-associated peritonitis caused by M. abscessus with distinct complications and outcome.
Case Description
Case 1 was a 44-year-old woman on PD for 8 years admitted for refractory exit-site infection. She had catheter replacement and shifted to hemodialysis preplanned for peritoneal resting. Fever and chills occurred from day 3 after the operation. Empirical treatment with piperacillin-tazobactam and vancomycin was ineffective. Ascites check-up on day 10 post-operation showed cell counts of 1,134/mm3, progressing to 53,512/mm3 on day 14, and both tests had positive smear stain of acid-fast bacilli (AFB). We started anti-tuberculosis drugs on day 10 and removed the catheter, but no significant improvement was seen. In the 4th week, the follow-up ascites study showed negative result of the tuberculosis-polymerase chain reaction (TB-PCR) test (Cobas TaqMan MTB PCR test, Roche Diagnostics, Basel, Switzerland), so we shifted the treatment to anti-NTM regimen with meropenem, amikacin and clarithromycin. The final culture result came out in the 5th week with M. abscessus infection. The patient later developed progressive nausea, vomiting, abdominal pain, and signs of bowel obstruction in the 3rd month. Her abdominal computed tomography (CT) showed peritoneal thickening with calcification, loculated ascites, and suspicious encapsulating peritoneal sclerosis (EPS). Exploratory laparotomy found no thick, sclerotic covering on visceral surfaces, but only great omentum adhesion to peritoneum with marked indurations. Pathological findings confirmed the NTM peritonitis (Figure 1). She expired in the 8th month due to left renal hemorrhage and complicated retroperitoneal infection.
Figure 1 —
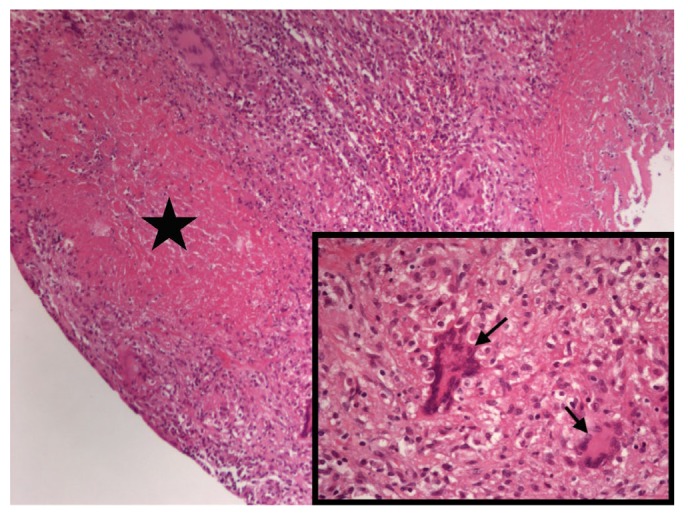
Peritoneal pathology in case 1. Chronic granulomatous inflammation with caseous necrosis (star) and Langhan’s giant cells (arrows) were noted. (Hematoxylin and eosin stain, magnification ×100 and ×400 in the lower right corner.)
Case 2 was a 58-year-old diabetic female on PD treatment for 5 years. Tunnel infections happened twice in 5 months before this admission. Wound debridement with PD catheter replacement was performed during the second episode of tunnel infection. Unfortunately, fever with chills and turbid dialysate (cell count: 286/mm3) occurred 3 weeks after the operation. However, a clean exit site without discharge was noted during this period, and negative results for all cultures were reported from both the exit site and the tunnel area. Empirical intra-peritoneal cefazolin and gentamicin were given and gram stain was negative. The Tenckhoff catheter was removed on the second day after admission due to refractory tunnel infection and PD-associated peritonitis. Acid fast bacilli stain of her peritoneal fluid revealed a positive result and tuberculosis-PCR showed negative. Following the previous experience from case 1, NTM infection was highly suspected and intravenous imipenem, amikacin, and oral clarithromycin were used. The dialysate culture proved to be M. abscessus 10 days later. Abdominal CT showed mesentery infiltration and abscess in the anterior low abdominal wall. After 2 debridements for the abdominal wall abscess and the anti-NTM therapy, she was discharged uneventfully and received another 6 months of oral antibiotics including clarithromycin, ciprofloxacin, and doxycycline, with no recurrence of infection in 12 months of follow-up.
Discussion
These 2 important cases highlight that PD-associated NTM peritonitis may have severe complications including mimicking EPS as in case 1, and timely diagnosis and treatment to localize the infection is life-saving. Encapsulating peritoneal sclerosis is a rare but a severe complication of PD. Long PD treatment duration and peritonitis episodes are important risk factors for EPS (3,4). Clinical symptoms of abdominal pain, nausea, vomiting, severe malnutrition, and body weight loss (5) with typical abdominal CT with peritoneal thickening, bowel tethering, peritoneal calcification and entrapped fluid collection are suggestive of EPS (5,6). Although our case 1 had a PD history for 8 years and a peritonitis episode with bowel obstruction and typical image findings, her laparotomy and peritoneal biopsy finally confirmed the NTM peritonitis. The intra-abdominal disseminated NTM infection proven by pathology findings may explain the ominous outcome of case 1.
The dramatic differences between the outcome of case 1 and case 2 might be the timing for initiating NTM-targeted treatment. We started treating case 1 as NTM peritonitis in the 4th week, which turned out to be disseminated intra-abdominal infection with poor outcome. Possible reasons for our delaying anti-NTM treatment included stopping PD and shifting to hemodialysis preplanned for peritoneal resting, initial vague peritoneal sign, and PD catheter removed later. More importantly, the ascites smear revealed positive for AFB, and delayed application of TB-PCR had misled us to suspect TB peritonitis. Based on the experience of case 1, we were highly alert when tests were positive for AFB and negative for prompt TB-PCR occurred in the 2nd case. Therefore, we were able to apply anti-NTM regimens on the 3rd day after symptom onset, and she recovered well after eradicating the relative localized infection. Another point worthy of discussion was the use of antibiotics. Basic regimen for NTM could be amikacin, clarithromycin, and imipenem. Nevertheless, severe neurologic complications like seizure have been reported in uremic patients during imipenem therapy (7), which is why we used meropenem in case 1. However, there is a high prevalence of meropenem resistance in M. abscessus in Taiwan. Meropenem had poorer potency against the NTM infection than imipenem (8). Based on the unfortunate experience of case 1 and the above report, we treated case 2 with imipenem.
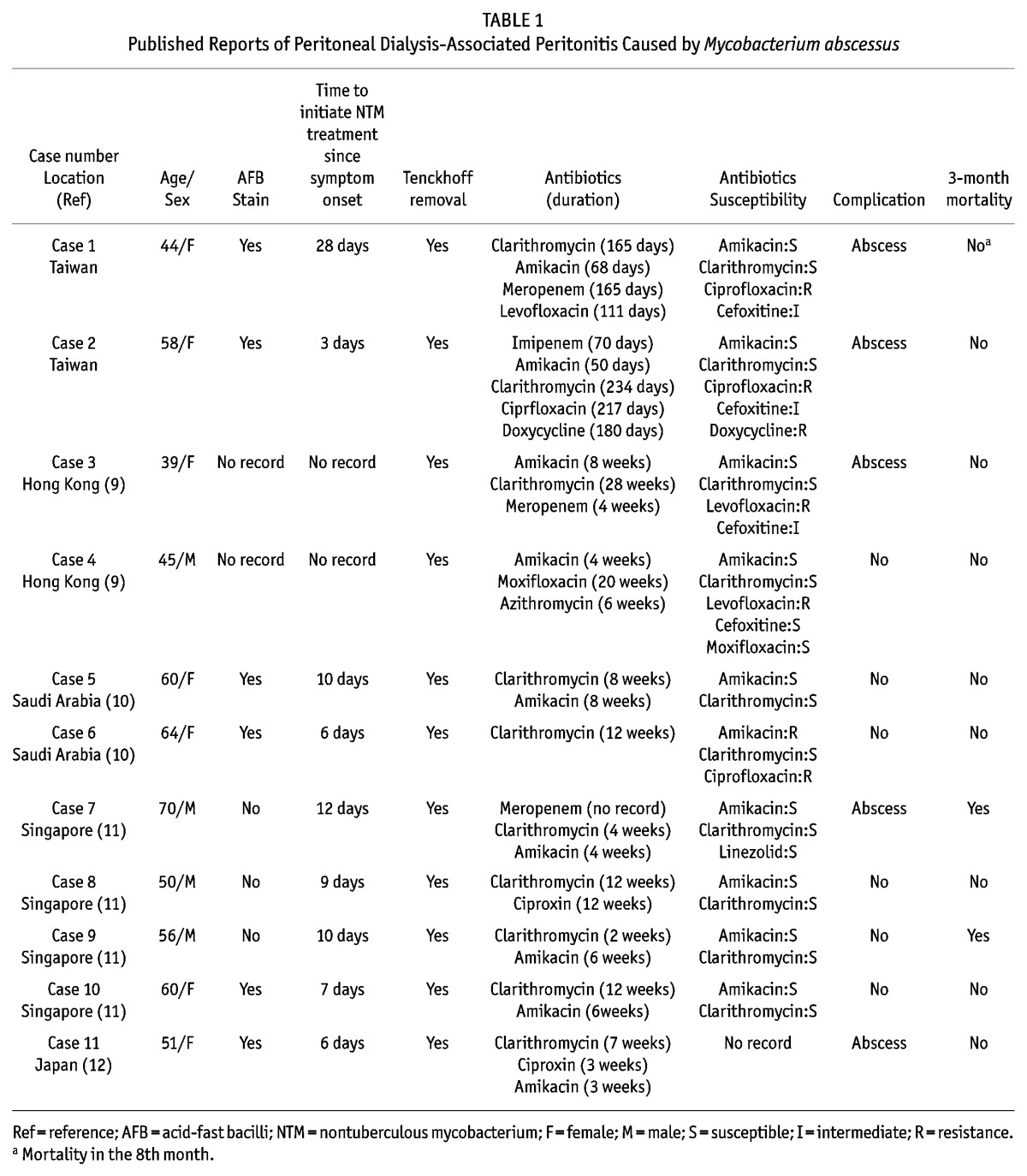
The characteristics of 9 cases of PD-associated peritonitis caused by M. abscessus by literature review and our 2 cases are summarized in Table 1 (9–12). All reports are from Asia. Only 6 of the 9 cases (66.7%, no record in 2 cases) had positive AFB stain, which was compatible with the previous findings that negative AFB stain in NTM peritonitis is common (2). A relatively higher complication rate (45.5%) and 3-month mortality (18.2%) of M. abscessus peritonitis compared with the rates of other NTM peritonitis cases were noted (2). The commonly utilized antibiotics were clarithromycin, amikacin, quinolones, and carbapenem. There is no consensus on the duration of NTM therapy which may vary from 2 to 12 months, based on clinical judgment according to the severity of infection (13).
TABLE 1.
Published Reports of Peritoneal Dialysis-Associated Peritonitis Caused by Mycobacterium abscessus
Conclusion
Nontuberculous mycobacteria is indeed a threat in immunocompromised groups, such as uremic patients. Nontuberculous mycobacteria should be highly suspected by positive AFB smear and negative TB-PCR results, and confirmed by timely identification of culture results, especially for those that grow quickly. Early initiation of proper treatment of NTM may prevent late and severe complications.
Disclosures
The authors declare no financial conflicts of interest to declare.
REFERENCES
- 1. Li PK, Szeto CC, Piraino B, Bernardini J, Figueiredo AE, Gupta A, et al. Peritoneal dialysis-related infections recommendations: 2010 update. Perit Dial Int 2010; 30:393–423. [DOI] [PubMed] [Google Scholar]
- 2. Song Y, Wu J, Yan H, Chen J. Peritoneal dialysis-associated nontuberculous mycobacterium peritonitis: a systematic review of reported cases. Nephrol Dial Transplant 2012; 27:1639–44. [DOI] [PubMed] [Google Scholar]
- 3. Rigby RJ, Hawley CM. Sclerosing peritonitis: the experience in Australia. Nephrol Dial Transplant 1998; 13:154–9. [DOI] [PubMed] [Google Scholar]
- 4. Wong YY, Wong PN, Mak SK, Chan SF, Cheuk YY, Ho LY, et al. Persistent sterile peritoneal inflammation after catheter removal for refractory bacterial peritonitis predicts full-blown encapsulating peritoneal sclerosis. Perit Dial Int 2013; 33:507–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nakamoto H. Encapsulating peritoneal sclerosis—a clinician’s approach to diagnosis and medical treatment. Perit Dial Int 2005; 25(Suppl 4):S30–8. [PubMed] [Google Scholar]
- 6. Lambie M, Braun N, Davies SJ. Towards standardized reporting in studies of encapsulating peritoneal sclerosis. Perit Dial Int 2013; 33:482–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Campise M. Neurological complication during imipenem/cilastatin therapy in uraemic patients. Nephrol Dial Transplant 1998; 13:1895–6. [PubMed] [Google Scholar]
- 8. Yang SC, Hsueh PR, Lai HC, Teng LJ, Huang LM, Chen JM, et al. High prevalence of antimicrobial resistance in rapidly growing mycobacteria in taiwan. Antimicrob Agents Chemother 2003; 47:1958–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lo MW, Mak SK, Wong YY, Lo KC, Chan SF, Tong GM, et al. Atypical mycobacterial exit-site infection and peritonitis in peritoneal dialysis patients on prophylactic exit-site gentamicin cream. Perit Dial Int 2013; 33:267–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Siddiqi N, Sheikh I. Peritonitis caused by mycobacterium abscesses in patients on continuous ambulatory peritoneal dialysis. Saudi J Kidney Dis Transpl 2012; 23:321–4. [PubMed] [Google Scholar]
- 11. Renaud CJ, Subramanian S, Tambyah PA, Lee EJ. The clinical course of rapidly growing nontuberculous mycobacterial peritoneal dialysis infections in asians: a case series and literature review. Nephrology (Carlton) 2011; 16:174–9. [DOI] [PubMed] [Google Scholar]
- 12. Kameyama H, Mori Y, Kimura T, Sugishita C, Adachi T, Sonomura K, et al. A case report of mycobacterium abscessus peritonitis in a peritoneal dialysis patient. Ther Apher Dial 2007; 11:449–51. [DOI] [PubMed] [Google Scholar]
- 13. Dunmire RB, 3rd, Breyer JA. Nontuberculous mycobacterial peritonitis during continuous ambulatory peritoneal dialysis: Case report and review of diagnostic and therapeutic strategies. Am J Kidney Dis 1991; 18:126–30. [DOI] [PubMed] [Google Scholar]


