Adoption of the Model for End-stage Liver Disease (MELD) score as a marker of chronic liver disease severity has led to shorter wait lists and wait times for liver transplantation, increases in the number of liver transplant procedures and lower mortality. However, given the considerable resource expenditure for patients with significantly elevated MELD score and the high wait list mortality in this group, transplant authorities have set a threshold value for this important metric. This single-centre, retrospective study investigated short- and long-term survival rates, in addition to several important resource-related variables in a group of patients who underwent liver transplantation over a 10-year period.
Keywords: Cirrhosis, End-stage liver disease, Liver transplant, MELD
Abstract
BACKGROUND:
Cirrhotic patients with Model for End-stage Liver Disease (MELD) score ≥40 have high risk for death without liver transplant (LT).
OBJECTIVE:
To evaluate these patients’ outcomes after LT.
METHODS:
The present study analyzed a retrospective cohort of 519 cirrhotic adult patients who underwent LT at a single Canadian centre between 2002 and 2012. Primary exposure was severity of liver disease measured by MELD score at LT (≥40 versus <40). Primary outcome was duration of first intensive care unit (ICU) stay after LT. Secondary outcomes were duration of first hospital stay after LT, rate of ICU readmission, re-LT and survival rates.
RESULTS:
On the day of LT, 5% (28 of 519) of patients had a MELD score ≥40. These patients had longer first ICU stays after LT (14 versus two days; P<0.001). MELD score ≥40 at LT was independently associated with first ICU stay after LT ≥10 days (OR 3.21). These patients had longer first hospital stays after LT (45 versus 18 days; P<0.001); however, there was no significant difference in the rate of ICU readmission (18% versus 22%; P=0.58) or re-LT rate (4% versus 4%; P=1.00). Cumulative survival at one month, three months, one year, three years and five years was 98%, 96%, 90%, 79% and 72%, respectively. There was no significant difference in cumulative survival stratified according to MELD score ≥40 versus <40 at LT (P=0.59).
CONCLUSIONS:
Cirrhotic patients with MELD score ≥40 at LT utilize greater postoperative health resources; however, they derive similar long-term survival benefit from LT.
Abstract
HISTORIQUE :
Les patients cirrhotiques ayant un score MELD (acronyme anglais de modèle de maladie hépatique en phase terminale) de 40 ou plus risquent fort de mourir sans avoir reçu de transplantation hépatique (TH).
OBJECTIF :
Évaluer les résultats de ces patients après une TH.
MÉTHODOLOGIE :
La présente étude portait sur une cohorte de 519 patients cirrhotiques adultes qui ont subi une TH dans un seul centre canadien entre 2002 et 2012. L’exposition primaire était la gravité de la maladie hépatique mesurée par le score MELD à la TH (40 ou plus par rapport à moins de 40). Le résultat primaire était la durée du premier séjour en soins intensifs (USI) après la TH, le taux de réadmissions à l’USI, une nouvelle TH et les taux de survie.
RÉSULTATS :
Le jour de la TH, 5 % des patients (28 sur 519) avaient un score MELD de 40 ou plus. Ces patients séjournaient plus longtemps à l’USI après la TH (14 jours par rapport à deux; P<0,001). Leur score MELD de 40 ou plus à la TH s’associait de manière indépendante à un premier séjour à l’USI de dix jours ou plus après la TH (RC 3,21). Le premier séjour hospitalier de ces patients était plus long après la TH (45 jours par rapport à 18; P<0,001), mais il n’y avait pas de différence significative dans le taux de réadmission à l’USI (18 % par rapport à 22 %; P=0,58) ou de nouvelle TH (4 % par rapport à 4 %; P=1,00). La survie cumulative au bout d’un mois, de trois mois, d’un an, de trois ans et de cinq ans s’élevait à 98 %, 96 %, 90 %, 79 % et 72 %, respectivement. Il n’y avait pas de différence significative dans la survie cumulative stratifiée selon le score MELD de 40 ou plus par rapport à celui de moins de 40 à la TH (P=0,59).
CONCLUSIONS :
Les patients cirrhotiques ayant un score MELD de 40 ou plus à la TH utilisaient plus de ressources de santé après l’opération, mais tiraient des bienfaits similaires de la TH à long terme sur le plan de la survie.
The Model for End-stage Liver Disease (MELD), a marker of chronic liver disease severity based on the patient’s serum bilirubin and creatinine levels, and international normalized ratio (1), has proven to be an accurate predictor of wait list for liver transplant (LT) three-month mortality (2).
Adoption of MELD to select and prioritize patients on the wait list for LT has contributed to a reduction in the number of patients waiting for LT, shorter wait times, an increase in the number of LT procedures and lower mortality (3).
Despite the continuous character of MELD, transplant authorities have decided to restrict its score to a maximum of 40. However, recent data have shown that patients with a MELD score ≥40 have higher wait list mortality than candidates with a lower MELD score (4,5).
While patients with end-stage liver disease and a high MELD score represent considerable resource expenditure for the health system, especially if transplanted, their outcomes have been reported to be acceptable (6–8).
We hypothesized that patients with a MELD score ≥40 at LT would consume greater health resources post-LT, but would achieve similar short- and long-term survival rates. Accordingly, our primary objective was to determine whether patients with a biochemical MELD score ≥40 at LT would have a longer duration of first intensive care unit (ICU) stay after LT. Our secondary objectives were to evaluate these patients’ duration of first hospital stay after LT, rate of ICU readmission, re-LT rate, and one-month, three-month, one-year, three-year and five-year survival rates.
METHODS
The present study adhered to the STROBE statement for observational studies (9). The local ethics committee approved the present study before commencement. The requirement for individual informed consent was waived.
Design, setting and participants
The present analysis was a single-centre, retrospective cohort study, which included all adult (≥18 years of age) cirrhotic patients who underwent LT at a Canadian transplant centre (University of Alberta Hospital [Edmonton, Alberta]) between January 1, 2002 and June 30, 2012. Patients were excluded if: they had concomitant transplant with another organ (eg, liver, kidney); their primary diagnosis was acute liver failure; or they were lost to follow-up.
Operational definitions
Cirrhosis was defined as bridging fibrosis on previous liver biopsy or a composite of clinical signs and findings provided by laboratory test results, endoscopy and radiological imaging (10).
Complications of cirrhosis included infection, variceal bleeding, hepatic encephalopathy, hepatorenal syndrome and hepatopulmonary syndrome. Although infection is not a specific complication of cirrhosis, it was considered as such due to its high prevalence and potential to alter disease course, being a common cause of acute decompensation and increased mortality (11). This definition included spontaneous bacterial peritonitis, bloodstream infection, urinary tract infection and pneumonia. Variceal bleeding was defined as any confirmed episode of acute bleeding originating from esophageal or gastric varices due to portal hypertension (12). Hepatic encephalopathy was defined as any acute confusional state in a patient with underlying liver disease, after excluding any potentially confounding metabolic, infectious or neurological disorders (13). Hepatorenal syndrome was defined as acute kidney injury in a patient with advanced liver disease in the absence of an identifiable cause based on the most recent criteria of the European Association for the Study of the Liver (14). Hepatopulmonary syndrome was defined as an oxygenation defect caused by pulmonary vascular dilation in the setting of portal hypertension, with the diagnosis being made by contrast-enhanced transthoracic echocardiography (15).
All MELD scores were calculated according to the United Network for Organ Sharing recommendations (16), without adjusting for serum sodium level or standardized exception points (17).
Variables
The primary exposure was the severity of end-stage liver disease measured by biochemical MELD score at LT (≥40 versus <40). The primary outcome was the duration of first ICU stay after LT. A prolonged first ICU stay following LT was defined as ≥10 days based on previous literature (8,18). The secondary outcomes were the duration of first hospital stay after LT, rate of ICU readmission, re-LT rate, and one-month, three-month, one-year, three-year and five-year patient cumulative survival rates.
Data collection
The LT program at the University of Alberta Hospital started in 1989 and, since 1995, has maintained a dedicated computerized database of all cases using the Organ Transplant Tracking Record (HKS Medical Information Systems, USA).
Eligible patients were initially identified using the Organ Transplant Tracking Record. Data regarding patients’ age, sex, race, body mass index, etiology of liver disease and its complications, comorbidities, laboratory parameters, the need for pretransplant ICU admission, severity aggregate scores (Sequential Organ Failure Assessment, Child-Turcotte-Pugh and MELD), time between listing for and receipt of LT, Donor Risk Index parameters (19), operative requirements of red blood cells and platelets, and outcomes were extracted from that database and from patients’ medical records.
Statistical analysis
Statistical analysis was performed using SPSS version 20.0 (IBM Corporation, USA). Categorical variables were presented as frequencies (percentages) and continuous variables as mean ± SD, if normally distributed, or median (interquartile range [IQR]) if non-normally distributed. In the event of missing values, data were not replaced or estimated.
Univariable analysis of outcomes was performed using χ2 or Fisher’s exact test (<5 events) for categorical variables, and Student’s t test (parametric) or Mann-Whitney test (nonparametric) for continuous variables; P<0.05 was considered to be statistically significant for all comparisons.
Crude survival analysis was performed using the Kaplan-Meier estimator (with Breslow test) and adjusted survival analysis with Cox proportional-hazards regression. In this context, potentially confounding factors were selected based on previous literature (20) and clinical rationale.
Logistic regression was performed to study the effect of a MELD score ≥40 at LT on the probability of experiencing a prolonged first ICU stay following LT, after adjustment for other patients’, donors’ and perioperative covariates. Variables initially included in the model were selected based on the following three features: minimum frequency of 85% of the total number of cases under analysis (21); P<0.15 on univariable analysis; and clinical rationale. A backward stepwise selection of variables was performed to build the final models. Potentially collinear variables were excluded. Models’ goodness of fit and discrimination were assessed using the χ2 statistic (with correspondent degrees of freedom) and the area under ROC curve, respectively.
RESULTS
Patients’, donors’ and perioperative characteristics
Of the 603 LT procedures performed at the University of Alberta Hospital during the study period, 519 met the eligibility criteria. Median follow-up time for the entire cohort was 3.6 years (IQR 1.5 to 6.4 years).
Patients’ pre-LT characteristics for the entire cohort and stratified according to MELD score categories at LT (≥40 versus <40) are shown in Table 1.
TABLE 1.
Analysis of patients’ pre-liver transplant (LT) characteristics for the entire cohort and according to Model for End-stage Liver Disease (MELD) score (≥40 versus <40) categories at LT
| Characteristic | Total | MELD score | P | |
|---|---|---|---|---|
|
| ||||
| <40 (n=491) | ≥40 (n=28) | |||
| Demographics | ||||
| Age, years, median (IQR) (n=519) | 54 (48–59) | 54 (48–59) | 53 (48–59) | 0.57 |
| Male sex, n (%) (n=519) | 353 (68) | 334/491 (68) | 19/28 (68) | 0.99 |
| Race (Caucasian), n (%) (n=515) | 441 (86) | 419/487 (86) | 22/28 (79) | 0.27 |
| Body mass index, kg/m2, median (IQR) (n=517) | 25 (22–28) | 25 (22–28) | 26 (23–31) | 0.047 |
| Indication for LT, n (%) (n=519) | ||||
| Hepatitis C | 124 (24) | 115/491 (23) | 9/28 (32) | 0.29 |
| Hepatitis B | 13 (3) | 10/491 (2) | 3/28 (11) | 0.028 |
| Hepatic malignancy | 109 (21) | 106/491 (22) | 3/28 (11) | 0.23 |
| Primary biliary cirrhosis or primary sclerosing cholangitis | 96 (18) | 90/491 (18) | 6/28 (21) | 0.68 |
| Alcohol | 70 (14) | 68/491 (14) | 2/28 (7) | 0.41 |
| Nonalcoholic steatohepatitis or cryptogenic | 41 (8) | 39/491 (8) | 2/28 (7) | 1.00 |
| Autoimmune | 20 (4) | 20/491 (4) | 0/28 (0) | 0.62 |
| Other | 46 (9) | 43/491 (9) | 3/28 (11) | 0.73 |
| Complications of cirrhosis, n (%) | ||||
| Infection (n=465) | 190 (41) | 172/438 (39) | 18/27 (67) | 0.005 |
| Variceal bleeding (n=517) | 235 (45) | 220/489 (45) | 15/28 (54) | 0.38 |
| Hepatic encephalopathy (n=519) | 312 (60) | 287/491 (59) | 25/28 (89) | 0.001 |
| Grades 3 to 4 | 90 (17) | 73/491 (15) | 17/28 (61) | <0.001 |
| Hepatorenal syndrome (n=456) | 75 (16) | 61/430 (14) | 14/26 (54) | <0.001 |
| Hepatopulmonary syndrome (n=454) | 12 (3) | 12/428 (3) | 0/26 (0) | 1.00 |
| Comorbidities, n (%) | ||||
| Coronary artery disease (n=519) | 24 (5) | 24/491 (5) | 0/28 (0) | 0.63 |
| Chronic obstructive pulmonary disease (n=519) | 22 (4) | 22/491 (5) | 0/28 (0) | 0.62 |
| Diabetes mellitus (n=519) | 110 (21) | 103/491 (21) | 7/28 (25) | 0.61 |
| Chronic kidney diease (GFR <60 mL/min) (n=374) | 78 (21) | 70/351 (20) | 8/23 (35) | 0.09 |
| Laboratory parameters (day of LT) | ||||
| Hemoglobin*, g/L, mean ± SD (n=188) | 100±20 | 101±20 | 83±6 | <0.001 |
| International normalized ratio, median (IQR) (n=519) | 1.4 (1.2–1.7) | 1.3 (1.2–1.6) | 2.8 (2.3–3.3) | <0.001 |
| Albumin, g/L, median (IQR) (n=486) | 34 (30–39) | 34 (30–39) | 38 (33–44) | 0.001 |
| Bilirubin, μmol/L, median (IQR) (n=519) | 50 (28–133) | 46 (27–104) | 757 (515–907) | <0.001 |
| Creatinine, μmol/L, median (IQR) (n=519) | 86 (68–112) | 84 (67–109) | 117 (100–196) | <0.001 |
| Sodium, mmol/L, median (IQR) (n=510) | 136 (133–138) | 136 (133–138) | 138 (134–139) | 0.07 |
| pH*, mean ± SD (n=188) | 7.39±0.06 | 7.39±0.07 | 7.40±0.05 | 0.64 |
| Lactate, mmol/L, median (IQR) (n=188) | 1.7 (1.2–2.7) | 1.7 (1.2–2.6) | 2.7 (1.4–4.2) | 0.09 |
| Intensive care unit stay before LT, n (%) (n=519) | 69 (13) | 46/491 (9) | 23/28 (82) | <0.001 |
| Mechanical ventilation† (n=45) | 34 (76) | 23/29 (79) | 11/16 (69) | 0.43 |
| Vasopressors† (n=45) | 32 (71) | 18/29 (62) | 14/16 (88) | 0.09 |
| Renal replacement therapy† (n=45) | 31 (69) | 17/29 (59) | 14/16 (88) | 0.09 |
| Severity aggregate scores (day of LT) | ||||
| Sequential Organ Failure Assessment*†, mean ± SD (n=45) | 16±4 | 16±4 | 17±3 | 0.14 |
| Child-Turcotte-Pugh, median (IQR) (n=519) | 10 (8–12) | 9 (7–12) | 13 (12–13) | < 0.001 |
Normal distribution;
Data available for 45 of 69 patients. IQR Interquartile range; GFR Glomerular filtration rate
On the day of LT, median MELD score was 15 (IQR 11 to 23); at that same time, 5% (28 of 519) of patients had a MELD score ≥40, with a correspondent median score of 43 (IQR 41 to 46). The proportions of these patients for the periods 2002 to 2006 and 2007 to 2012 were similar (4% versus 7%; P=0.20). While median age (54 versus 53 years) and male sex proportion (68% versus 68%) were similar between the two MELD score categories (P>0.50 for both comparisons), patients with a MELD score ≥40 at LT had a marginally significant greater median body mass index (26 kg/m2 versus 25 kg/m2; P=0.047).
The most common indications for LT were hepatitis C (32% versus 23%) and hepatic malignancy (11% versus 22%), and their proportions were similar between the two MELD score categories (P>0.20 for both comparisons). Patients with a MELD score ≥40 at LT were more likely to have infection (67% versus 39%; P=0.005), hepatic encephalopathy (89% versus 59%; P=0.001) and hepatorenal syndrome (54% versus 14%; P<0.001) before LT. ICU admission before LT was required for 13% (69 of 519) of patients and occurred more commonly in patients with a MELD score ≥40 at transplant (82% versus 9%; P<0.001). Organ support was necessary for the majority of patients in the ICU: mechanical ventilation in 76% (34 of 45); vasopressors in 71% (32 of 45); and renal replacement therapy in 69% (31 of 45). Among patients in ICU, there were nonsignificant trends toward greater use of vasopressors (88% versus 62%) and renal replacement therapy (88% versus 59%) in those with a MELD score ≥40 at transplant (P=0.09 for both comparisons). On the day of LT, patients in ICU had a mean (± SD) Sequential Organ Failure Assessment score of 16±4, which was similar for the two MELD score categories (17 versus 16; P=0.14).
Donors’ and perioperative characteristics for the entire cohort and stratified according to MELD score categories at LT (≥40 versus <40) are presented in Appendix 1. Median time between listing for and receipt of LT was significantly lower for patients with a MELD score ≥40 at LT (1.3 versus 6.2 months; P<0.001). Due to their liver disease severity, these patients received an organ from a national source more often (64% versus 36%; P=0.003). The median Donor Risk Index score was similar for the two MELD score categories (1.5 versus 1.4; P=0.19).
Primary outcome
Overall, median length of first ICU stay following LT was two days (IQR one to six days) days. Patients with a MELD score ≥40 at LT had significantly greater median length of first ICU stay after LT (14 days [IQR five to 24 days) versus two days (IQR one to five days); P<0.001 (Figure 1).
Figure 1).
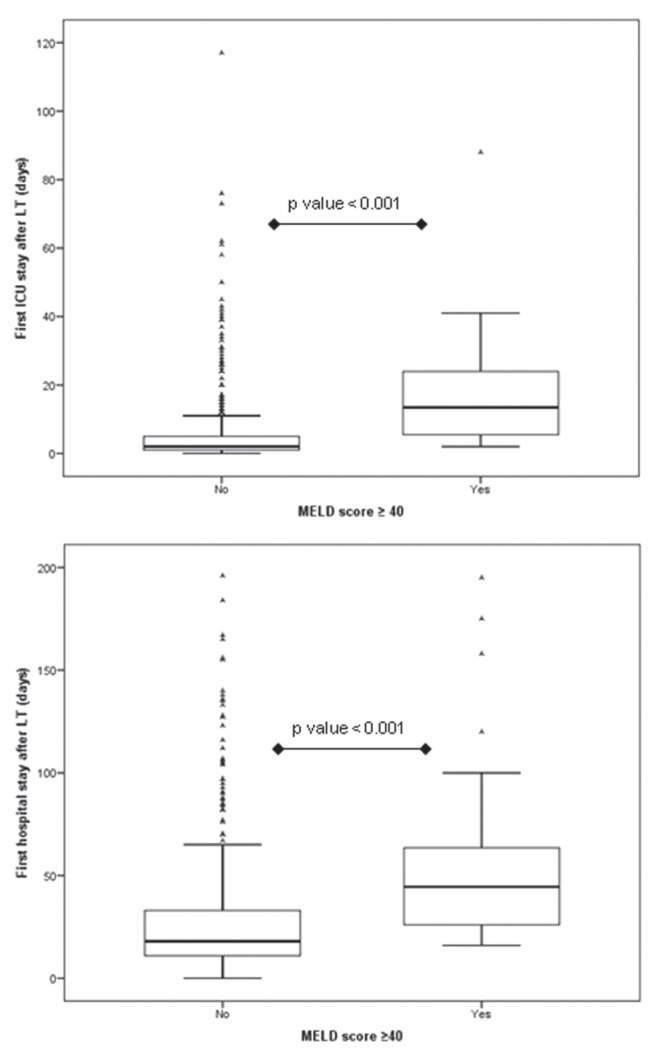
Median length of first intensive care unit and hospital stays according to biochemical Model for End-stage Liver Disease (MELD) score categories (≥40 versus <40) at liver transplant (LT)
Factors associated with a prolonged first ICU stay after LT
Univariable analysis for patients’, donors’ and perioperative characteristics stratified according to the duration of first ICU stay after LT is presented in Table 2.
TABLE 2.
Analysis of patients’, donors’ and perioperative characteristics for the entire cohort and according to duration of the first intensive care unit stay after liver transplant (≥10 days versus <10 days)
| Characteristic | Intensive care unit stay | P | |
|---|---|---|---|
|
| |||
| <10 days (n=427) | ≥10 days (n=86) | ||
| Demographics | |||
| Age, years, median (IQR) (n=519) | 54 (48–59) | 54 (50–59) | 0.42 |
| Male sex, n/n (%) (n=519) | 292/427 (68) | 57/86 (66) | 0.70 |
| Race (Caucasian), n/n (%) (n=515) | 369/424 (87) | 68/85 (80) | 0.09 |
| Body mass index, kg/m2, median (IQR) (n=517) | 24 (22–28) | 25 (23–30) | 0.045 |
| Indication for liver transplant, n/n (%) (n=519) | |||
| Hepatitis C | 107/427 (25) | 17/86 (20) | 0.30 |
| Hepatitis B | 12/427 (3) | 1/86 (1) | 0.71 |
| Hepatic malignancy | 87/427 (20) | 19/86 (22) | 0.72 |
| Primary biliary cirrhosis or primary sclerosing cholangitis | 79/427 (19) | 16/86 (19) | 0.98 |
| Alcohol | 56/427 (13) | 12/86 (14) | 0.83 |
| Complications of cirrhosis, n/n (%) | |||
| Infection (n=465) | 150/382 (39) | 39/77 (51) | 0.06 |
| Variceal bleeding (n=517) | 194/425 (46) | 38/86 /44) | 0.81 |
| Hepatic encephalopathy (n=519) | 246/427 (58) | 63/86 (63) | 0.007 |
| Grades 3 to 4 | 56/427 (13) | 34/86 (40) | <0.001 |
| Hepatorenal syndrome (n=456) | 54/372 (15) | 21/78 (27) | 0.008 |
| Hepatopulmonary syndrome (n=454) | 6/370 (2) | 6/78 (17) | 0.003 |
| Comorbidities, n/n (%) | |||
| Coronary artery disease (n=519) | 20/427 (5) | 4/86 (5) | 1.00 |
| Chronic obstructive pulmonary disease (n=519) | 19/427 (4) | 3/86 (4) | 1.00 |
| Diabetes mellitus (n=519) | 93/427 (22) | 17/86 (20) | 0.68 |
| Chrnic kidney disease (GFR <60 mL/min) (n=374) | 60/300 (20) | 16/69 (23) | 0.56 |
| Laboratory parameters (day of liver transplant) | |||
| Hemoglobin*, g/L, mean ± SD (n=188) | 101±20 | 94±19 | 0.037 |
| International normalized ratio, median (IQR) (n=519) | 1.3 (1.2–1.7) | 1.6 (1.2–2.8) | <0.001 |
| Albumin, g/L, median (IQR) (n=486) | 34 (30–39) | 36 (31–41) | 0.08 |
| Bilirubin, μmol/L, median (IQR) (n=519) | 47 (27–105) | 96 (36–456) | <0.001 |
| Creatinine, μmol/L, median (IQR) (n=519) | 86 (67–112) | 88 (71–122) | 0.62 |
| Sodium, mmol/L, median (IQR) (n=510) | 136 (133–138) | 136 (133–139) | 0.65 |
| pH* (n=88), mean ± SD | 7.39±0.06 | 7.39±0.07 | 0.70 |
| Lactate, mmol/L, median (IQR) (n=188) | 1.6 (1.2–2.5) | 2.6 (1.4–4.1) | 0.002 |
| Intensive care unit stay before liver transplant, n/n (%) (n=519) | 39/427 (9) | 30/86 (35) | <0.001 |
| Mechanical ventilation† (n=45) | 14/22 (64) | 20/23 (87) | 0.09 |
| Vasopressors† (n=45) | 13/22 (59) | 19/23 (83) | 0.11 |
| Renal replacement therapy† (n=45) | 11/22 (50) | 20/23 (87) | 0.011 |
| Severity aggregate scores (day of liver transplant) | |||
| Sequential Organ Failure Assessment*†, mean ± SD (n=45) | 14±3 | 18±3 | 0.001 |
| Child-Turcotte-Pugh, median (IQR) (n=519) | 9 (7–11) | 12 (9–13) | <0.001 |
| Model for End-stage Liver Disease score (n=519) | 15 (10–22) | 22 (11–35) | <0.001 |
| Donor risk index (n=455) | 1.4 (1.2–1.7) | 1.5 (1.2–1.7) | 0.78 |
| Red blood cells in operating room, units, median (IQR) (n=519) | 2 (0–5) | 5 (0–12) | <0.001 |
| Platelets in operating room, units, median (IQR) (n=519) | 0 (0–4) | 2 (0–7) | <0.001 |
Normal distribution;
Data available for 45 of 69 patients. IQR Interquartile range; GFR Glomerular filtration rate
The predicted probability of experiencing a prolonged ICU stay after LT was associated with the continuous MELD score at LT (Figure 2). On unadjusted analysis, a MELD score ≥40 at LT was significantly associated with a length of first ICU stay after LT ≥10 days (OR 6.73 [95% CI 3.07 to 14.7]; P<0.001) (Table 3). After adjusting for demographics (age and sex), pre-LT complications of cirrhosis (high-grade [3 to 4] hepatic encephalopathy, hepatorenal syndrome and hepatopulmonary syndrome), ICU stay before LT, standardized quality assessment of the liver received (Donor Risk Index) and the volume of red blood cells used during the LT surgery, the OR for this association remained significant (OR 3.21 [95% CI 1.12 to 9.20]; P=0.030).
Figure 2).
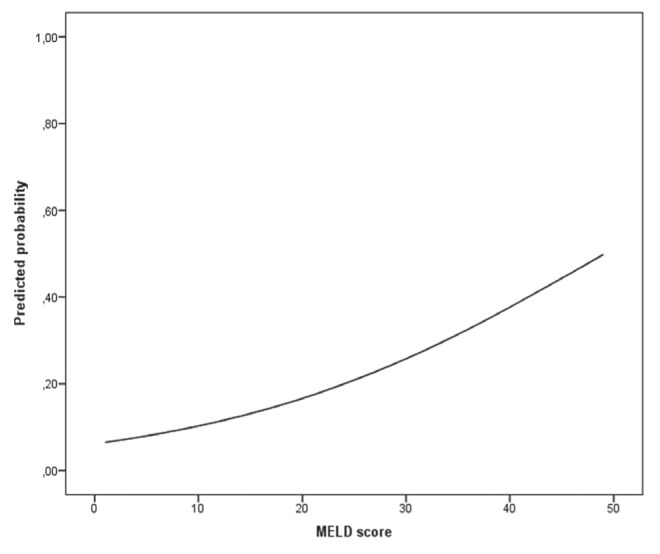
Unadjusted predicted probabilities of a length of first intensive care unit stay after liver transplant ≥10 days according to continuous biochemical Model for End-stage Liver Disease (MELD) score at liver transplant
TABLE 3.
Logistic regression analysis: variables associated with a length of first intensive care unit stay after liver transplant (LT) ≥10 days
| Variable | Unadjusted | Model 1 | Model 2 | |||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| Age, years | 1.01 (0.99–1.04) | 0.37 | 1.01 (0.99–1.04) | 0.30 | 1.01 (0.98–1.04) | 0.61 |
| Male sex | 0.91 (0.56–1.49) | 0.70 | 0.90 (0.54–1.50) | 0.69 | 0.85 (0.46–1.56) | 0.59 |
| Biochemical MELD score at LT (≥40) | 6.73 (3.07–14.7) | <0.001 | 6.86 (3.12–15.1) | <0.001 | 3.21 (1.12–9.20) | 0.030 |
| Hepatic encephalopathy (grades 3 to 4) | 4.33 (2.59–7.25) | <0.001 | 2.29 (1.14–4.60) | 0.020 | ||
| Hepatorenal syndrome | 2.17 (1.22–3.87) | 0.009 | 0.81 (0.37–1.74) | 0.58 | ||
| Hepatopulmonary syndrome | 5.06 (1.59–16.1) | 0.006 | 4.86 (1.23–19.2) | 0.024 | ||
| Intensive care unit admission before LT | 5.33 (3.07–9.26) | <0.001 | 2.42 (1.02–5.71) | 0.044 | ||
| Donor risk index | 1.08 (0.57–2.07) | 0.81 | 0.76 (0.36–1.60) | 0.46 | ||
| Red blood cells in operating room (≥5 units) | 3.08 (1.92–4.96) | <0.001 | 1.75 (0.97–3.16) | 0.06 | ||
Model 1 goodness of fit (n=513): χ2 22 (degrees of freedom ([Df], 3); P<0.001; Area under the ROC curve (AUROC) 0.61 (95% CI 0.54 to 0.67). Model 2 goodness of fit (n=399): χ2 49 (Df, 9); P<0.001; AUROC 0.75 (95% CI 0.68 to 0.81). MELD Model for End-stage Liver Disease
In the same multivariable analysis, other factors that showed a significant association with a prolonged first ICU stay after LT were high-grade hepatic encephalopathy (OR 2.29 [95% CI 1.14 to 4.60]; P=0.020), hepatopulmonary syndrome (OR 4.86 [95% CI 1.23 to 19.2]; P=0.024) and preoperative ICU admission (OR 2.42 [95% CI 1.02 to 5.71]; P=0.044) before LT. Additionally, transfusion of ≥5 units of red blood cells during the LT surgery showed a nonsignificant trend toward a higher likelihood of a prolonged first ICU stay after LT (OR 1.75 [95% CI 0.97 to 3.16]; P=0.06).
Secondary outcomes
Overall, the median length of first hospital stay following LT was 19 days (IQR 12 to 36 days). Patients with a MELD score ≥40 at LT experienced significantly greater median length of first hospital stay after LT (45 days [IQR 26 to 66 days]) versus 18 days [IQR 11 to 34 days]; P<0.001) (Figure 1).
For the entire follow-up period, the rate of ICU readmission was 22% (113 of 513). Of all ICU readmissions, 36% (41 of 113) represented at least a second readmission. Overall, median time to first ICU readmission was 16 days (IQR five to 346 days). The rate of ICU readmission was similar for the two MELD score categories (18% [five of 28] versus 22% [108 of 485]; P=0.58).
For the entire period of follow-up, the re-LT rate was 4% (18 of 519). Causes of re-LT were hepatic artery thrombosis (56% [10 of 18]), primary nonfunction (17% [three of 18]), acute rejection (6% [one of 18]), chronic rejection (6% [one of 18]) and others (17% [three of 18]). The re-LT rate was similar for the two MELD score categories (4% [one of 28] versus 4% [17 of 491]; P=1.00).
During the study period, 139 deaths occurred. Causes of death were recurrence of underlying liver disease (32% [45 of 139]), sepsis (24% [33 of 139]), cardiovascular events (18% [25 of 139]), de novo malignancy (12% [16 of 139]), chronic rejection (4% [five of 139]) and others (11% [15 of 139]).
Kaplan-Meier cumulative survival at one month, three months, one year, three years and five years was 98%, 96%, 90%, 79% and 72%, respectively. Kaplan-Meier curves were similar for the two MELD score categories (P=0.59 [Figure 3]). After adjusting for confounding factors, including age, sex, etiology of liver disease (hepatitis C versus other) and ICU admission before LT, a MELD score ≥40 at LT was not significantly associated with survival (HR 0.76 [95% CI 0.33 to 1.73]; P=0.51). Advanced age was the only factor significantly associated with worse survival after LT (HR 1.03 per incremental year [95% CI 1.01 to 1.06]; P=0.002 (model not shown [n=519]: χ2 25 [degrees of freedom 6]; P<0.001).
Figure 3).
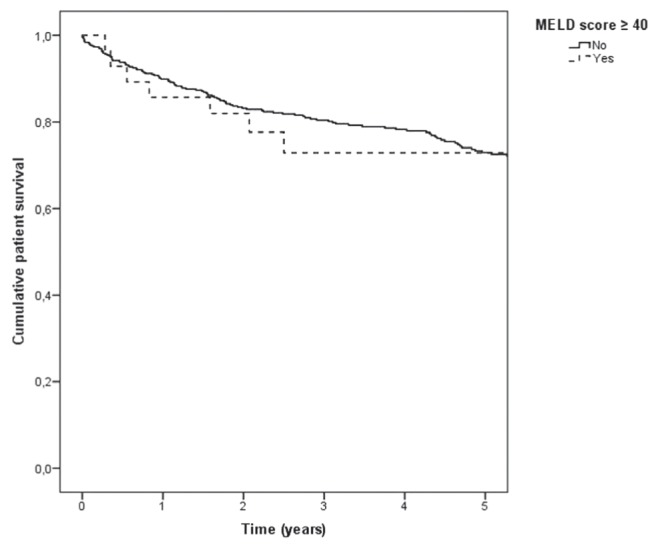
Kaplan-Meier patient survival curve for biochemical Model for End-stage Liver Disease (MELD) score categories (≥40 versus <40) at liver transplant (P=0.59)
DISCUSSION
The purpose of the present study was to characterize post-LT outcomes of patients with end-stage liver disease and a MELD score ≥40 at LT.
Key results
In a large single-centre cohort of adult cirrhotic patients who underwent LT, we found that patients with a biochemical MELD score ≥40 at LT had significantly greater length of first ICU and hospital stays following LT, but similar short- and long-term survival. We also found that a biochemical MELD score ≥40 at LT, high-grade hepatic encephalopathy, hepatopulmonary syndrome and ICU admission before LT, were all independently associated with a length of first ICU stay after LT ≥10 days.
Comparison with previous studies
Our findings that a MELD score ≥40 at LT was significantly associated with a prolonged first ICU stay after LT, but not with survival, are consistent with results from previous studies. Oberkofler et al (8) found that a MELD score >23 was significantly associated with a length of ICU stay after transplant >10 days, but not with survival. Conclusions of this study may not be readily comparable with ours because they used the MELD corrected for exceptions (hepatocellular carcinoma and hepatopulmonary syndrome), instead of the biochemical MELD, which resulted in greater median MELD scores (19 [IQR eight to 40]) and, possibly, overestimation of liver disease severity. Similarly to our study, Alexopoulos et al (7) reported, for patients with an unspecified MELD score ≥40 at LT, Kaplan-Meier cumulative survival at one year and three years of 89% and 77%, respectively. In contrast to our study, they included patients receiving both LT and kidney transplants, and excluded those who received a split graft; however, outcomes for these patients have been reported to be similar to those of whole liver-only recipients (22,23). Further evidence regarding the absence of a significant association between MELD score and survival after LT was provided by Sharma et al (5), who found that cirrhotic patients with a biochemical MELD score ≥40 at listing had a post-LT survival similar to transplant recipients for fulminant hepatic failure, the highest status group of patients for liver allocation. Overall, these results consistently indicate that patients with a MELD score ≥40 at LT are at higher risk for a longer duration of the first ICU stay postoperatively; however, their short- and long-term survival is expected to be similar to other LT recipients.
The significant association of pre-LT high-grade hepatic encephalopathy with a prolonged first ICU stay after LT found in our cohort has not been reported in the literature. In fact, pre-LT hepatic encephalopathy has been associated with post-LT neurocognitive changes (24,25), but its role as a predictor of outcomes after LT has not been well characterized. In a study involving cirrhotic patients admitted to the ICU for high-grade hepatic encephalopathy, but without receiving LT, Fichet et al (26) reported a median length of stay of six days (IQR two to 10 days) and an ICU mortality (35% [25 of 71]) greater than what has been reported for other cirrhotic patients admitted to the ICU.
Although we found pre-LT hepatopulmonary syndrome to be significantly associated with a prolonged first ICU stay after LT, possibly in the context of these patients’ high dependence on oxygen, outcomes of hepatopulmonary syndrome reported in the literature are controversial, with some studies suggesting acceptable outcomes (27,28) and others suggesting that these patients experience poor outcomes after LT (29,30).
The association between ICU admission before LT and the duration of hospital stay after transplant has been studied. Smith et al (31) found that ICU admission before LT was independently associated with a longer hospital stay after transplant; however, this study did not specifically evaluate the utilization of ICU resources postoperatively. Oberkofler et al (8) did not find any significant association between ICU admission before LT and a length of ICU stay after LT >10 days.
While we found a nonsignificant trend between a consumption of ≥5 units of red blood cells during transplant surgery and a prolonged first ICU stay after LT, Oberkofler et al (8) reported that patients who underwent LT and were transfused with >7 units of red blood cells during the procedure had a significantly higher likelihood of experiencing a length of ICU stay after LT of >10 days.
Overall, these results suggest that patients with one or more factors found to independently increase the risk for a prolonged first ICU stay after LT should be expected to consume a greater volume of hospital resources; therefore, specific institutional policies should be developed to improve the efficiency of care provided to them.
Study limitations
The results of the present study need to be considered in the context of the following limitations. First, it was a retrospective analysis of prospectively collected data from a single transplant centre and may be prone to selection bias. Second, individual transplant centres, especially from different countries, have diverse groups of patients and practices regarding enlistment for transplant, allocation of organs, and medical and surgical management strategies; therefore, results may, in part, reflect those specific realities. Despite these limitations, our study was one of the few to characterize the health resource utilization, and short- and long-term outcomes of cirrhotic patients with a MELD score ≥40 at LT. In future studies, an effort should be made to standardize the most valuable markers of post-LT morbidity and survival to further help decision-making on organ allocation and peri-LT care.
CONCLUSIONS
While cirrhotic patients with a MELD score ≥40 at LT experienced significantly greater length of first ICU and hospital stays after LT, they derived similar long-term survival benefit. Despite representing a higher burden of care and costs for the health system, these patients seemed to similarly benefit from LT.
Acknowledgments
Dr Bagshaw is supported by a Canada Research Chair in Critical Care Nephrology and Clinical Investigator Award from Alberta Innovates – Health Solutions. Drs Cardoso and Fidalgo are supported by an unrestricted educational grant donated by Gambro Inc.
APPENDIX 1. Analysis of donors’ and perioperative characteristics for the entire cohort and according to Model for End-stage Liver Disease (MELD) score (≥40 versus <40) categories at liver transplant (LT)
| Total (n=519) | MELD score | P | ||
|---|---|---|---|---|
|
| ||||
| <40 (n=491) | ≥40 (n=28) | |||
| Time between listing for and receipt of LT, months, median (IQR) (n=519) | 5.6 (1.6–12.6) | 6.2 (1.8–13.2) | 1.3 (0.4–4.1) | <0.001 |
| Donor risk index, median (IQR) (n=455) | 1.4 (1.2–1.7) | 1.4 (1.2–1.7) | 1.5 (1.3–1.8) | 0.19 |
| Split graft, n (%) (n=519) | 70 (13) | 69/491 (14) | 1/28 (4) | 0.16 |
| Live donor, n (%) (n=519) | 64 (12) | 63/491 (13) | 1/28 (4) | 0.23 |
| Cold ischemia time*, h, mean ± SD (n=517) | 5.5±2.9 | 5.4±2.9 | 7.1±2.8 | 0.003 |
| Organ from national source, n/n (%) (n=519) | 195 (38) | 177/491 (36) | 18/28 (64) | 0.003 |
| Red blood cells in operating room, units, median (IQR) (n=519) | 2 (0–6) | 2 (0–5) | 8 (4–13) | <0.001 |
| Platelets in in operating room, units, median (IQR) (n=519) | 0 (0–5) | 0 (0–4) | 5 (2–10) | <0.001 |
Normal distribution. IQR Interquartile range
Footnotes
DISCLOSURES: The authors have no financial disclosures or conflicts of interest to declare.
CONSENT: Given that this was a retrospective study, the Institutional Review Board at the University of Alberta waved the requirement for informed consent. While this was a retrospective study and no patients were in contact with study investigators, ethical standards were in accordance with the Helsinki Declaration of 1975, as revised in 2000 and 2008.
REFERENCES
- 1.Edwards EB, Harper AM. Application of a continuous disease severity score to the OPTN liver waiting list. Clin Transpl. 2001:19–24. [PubMed] [Google Scholar]
- 2.Wiesner RH, Mcdiarmid SV, Kamath PS, et al. MELD and PELD: Application of survival models to liver allocation. Liver Transpl. 2001;7:567–80. doi: 10.1053/jlts.2001.25879. [DOI] [PubMed] [Google Scholar]
- 3.Bernardi M, Gitto S, Biselli M. The MELD score in patients awaiting liver transplant: Strengths and weaknesses. J Hepatol. 2011;54:1297–306. doi: 10.1016/j.jhep.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 4.Wiesner R, Edwards E, Freeman R, et al. Model for End-stage Liver Disease (MELD) and allocation of donor livers. Gastroenterology. 2003;124:91–6. doi: 10.1053/gast.2003.50016. [DOI] [PubMed] [Google Scholar]
- 5.Sharma P, Schaubel DE, Gong Q, Guidinger M, Merion RM. End-stage liver disease candidates at the highest Model for End-stage Liver Disease scores have higher wait-list mortality than status-1A candidates. Hepatology. 2012;55:192–8. doi: 10.1002/hep.24632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shawcross DL, Austin MJ, Abeles RD, et al. The impact of organ dysfunction in cirrhosis: Survival at a cost? J Hepatol. 2012;56:1054–62. doi: 10.1016/j.jhep.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 7.Alexopoulos S, Matsuoka L, Cho Y, et al. Outcomes after liver transplantation in patients achieving a Model for End-stage Liver Disease score of 40 or higher. Transplantation. 2013;95:507–12. doi: 10.1097/TP.0b013e3182751ed2. [DOI] [PubMed] [Google Scholar]
- 8.Oberkofler CE, Dutkowski P, Stocker R, et al. Model for End-stage Liver Disease (MELD) score greater than 23 predicts length of stay in the ICU but not mortality in liver transplant recipients. Crit Care. 2010;14:R117. doi: 10.1186/cc9068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. BMJ. 2007;335:806–8. doi: 10.1136/bmj.39335.541782.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moreau R, Jalan R, Gines P, et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144:1426–37. e1–9. doi: 10.1053/j.gastro.2013.02.042. [DOI] [PubMed] [Google Scholar]
- 11.Arvaniti V, D’amico G, Fede G, et al. Infections in patients with cirrhosis increase mortality four-fold and should be used in determining prognosis. Gastroenterology. 2010;139:1246–56. 56:e1–5. doi: 10.1053/j.gastro.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 12.Garcia-Tsao G, Sanyal AJ, Grace ND, Carey W. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology. 2007;46:922–38. doi: 10.1002/hep.21907. [DOI] [PubMed] [Google Scholar]
- 13.Blei AT, Cordoba J. Hepatic encephalopathy. Am J Gastroenterol. 2001;96:1968–76. doi: 10.1111/j.1572-0241.2001.03964.x. [DOI] [PubMed] [Google Scholar]
- 14.European Association for the Study of the Liver EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol. 2010;53:397–417. doi: 10.1016/j.jhep.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 15.Machicao VI, Fallon MB. Hepatopulmonary syndrome. Semin Respir Crit Care Med. 2012;33:11–6. doi: 10.1055/s-0032-1301730. [DOI] [PubMed] [Google Scholar]
- 16.UNOS MELD/PELD Calculator Documentation. < www.unos.org/docs/MELD_PELD_Calculator_Documentation.pdf> (Accessed February 11, 2015).
- 17.Massie AB, Caffo B, Gentry SE, et al. MELD exceptions and rates of waiting list outcomes. Am J Transplant. 2011;11:2362–71. doi: 10.1111/j.1600-6143.2011.03735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karvellas C J, Lescot T, Goldberg P, et al. Liver transplantation in the critically ill: A multicenter Canadian retrospective cohort study. Crit Care. 2013;17:R28. doi: 10.1186/cc12508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feng S, Goodrich NP, Bragg-Gresham JL, et al. Characteristics associated with liver graft failure: The concept of a donor risk index. Am J Transplant. 2006;6:783–90. doi: 10.1111/j.1600-6143.2006.01242.x. [DOI] [PubMed] [Google Scholar]
- 20.Singal AK, Hmoud BS, Guturu P, Kuo YF. Outcome after liver transplantation for cirrhosis due to alcohol and hepatitis C: Comparison to alcoholic cirrhosis and hepatitis C cirrhosis. J Clin Gastroenterol. 2013;47:727–33. doi: 10.1097/MCG.0b013e318294148d. [DOI] [PubMed] [Google Scholar]
- 21.Groenwold RH, Donders AR, Roes KC, Harrell FE, Jr, Moons KG. Dealing with missing outcome data in randomized trials and observational studies. Am J Epidemiol. 2012;175:210–7. doi: 10.1093/aje/kwr302. [DOI] [PubMed] [Google Scholar]
- 22.Doyle MB, Maynard E, Lin Y, et al. Outcomes with split liver transplantation are equivalent to those with whole organ transplantation. J Am Coll Surg. 2013;217:102–12. doi: 10.1016/j.jamcollsurg.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 23.Ruiz R, Kunitake H, Wilkinson AH, et al. Long-term analysis of combined liver and kidney transplantation at a single center. Arch Surg. 2006;141:735–41. doi: 10.1001/archsurg.141.8.735. [DOI] [PubMed] [Google Scholar]
- 24.Sotil EU, Gottstein J, Ayala E, Randolph C, Blei AT. Impact of preoperative overt hepatic encephalopathy on neurocognitive function after liver transplantation. Liver Transpl. 2009;15:184–92. doi: 10.1002/lt.21593. [DOI] [PubMed] [Google Scholar]
- 25.Kanwal F, Chen D, Ting L, et al. A model to predict the development of mental status changes of unclear cause after liver transplantation. Liver Transpl. 2003;9:1312–9. doi: 10.1016/j.lts.2003.09.023. [DOI] [PubMed] [Google Scholar]
- 26.Fichet J, Mercier E, Genee O, et al. Prognosis and 1-year mortality of intensive care unit patients with severe hepatic encephalopathy. J Crit Care. 2009;24:364–70. doi: 10.1016/j.jcrc.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 27.Deberaldini M, Arcanjo AB, Melo E, et al. Hepatopulmonary syndrome: Morbidity and survival after liver transplantation. Transplant Proc. 2008;40:3512–6. doi: 10.1016/j.transproceed.2008.08.134. [DOI] [PubMed] [Google Scholar]
- 28.Gupta S, Castel H, Rao RV, et al. Improved survival after liver transplantation in patients with hepatopulmonary syndrome. Am J Transplant. 2010;10:354–63. doi: 10.1111/j.1600-6143.2009.02822.x. [DOI] [PubMed] [Google Scholar]
- 29.Arguedas MR, Abrams GA, Krowka MJ, Fallon MB. Prospective evaluation of outcomes and predictors of mortality in patients with hepatopulmonary syndrome undergoing liver transplantation. Hepatology. 2003;37:192–7. doi: 10.1053/jhep.2003.50023. [DOI] [PubMed] [Google Scholar]
- 30.Schiffer E, Majno P, Mentha G, et al. Hepatopulmonary syndrome increases the postoperative mortality rate following liver transplantation: A prospective study in 90 patients. Am J Transplant. 2006;6:1430–7. doi: 10.1111/j.1600-6143.2006.01334.x. [DOI] [PubMed] [Google Scholar]
- 31.Smith JO, Shiffman ML, Behnke M, et al. Incidence of prolonged length of stay after orthotopic liver transplantation and its influence on outcomes. Liver Transpl. 2009;15:273–9. doi: 10.1002/lt.21731. [DOI] [PubMed] [Google Scholar]


