Abstract
Efficient functioning of the peripheral vasculature is an essential component in healthy cardiovascular regulation. Alterations in this functioning have been linked to the etiology and pathophysiological course of cardiovascular disease (CVD), especially hypertension. Given its significant role in the maintenance of both healthy and pathological blood pressure, total peripheral resistance (TPR), an index of the vasoconstrictive and elastic properties of the peripheral vasculature, has received much attention in this regard. However, obtaining a reliable estimate of TPR remains a complex and costly endeavor, primarily due to the necessity for sophisticated instrumentation as well as associated limitations in deriving cardiac output (CO). We have previously described a simple estimation method for CO using only arterial blood pressure and heart rate (Hill et al, 2012). In the present study we extend this technique to the estimation of TPR using beat-to-beat blood pressure data from the same sample of 67 young (mean age = 20.04± 2.8), healthy men (n = 30) and women (n = 37). Estimated TPR (TPRest) was calculated from the computationally-derived estimate of CO and mean arterial pressure (MAP). Correlation between TPR obtained via the validated Model-Flow technique and TPRest was moderate (r =.73, p <. 000) and stronger in men (r =.78, p <. 000) compared to women (r =.66, p <. 001). These data further suggest that reconstructed measures of hemodynamic functioning may be validly/adequately estimated from limited data sources.
Keywords: Total Peripheral Resistance, Cardiac Output, Cardiovascular Disease
INTRODUCTION
Efficient functioning of the peripheral vasculature is an essential component in healthy cardiovascular regulation. Alterations in this functioning have been linked to the etiology and pathophysiological course of cardiovascular disease (CVD), especially hypertension. Hypertension, or chronically elevated blood pressure, is a pervasive and costly public health concern affecting millions of individuals domestically and worldwide [1].
Additionally, while diagnosis of hypertension is primarily based on clinical interpretation of arterial indices, (i.e. systolic and diastolic blood pressure) underlying hemodynamics such as the cardiac output (CO) and total peripheral resistance are also important. Given its significant role in the maintenance of both healthy and pathological blood pressure, total peripheral resistance (TPR), an index of the vasoconstrictive and elastic properties of the peripheral vasculature, has received much attention in this regard.
For example, Julius and colleagues [2-3] have proposed the ‘Hyperkinetic’ model of Hypertension, which implicates elevated TPR as the maintaining/sustaining factor in disease progression from initial to later, more severe stages. Consistent with this conceptualization researchers have reported a greater risk for cardiovascular events and death in both normotensive and hypertensive individuals with elevated blood pressure maintained by increased TPR compared to CO [3-4].
These two parameters are quantitatively related as the determinants of blood pressure, wherein the mean arterial or average blood pressure (MAP) is determined as the product of blood flow from the heart (CO) and the resistance (TPR), or friction generated as blood comes into contact with the vessel walls and is pumped and throughout the vasculature. Equation 1 represents an analogous form of Ohm’s law commonly applied in cardiovascular physiology [5].
| (eq.1) |
However, despite its noted importance and clinical value/relevance, obtaining a reliable estimate of TPR remains a complex and costly endeavor, primarily due to the necessity for sophisticated instrumentation as well as associated limitations in deriving cardiac output (CO).
We have previously described a simple estimation method for CO using only arterial blood pressure and heart rate [6]. Specifically, we reported that applying a correction factor (.002) to the product of pulse pressure (PP) and heart rate (HR), yielded an estimate of cardiac output that is comparable to measurements obtained via the model-flow method [6]. The aim of the present research is to extend this technique to the estimation of TPR (i.e. TPRest) and to compare this estimate with values derived via the validated, ModelFlow method [7].
METHODS
Continuous beat-to-beat blood pressure (BP) data from our original sample of 67 young, healthy men and women was used in the present study. Participants completed a seated resting baseline recording period and several laboratory tasks including standing, reading and speaking. Each period was 5 minutes in duration.
Data were recorded using the Finometer® Model-2, non-invasive blood pressure monitoring device (FMS Medical Systems, The Netherlands), which estimates hemodynamic parameters using the Modelflow method based on a three-element Windkessel model [7]. Total peripheral resistance (TPR) is determined as the quotient of ModelFlow-derived MAP divided by CO.
After processing of the raw data using the proprietary Beatscope© software, the unit of measurement for ModelFlow TPR is millimeters of mercury per milliliter per second (mmHg.s/ml).
TPRest was obtained as the quotient of mean arterial pressure in millimeters of mercury (mmHg) divided by cardiac output in liters per minute (L/min) [Equation 2]. Given our derived estimate of cardiac output (CO) [6], mean arterial pressure was calculated from systolic (SBP) and diastolic blood pressure (DBP) using a commonly employed algorithm [Equation 3].
| (eq.2) |
| (eq.3) |
To obtain TPRest values with a comparable unit of measurement to the ModelFlow value, metric conversion was necessary [8]. This was accomplished by multiplying TPRest (mmHg.min/L) by .06 s/ml, which was obtained by converting (min/L) to (s/ml) and dividing ((i.e 1 minute = 60 seconds and 1 liter = 1000 milliliters, 60s/1000ml = .06s/ml).
Pearsons correlation (r) was used to evaluate the association between baseline total peripheral resistance (TPR) derived via the Finometer and the estimate (TPRest). Bland-Altman plots were constructed to examine the level of agreement between both measures in the total sample, as well as separately by gender. We additionally assessed the comparability of TPRest to TPR during performance of several laboratory tasks involving physical and mental.
RESULTS
Participants were comparable for all parameters (see Table 1). Males and females did not differ significantly on either TPR or TPRest. The difference between TPR and TPRest was statistically significant in the total sample, as well as separately by gender (all p’s < .001). TPRest yielded a smaller value compared to Modelflow-derived TPR. This difference may be attributable to our use of estimates for both CO and MAP. As others have suggested composite hemodynamic measures may likely exhibit less reliability than their component factors as such estimates incorporate the independent measurement variance of each constituent parameter [9].
Table 1.
Total and subsample n’s, means and standard deviations for resting beat-to-beat data.
| M(SD) | Men | Women | Total |
|---|---|---|---|
| n | 30 | 37 | 67 |
| Age (yrs) | 19.97 (2.02) | 20.11 (3.39) | 20.04 (2.82) |
| SBP (mmHg) | 125.23 (15.55)a | 116.54 (13.05) | 120.33 (14.87) |
| DBP (mmHg) | 69.54 (11.22) a | 63.98 (9.73) | 66.45 (10.71) |
| MAP (mmHg) | 88.11 (12.04) a | 81.50 (9.96) | 84.41 (11.39) |
| COest (L/min) | 7.81 (1.53) | 7.93 (1.78) | 7.87 (1.64) |
| TPR (mmHg.s/ml) | 0.87 (0.19)b | 0.91 (0.25) b | 0.89 (0.22)b |
| TPRest (mmHg.s/ml) | 0.67 (0.14) | 0.64 (0.16) | 0.65 (0.15) |
| Mean Difference(TPR-TPRest) | 0.21 (0.14) | 0.28 (0.21) | 0.25 (0.19) |
denotes significant difference p < .05,
denotes significant TPR vs TPRest difference p < .001.
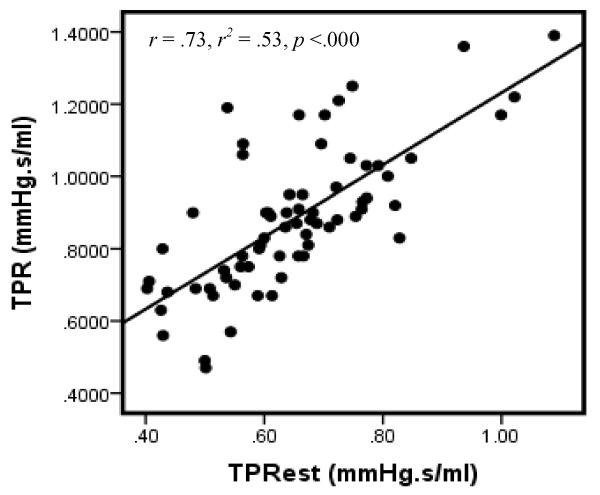
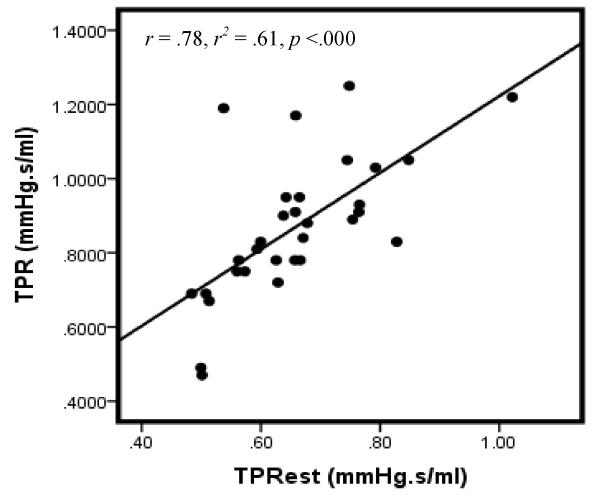
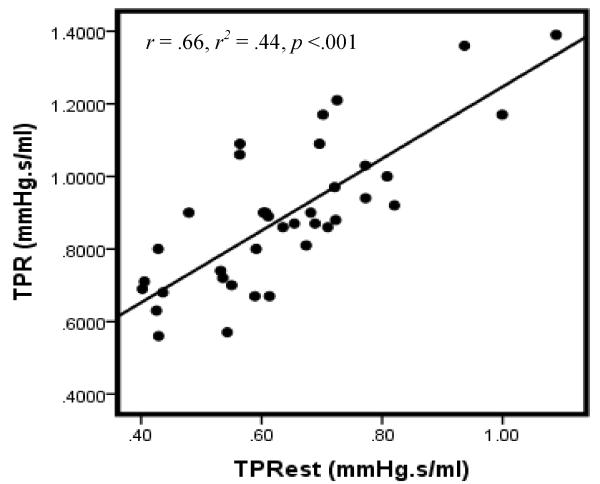
Examination of the correlation between TPR and TPRest does, however, suggest reasonable reliability between the two indices. Particularly, correlation between the measures is relatively robust in the total sample (Figure 1) as well as when considered separately for males (Figure 2) and females (Figure 3). These associations are notable larger than those reported in our previous investigation of computationally-derived CO compared with CO obtained using the ModelFlow method.
Figure 1.
Scatterplot of TPR & TPRest: All
Note: Data for 2 participants excluded due to missing gender code.
Figure 2.
Scatterplot of TPR & TPRest: Males
Note: Data for 2 participants excluded due to missing gender code.
Figure 3.
Scatterplot of TPR & TPRest: Females
Note: Data for 2 participants excluded due to missing gender code.
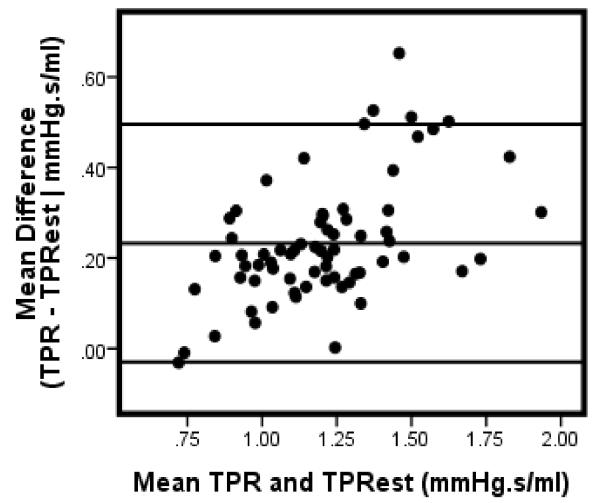
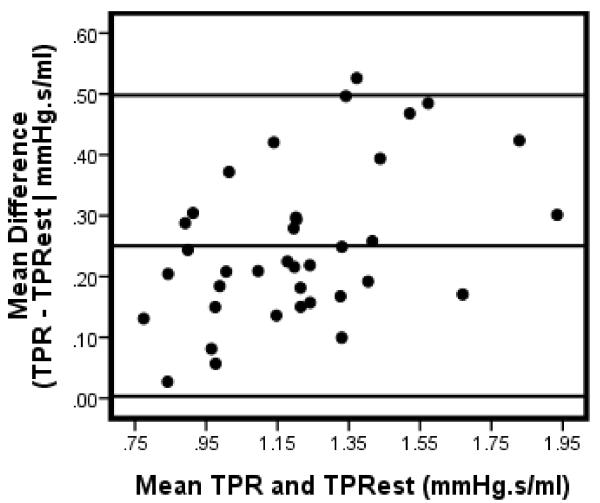
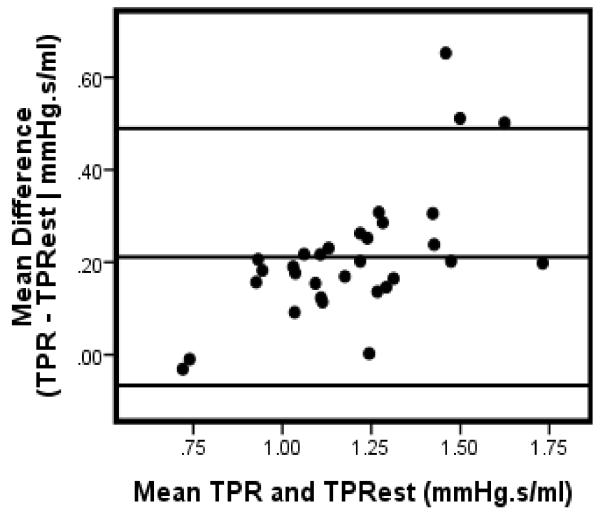
Bland-Altman analysis [9] were conducted to further assess the level of agreement between TPR and TPRest . The mean difference (Table 1) between TPR and TPRest ranged between .21 to .28 with the smallest margin occurring in males and the largest difference in females. As depicted in Figure 4, 3 cases (4%) fell beyond the upper limit of agreement (approximatley 2 standard deviations) above the mean difference of the total sample. In men, only one exceeded the limits of agreement (Figure 5); while 2 (5%) cases were completely above the limit with one additional case falling partially on the margin (Figure 6).
Figure 4.
Bland-Altman plot: TPR & TPRest: All
Note: Data for 2 participants excluded due to missing gender code.
Figure 5.
Bland-Altman plot: TPR & TPRest: Males
Note: Data for 2 participants excluded due to missing gender code.
Figure 6.
Bland-Altman plot: TPR & TPRest: Females
Note: Data for 2 participants excluded due to missing gender code.
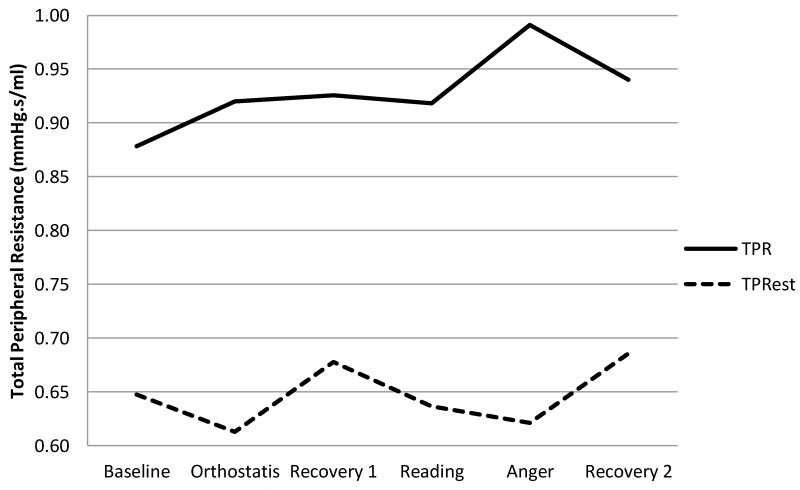
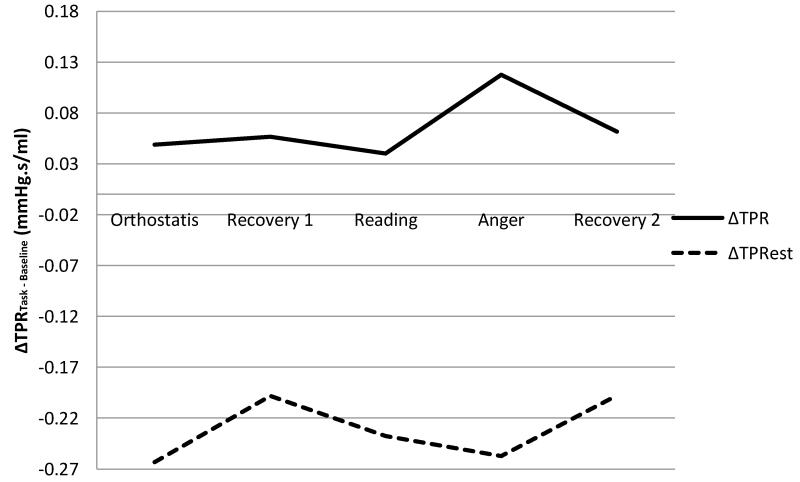
TPRest was consistently lower than ModelFlow TPR both in terms of absolute levels (Figure 7) as well as in terms of change (Δ) scores calculated as the mean value of CO (COest) for a given task minus the baseline value (Figure 8). Collectively these results suggest that TPRest may represent a conservative, lower-bound estimate of total peripheral resistance.
Figure 7.
Mean TPR & TPRest across a series of laboratory tasks.
Figure 8.
Difference Scores (Task – Baseline) for TPR & TPRest across a series of laboratory tasks.
Note: Plotted values represent data averaged across all subjects.
DISCUSSION
Total peripheral resistance is an important index of cardiovascular health and functioning and plays a significant role in the maintenance and progression of Hypertension. While direct measurement of this and other physiological phenomena is preferable, refined computational methods such as the ModelFlow algorithm have shown remarkable utility in producing reliable estimates, non-invasively.
However, in both clinical and research contexts factors such as equipment and software cost, methodological and interpretive complexity and clinician/researcher time-constraints must be considered. Simple quantitative estimates provide a practical, inexpensive alternative to more complex methods of hemodynamic parameter estimation.
Others have demonstrated the feasibility of obtaining similar estimates of CO and TPR in an ambulatory context [11]. The results of both our previous work and the present investigation support the notion of valid estimation of hemodynamic parameters from heart rate, systolic and diastolic blood pressure which are commonly obtained in ambulatory investigations.
The current results indicate a moderate association between TPR and TPRest though the latter value is notably more conservative under both tonic and phasic conditions. Several factors may help to explain this modest discrepancy including the fact that estimated CO (i.e. COest) was found to be consistently higher than ModelFlow estimate [6] which would in turn yield a smaller value for TPRest given TPRest = MAP/COest.
CONCLUSIONS
We extended our previous work to evaluate the concordance of a computationally-derived estimate of total peripheral resistance with TPR obtained using an advanced, ModelFlow algorithm. Correlation between TPRest and ModelFlow TPR was relatively robust and stronger in males compared to females. TPR was signficantly larger than TPRest; however, 96% of cases fell within the limits of agreement around the mean difference between the two measures.
The ModelFlow algorithm produces CO values which are based on an estimate of aortic area derived via population-based averages that account for subject characteristics such as age and gender among others. As such, our estimate of TPR should be considered an ‘uncalibrated’ index. Future studies should evaluate the agreement of TPRest assessed using an independent device such as a mercury sphygmomanometer or automated auscultatory monitor with ModelFlow TPR.
Given the dynamic compensatory interaction of CO and TPR in the maintanence and regulation of both normal and pathologic blood pressure, retrospective estimation of these values from pre-existing data may yield further insights into additional factors that both alter and/or enhance cardiovascular health and functioning. The proposed estimate may serve as a useful starting point in this regard.
ACKNOWLEDGMENTS
We would like to thank all of the participants at The Ohio State University.
REFERENCES
- [1].Mittal BV, Singh AK. Hypertension in the Developing World: Challenges and Opportunities. American Journal of Kidney Diseases. 2010;55(3):590–598. doi: 10.1053/j.ajkd.2009.06.044. [DOI] [PubMed] [Google Scholar]
- [2].Julius S, Pascual AV, Sannerstedt R, Mitchell C. Relationship Between Cardiac Output and Peripheral Resistance in Borderline Hypertension. Circulation. 1971;43(3):382–90. doi: 10.1161/01.cir.43.3.382. [DOI] [PubMed] [Google Scholar]
- [3].Julius S. Transition from high cardiac output to elevated vascular resistance in hypertension. American Heart Journal. 1988;116(2):600–606. doi: 10.1016/0002-8703(88)90557-1. [DOI] [PubMed] [Google Scholar]
- [4].Thayer JF, Hansen AL, Johnsen BH. Noninvasive Assessment of Autonomic Influences on the Heart: Impedance Cardiography and Heart Rate Variability. In: Gallo LC, Lueken LJ, editors. Handbook of Physiological Research methods in Health Psychology. Sage Publications; 2007. pp. 183–209. [Google Scholar]
- [5].Guyton, Hall . Textbook of Medical Physiology. Tenth Edition W.B. Saunders Co.; 2001. [Google Scholar]
- [6].Hill LK, Sollers JJ, Iii, Thayer JF. Evaluation of a simple estimation method for the derivation of cardiac output from arterial blood pressure and heart rate. Biomed Sci Instrum. 2012;48:165–70. [PubMed] [Google Scholar]
- [7].Finapres Medical Systems . BeatScope 1.1. User’s Guide. Finapres Medical Systems; Amsterdam, the Netherlands: 2002. [Google Scholar]
- [8].Westerhof N, Stergiopulos N, Noble MIM. Snapshots of Hemodynamics: An Aid for Clinical Research and Graduate Education, SpringerLink: Bücher. 2010 [Google Scholar]
- [9].Smith TW, Uchino BN. Measuring Physiological Processes in Biopsychosocial Research: Basic Principles Amid Growing Complexity. In: Gallo LC, Lueken LJ, editors. Handbook of Physiological Research methods in Health Psychology. Sage Publications; 2007. pp. 11–33. [Google Scholar]
- [10].Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1986;327:307–10. [PubMed] [Google Scholar]
- [11].Haslam B, Gordhandas A, Ricciardi C, Heldt T, Verghese G. Relating noninvasive cardiac output and total peripheral resistance estimates to physical activity in an ambulatory setting; AAAI 2011 Spring Symposium on Computational Physiology (SS-11-04). AAAI; 2011.pp. 27–31. [Google Scholar]