Abstract
The nematode Caenorhabditis elegans has been a powerful model system for the study of key muscle genes relevant to human neuromuscular function and disorders. The behavioral robustness of C. elegans, however, has hindered its use in the study of certain neuromuscular disorders because many worm models of human disease show only subtle phenotypes while crawling. By contrast, in their natural habitat, C. elegans likely spends much of the time burrowing through the soil matrix. We developed a burrowing assay to challenge motor output by placing worms in agar-filled pipettes of increasing densities. We find that burrowing involves distinct kinematics and turning strategies from crawling that vary with the properties of the substrate. We show that mutants mimicking Duchenne muscular dystrophy by lacking a functional ortholog of the dystrophin protein, DYS-1, crawl normally but are severely impaired in burrowing. Muscular degeneration in the dys-1 mutant is hastened and exacerbated by burrowing, while wild-type shows no such damage. To test whether neuromuscular integrity might be compensated genetically in the dys-1 mutant, we performed a genetic screen and isolated several suppressor mutants with proficient burrowing in a dys-1 mutant background. Further study of burrowing in C. elegans will enhance the study of diseases affecting neuromuscular integrity, and will provide insights into the natural behavior of this and other nematodes.
Keywords: Behavior, Caenorhabditis elegans, burrowing, nematode, dystrophin
Introduction
For over half a century the nematode Caenorhabditis elegans has been successfully used to study the cellular, molecular and genetic basis of neuromuscular function and disease (Dougherty and Calhoun, 1948; Culetto and Sattelle, 2000). Most of this research has relied on assessing worms crawling on an agar-filled plate as the primary behavioral diagnostic of neuromuscular integrity (Hart, 2006). Crawling offers an amenable behavioral readout in the study of key genes expressed in neurons and muscle. In fact, many human genes, such as distinct myosins and neurotransmitter transporters, were first identified in C. elegans as mutants deficient in crawling, the so-called uncoordinated (unc) phenotype (Brenner, 1974; Harris and Epstein, 1977; McIntire et al., 1997). However, C. elegans appears less suited to study phenomena like Muscular dystrophy (MD) where the neuromusculature of animals is not significantly challenged by the relatively facile task of crawling on an agar surface. Indeed, previous studies have struggled to identify severe phenotypes in worms modeling MD by carrying a loss-of-function mutations in the C. elegans dystrophin ortholog, dys-1, responsible for Duchenne muscular dystrophy (DMD). The dys-1 mutant displays only moderate behavioral phenotypes, such as a slightly crooked neck; however, the dys-1 mutant can crawl normally (Giugia et al., 1999). This unpairing of genotype and phenotype is not common to the worm model of the disease. Indeed, fly (Shcherbata et al., 2007) and mouse (Torres and Duchen, 1987; Durbeej and Campbell, 2002) systems that model DMD through disruption of dystrophin also have failed to model the extreme behavioral phenotype observed in humans. Many groups working on worms (e.g. Gieseler et al., 2000; Mariol and Ségalat, 2001), flies (e.g. Kucherenko et al., 2008), and mice (Deconinck et al., 1997) have thus turned to study sensitized strains (e.g. Mdx mutant background) in an attempt to recapitulate the human phenotype. However, genetic sensitization may limit the applicability of results to MD which occurs in humans with mutations in dystrophin alone (Monaco et al., 1987).
The lack of acute behavioral phenotypes in these models may be a result from compensatory pathways (Michele et al., 2002; Moore et al., 2002) or alternatively, it may be the product of the behavioral paradigms used to evaluate function. Petrof et al. (1993) showed that the degree of muscular degeneration in Dmdmdx mice was directly correlated with the strength (and not the frequency) of muscle contraction. To investigate if behavioral paradigms exposing dys-1 worms to increased strength of muscular exertion could better recapitulate the ethology of DMD we set out to study burrowing in worms. As a prelude, we provide an initial description of the kinematics of the burrowing behavior of C. elegans, and compare burrowing to crawling and swimming behaviors. We then investigate the potential usefulness of burrowing as a diagnostic of neuromuscular integrity by comparing muscular degeneration in dys-1 animals reared in a burrowing versus a crawling regiment. Lastly, we use this behavior to perform the first behavioral suppression screen on worms modeling muscular dystrophy.
Materials and Methods
Animals
C. elegans strains wild-type N2, dys-1(eg33), islo1(eg978), and dys-1(cx18) were obtained from the Caenorhabditis Genetic Center or were gifts from Dr. Hoky Kim. Oh and Kim (2013) report that the eg33 allele has a nonsense mutation at position 3287, and that the cx18 allele has a nonsense mutation at position 2721. Animals were cultured on nematode growth media (NGM) agar plates and fed OP50 strain E. coli at 20°C as described (Brenner, 1974). Wild-type and dys-1 mutants expressing nuclear and mitochondrial GFP in body wall muscles were the HKK5 and HKK22 strains as previously described (Oh and Kim, 2013). Mitochondria and nuclei in the muscles were labeled with green fluorescent protein via the integrated transgene array ccIs4251[Pmyo-3GFP-NLS, Pmyo-3 GFP-mit] (Oh and Kim, 2013). Out of the 4 different dys-1 suppressor mutant strains isolated in this study, JPS518 was the only one that successfully mated with the dys-1;ccIs4251 strain allowing us to analyze its subcellular muscle integrity. The dys-1 gene was knocked down via RNA interference as originally described (Timmons & Fire, 1998).
Behavioral analyses
Adult (day one) animals were picked into a 1-μl droplet of Nematode Growth Medium (NGM) buffer (Hart, 2006). A glass capillary was used to transfer by injection the worms into a 1.5-ml glass pipette prefilled with agar of 0.5%, 1.5%, 3%, 6%, and 9% densities in chemotaxis buffer (Hart, 2006). Following a two-minute acclimation period, ten animals in each condition were filmed for five minutes at 10 frames/s, 344 pixels/mm using a Flea2 camera (Point Grey Research, Richmond, BC, Canada) mounted on a dissecting microscope and using StreamPix software (NorPix, Montreal, Canada).
Burrowing proficiency
Worms were injected into one end of a 10-mL plastic pipette and sealed with Parafilm wax. The opposite end of the plastic pipette had an attractant, diacetyl, to encourage directed burrowing. To quantify proficiency at burrowing, we counted the number of worms on either side of the 4-cm mark. The majority of wild-type worms migrate past this position (Figure 4d). Worms did not make sufficient progress towards the opposite side without the attractant.
Figure 4.

Burrowing is an ideal behavior to assess neuromuscular integrity. We tested two dys-1 loss-of function alleles that model Duchenne muscular dystrophy (MD). Crawling behavior failed to produce striking phenotypes for these animals. For example, dys-1 worms display no defect in head-bend frequency (a), reversal rate (b), and velocity (c) when crawling. The same dys-1 mutant strains, however, were severely impaired in burrowing (d). Mean and s.e.m. reported for samples of 30 worms for the crawling, and 65 animals for the burrowing experiments. **P < 0.001 two-tailed t-test. (e) Unlike wild-type (top), burrowing dys-1 mutants had crawl-like kinematics (middle) interspersed with periods of immobility (bottom) as evident in the curvature matrices (10 sec each). Average curvature plots for corresponding strain on right.
Single animals
Animal midlines (13 points) of single worms were derived as previously described using a custom image analysis algorithm available upon request (Pierce-Shimomura et al., 2008) (ImagePro; Media Cybernetics, Rockville, MD, USA). The series of 11 angles formed by the midline was represented in a color-coded “curvature column” (Figure 1B). A time series of curvature columns formed a “curvature matrix” in which blue and red stripes represent the waves of dorsal and ventral curvature, respectively, passing along the body. Curvature matrices and average phase cycles were obtained and plotted using IgorPro (Wave Metrics, Lake Oswego, OR, USA). The quality of each digitized worm midline was manually checked, superimposing it on the original video frame, and corrected if necessary for every video frame used in this study. For each condition, ten worms were used.
Figure 1.

Kinematic analysis of the burrowing behavior of C. elegans. (a) We used 1.5-ml glass pipettes filled with agar of varying densities to film the burrowing behavior of individual worms. Adults were injected into one end of the pipette and filmed as they burrowed to an attractant (diacetyl) placed at the opposite end (inset). (b) Our custom algorithm implemented in ImagePro detected and digitized worms while freely behaving (top). The midline of animals were then divided into eleven sections of equal length (middle) and the angle between these was measured and assigned a color ranging from red for 70° ventral, to white for 0°, to blue for 70° dorsal. Here a burrowing worm is shown. Three color-coded frames are followed through the spinning process, which results in the creation of a behavioral matrix that describes the entire behavior of the animal over time (bottom). (c) A spined worm is shown (i) to illustrate the angles represented in the curvature matrix. A dorsal angle is presented as a shade of blue (ii), straight angles appear as white (iii), and ventral angles as shades of red (iv). In this and all curvature matrices, anterior is down and posterior is up.
Groups of worms
For parameters listed below, we analyzed the behavior of groups of ten worms confined within a square copper frame (1.4 cm per side) on a blank NGM agar plate was recorded as before (Vidal-Gadea et al., 2012). Worm centroids were detected and tracked using ImagePro. For each condition, three assays containing ten worms each were conducted (N = 30). Worms were filmed for three minutes following a two-minute acclimation period to the arena consisting of a copper frame fused to the agar surface to contain the worms.
Parameters measured
Head bend frequency
Measured using the time required for ten consecutive, and uninterrupted, head bends.
Velocity
Distance travelled in 1 min by body centroid in the direction of locomotion.
Turning rate
Number of direction changes per minute.
Reversals
Consist of changes in forward trajectory preceded by reversed locomotion.
Omega bends
Changes in trajectory produced by a single full ventral bend where the anterior and posterior ends of the animals come in proximity adopting a shape reminiscent of the Greek letter naming the maneuver (Ω).
Deep bends
Changes in trajectory produced by a single body bend of large amplitude.
Lateral bends
Consist of changes in trajectory orthogonal to the plane of locomotion produced by a laterally directed deep bend.
Chemotaxis
Worms were assessed for chemotaxis performance according to Bargmann et al. (1991).
Suppressor screen
We conducted our suppressor screen according with the protocol described in Jorgensen and Mango (2002) but using 3-μM N-ethyl-N-nitrosourea as the mutagen. Approximately 100 haploid genomes were screened by re-plating the progeny of 50 mutagenized F1 worms. To make sure that food was available to worms in the pipettes, we centrifuged 1.5 ml of OP50 liquid culture and pipette half of each pellet 5 cm away from the start point, and the other half 10 cm away from the start point.
Imaging and Analysis
Worms were removed from the burrowing pipettes by ejecting the agar and immersing in NGM liquid. This allowed the recovery of worms with motor deficits. After recovery animals were mounted onto 2% agar pads containing 30-mM sodium azide. Optical stacks were acquired using a Zeiss 710 Laser Scanning Confocal Microscope connected to a fluorescent light source. Images were processed using ImageJ (NIH, Bethesda, MD, USA) software. Although we observed muscle degeneration throughout the worm, we focused our analysis on the midbody and posterior half of the animal due to the simpler muscle structure.
Statistical analysis
Sigmaplot 12.5 was used for all statistical analyses to determine significance (p ≤ 0.05, two-tailed) between two or more groups. If the groups being compared passed the Shapiro-Wilk normality test, they were analyzed using standard t- or ANOVA tests where appropriate. Groups that failed to pass the normality or equal variance test were compared using Mann-Whitney Sum of Rank tests. In every figure means and s.e.m. are reported.
Results
Development of a novel burrowing assay
To study the kinematics of burrowing behavior for individual C. elegans we used agar-filled pipettes (Fig. 1a). We injected a liquid-solution of worms into the agar 1 cm away from one end of the pipette. We then filmed worms while they burrowed towards a drop of the attractant diacetyl placed on the opposite end of the pipette. We manually adjusted the pipette to film worms, adjusting the focal plane and repositioning the pipette as necessary to keep them in view.
Burrowing kinematics were obtained by using a custom-built algorithm (Pierce-Shimomura et al., 2008) that divided the midline of the worm into eleven equal segments and plotted the measured angles between successive segments (Fig. 1b). In this way, the entire behavior of the animal could be analyzed and displayed for comparison as a color-coded matrix where the extent of dorsal and ventral flexion are correlated with the intensity of the blue and red color respectively (Fig. 1c). Video segments were only analyzed if the worm maintained its dorsoventral bending along the focal plane of the video camera.
Burrowing kinematics are distinct from swimming and crawling kinematics
C. elegans locomotes forward by propagating dorsoventral bends down the length of their bodies anterior to posterior. We found that the frequency of head bends during burrowing was lower than the frequency during crawling and swimming over the range of agar densities tested (between 0.5% and 9%, Fig. 2a). Head bend frequency displayed a bell-shaped curve with agar density. This might be explained by different forms of movement across agar densities. Worms appeared to primarily exhibit a form of motion resembling “slow crawling” in agar less dense than 1.7%. For denser agar conditions, however, worms appeared to adopt a distinct “burrowing” form of motion described in more detail below. Note that even the lowest density gel (0.5% provided significantly more resistance than water. The head-bend variability observed through the range of substrate densities (reported as s.e.m. in Fig. 2a) was substantially smaller for burrowing than for swimming or crawling.
Figure 2.

Burrowing is distinct from swimming and crawling behaviors. (a) Burrowing head-bend frequency in a range of agar densities was lower than crawling frequency (mean and s.e.m. shown in green), and swimming (mean and s.e.m. shown in blue). N = 15 worms, 10 head-bends each, for swimming and crawling, and N = 10 worms, 10 head-bends each, for each agar density during burrowing. (b) Curvature matrix plot of a representative swimming bout 10 seconds long (i). The phase plot of matrix data averaged across individual head-bend cycles (ii) shows how the worm switches from a ventrally bent “C” shaped posture, pictured on the right (iii), to a dorsally bent C shaped posture and back. (c) Crawling worms moved by means of a persistent but propagating “S” shape (i), as depicted by the average cycle plot (ii) and picture (iii). (d) Across densities, worms burrowed with a continuous “M” (or “W”) shape. Burrowing is also distinct from swimming and crawling in lacking the dampening of posteriorly directed bends. All curvature matrices are ten seconds long. N = 10 worms with minimum of 10 cycles each for every condition, representative examples shown.
Qualitative differences in burrowing kinematics
Aside from their decreased bend frequency, burrowing was also kinematically distinct from crawling and swimming. Across behaviors, bending amplitude (represented by the depth of the blue and red colors in the matrix plots) appeared inversely related to bending frequency (Fig. 2bi–di). Average maximal neck bend amplitudes were 28.4 ± 3.11° s.d. for crawling, 75.3 ± 10.1° s.d. for swimming, and 88.7 ± 3.2° s.d. for burrowing (n = 8 each). Swimming and crawling exhibited posterior dampening of the propagated bend, a phenomenon where the amplitude of the body bend is greatest near the head and gradually dampens as it reaches the tail (Supplemental Figure 1). This is evident on the representative curvature matrices and average cycle plots as the darker blue and red at the bottom (anterior) of the plots when compared to the lighter colors at the top (posterior) (Fig. 2bi & ii, 2ci & ii). It is also evidence when plotting neck and tail curvature time series and computing the average change in maximal bend (Supplemental Figure 1). By contrast, burrowing worms did not exhibit as significant dampening of bends (Fig. 2di & ii; Supplemental Figure 1). The average cycle plots (ii) and the accompanying screen shots (iii) also illustrate the distinct shapes worms assume in each behavior. Swimming animals can be said to locomote by alternating dorsal and ventral “C”-shaped body postures, while crawling and burrowing rely on persistent “S” and “W” shaped postures respectively (Fig. 2 biii–ciii). This is evident after noting that most columns in the curvature matrices include two changes in color for crawling, and always at least three changes for burrowing. By contrast, the dorsal and ventral C-shaped postures during swimming are apparent as curvature columns with only red or blue coded colors at the beginning and middle of each head-bend cycle.
Worms modulate their turning strategies according to their environment
While migrating toward or away from a stimulus, C. elegans has several ways to alter their trajectory (Ward, 1973; Croll, 1975; Pierce-Shimomura et al., 1999; Iino et al., 2009). Worms can perform a reversal, which consists of one to several bends that propagate from tail to head before a forward change in direction. During an omega bend the worm bends its head ventrally to touch its posterior end, causing its body to form an Ω shape, before heading in the opposite direction. Deep bends are changes in trajectory produced by single bends of increased amplitude, resulting in a new anterior-directed heading.
We found that while swimming worms changed direction primarily by using omega bends and deep bends, crawling worms relied heavily on reversals (Fig. 3a), consistent with our previous report (Vidal-Gadea et al., 2012). Burrowing worms modified their turning strategy according to the density of the substrate (Fig. 3a, b). At low densities, burrowing worms used all strategies also used during swimming and crawling together with an additional turning strategy we defined as lateral bends (see Video 1). During a lateral bend, the worm changes direction by virtue of a single left or right bend that causes the worm to now travel in an orthogonal plane to their ongoing dorsoventral movement. Like reversals and deep bends, lateral bends were observed for animals burrowing at densities below 3%. Above this density animals relied almost entirely on omega bends.
Figure 3.

Worms alter their trajectory by different means depending on substrate properties. (a) Wild-type worms showed distinct strategies to change their direction depending on their environment. Swimming animals changed direction by producing deep bends and omega bends. Crawling worms favored reversals and burrowing worms rely on deep and lateral bends. (b) As substrate density increases, worms decreased their turning rate and relied increasingly on omega bends. At lower densities however worms used several turning strategies including lateral bends characterized by three-dimensional waves. Mean and s.e.m. reported for 3-min observation period after 30-min of acclimation for 30 worms in each condition.
Worms modeling muscular dystrophy are impaired at burrowing
To determine if the burrowing behavior could be used as a read-out of neuromuscular integrity we compared the burrowing and crawling abilities of wild-type worms to that of two strains carrying loss-of-function mutations in the dys-1 gene. The dys-1 gene is orthologous to the human dystrophin gene responsible for Duchene’s muscular dystrophy when mutated. We were motivated to test the burrowing ability of the dys-1 mutant because previous reports found that they were grossly normal in crawling (Giugia et al., 1999). Consistent with this, we found that both dys-1 mutant strains showed no deficit in crawl frequency (Fig. 4a). Their rate of reversal and crawling velocity also appeared unimpaired (Fig. 4b,c).
To compare the burrowing ability of different strains, we injected worms into 5-ml agar-filled plastic pipettes and allowed the worms to burrow towards an attractant for two hours (Fig. 1a). After this time, worms were scored according to their progress along the chemoattractant gradient (Fig. 1b). In contrast to their proficient crawling, both of the dys-1 strains were severely impaired at burrowing (Fig. 4d, U = 0.000, P < 0.001 for WT vs. both cx18 and eg33). Individual dys-1 mutants often exhibit periods of immobility as apparent as static pattern in the curvature matrix (Fig. 4e bottom, Video 2). When moving, however, individual dys-1 mutants burrowed using kinematics reminiscent of crawling. Bends were faster, more variable in amplitude, and dampened posteriorly (Fig. 4e middle) compared with wild-type burrowing (Fig. 4e top).
Because dys-1 is expressed in both muscles and neurons, inefficient burrowing of the dys-1 mutant might be explained by a neuronal deficit in sensing the attractant and/or orienting towards the attractant. However, we found that the dys-1 mutant readily performed chemotaxis when crawling in a standard assay (Supplemental Figure 2a). Additionally, to confirm that the burrowing defect reflected a loss of dys-1 function, we assayed how knock-down of dys-1 via RNA interference affected burrowing. We found that compared to control-treated worms, dys-1-RNAi treated worms were profoundly defective in burrowing (Supplemental Figure 2b). Lastly, we tested whether the burrowing defect extended to additional members of the DGC complex in C. elegans muscle, by assaying the mutant islo-1. The ISLO-1 protein physically links DYS-1 to the highly conserved BK potassium channel throughout muscle to preserve calcium activation of the channel (Kim et al., 2009). We found that the islo-1 mutant was similarly defective in burrowing but not crawling like the dys-1 mutant (Supplemental Figure 3).
Taking the above results together, we conclude that dys-1 gene is likely required for proper muscle function for efficient burrowing.
Burrowing increases the rate and extent of muscular degeneration in animals modeling MD
Work on mouse models of DMD suggests that the extent of muscular degeneration directly correlates with the strength of muscle contractions (Petrof et al., 1993). Crawling dys-1 mutant worms show normal muscle morphology in young adulthood, with a small subset of seemingly random muscle cells dying only after advanced age (>10 days, Oh and Kim, 2013). We hypothesized that muscle degeneration may be more readily observed in the dys-1 mutant if its muscles were challenged by forcing worms to burrow rather than to crawl (as they do under standard culture conditions). We raised wild-type and dys-1 mutant worms in conditions where they were required to obtain food by crawling (standard conditions) or by burrowing (similar to described above). Specifically, we placed twenty young (day 3, L4-larval stage) wild-type and dys-1 mutant worms on agar plates seeded with bacterial lawns for standard conditions, and inside 6% agar-filled pipettes also seeded with bacterial food at the opposite end. Both wild-type and dys-1 strains carried integrated GFP transgenes to label muscle nuclei and mitochondria to observe muscular integrity after being raised in each condition.
After four days, adult worms were retrieved from crawling or burrowing conditions and assessed for muscular and behavioral integrity (Fig. 5). Although some degeneration was seen on dys-1 mutants grown in plates, as reported previously (Oh and Kim, 2013), dys-1 mutants forced to burrow in pipettes showed extensive muscle cell degeneration. This was evident by the loss of distinct circular appearance of GFP-labeled muscle cell nuclei, the absence of clearly linear arrangements of GFP-labeled mitochondria, together with the development of small aggregates of GFP indicating cell degeneration (representative confocal-stack images, Fig. 5a).
Figure 5.

Burrowing hastens muscular degeneration in worms modeling muscular dystrophy. Young (L4) wild-type and dys-1 mutant worms expressing GFP-tagged muscle nuclei and mitochondria (ccIs4251[Pmyo-3::GFP-NLS + Pmyo-3::GFP-mit]) were grown on either agar plates with bacteria, or in 6% agar pipettes with bacteria at opposite and half-way points to force animals to crawl or burrow respectively. After four days, crawling ability was tested and their musculature was subsequently imaged. Wild-type and dys-1 mutant worms showed only limited muscle degeneration when raised in the crawling condition ((a) top row); however, dys-1 mutants showed marked muscular degeneration when raised in the burrowing condition ((a) bottom row). Arrowheads point to areas of accumulation of GFP, indicative of muscle cell degeneration. Three representative worms are shown for each condition. The muscular degeneration observed for burrowing dys-1 mutants was reflected in their locomotor dysfunction. (b) dys-1(eg33) mutant worms raised in the burrowing condition showed a marked decrease in crawling velocity compared to their sisters raised in the crawling condition. (c) Although an increase in reversal frequency might be associated with the measured decrease in velocity for wild-type, this was not the case for dys-1 mutants. Instead, decreases in dys-1 crawling velocity seemed to be related to lower bending frequency (d). Here we report the mean and s.e.m. for N = 30 worms for each condition. ** P < 0.001 two-tailed t-test.
As a complementary method to assess muscular integrity, same-age (day 00) worms reared in the burrowing condition were allowed to exit the pipette and crawl on an agar plate. Paralleling our imaging results of muscle cell integrity, we found that dys-1 worms from the burrowing condition showed a marked reduction in crawling velocity when compared to wild-type worms under the same treatment (Fig. 5b, U = 195, P < 0.001 vs. F59 = 1.98, P = 0.052 respectively). While a modest reduction in overall crawling velocity could perhaps be attributable to an increased rate of reversals in wild-type (U = 285, P = 0.008), this was unlikely to be the case for the dys-1 mutants (Fig. 5c, U = 625, P = 0.658). Instead, the decreased crawl velocity may be explained by a reduced rate of head bends during crawling (Fig. 5d, WT: F38 = 1.58, P = 0.123 vs. dys-1: U = 99.5, P = 0.001). We conclude that burrowing presents an appropriate behavioral paradigm to evaluate the neuromuscular integrity of wild-type and neuromuscularly impaired animals.
Development of a suppressor screen for muscular dystrophy
The strong behavioral and anatomical phenotypes obtained from our burrowing assay led us to develop a genetic screen to search for mutations capable of suppressing the burrowing deficit of dys-1 mutants (Fig. 6). In brief, the screen consisted of exposing dys-1(eg33) mutant worms to a mutagen to induce random mutations in their gametes. We assessed approximately 100 individual F2 progeny of 50 mutagenized F1 worms suggesting coverage of 100 haploid genomes. Any individual that could burrow through 1.5% agar at wild-type level by burrowing further than 10 cm in 3 hours (thus suppressing the dys-1 phenotype) was re-tested to confirm the heritability of the phenotype, and then isolated for further characterization (Fig 6a).
Figure 6.

Suppression screen to identify mutations capable of rescuing the defective burrowing phenotype of worms modeling muscular dystrophy (dys-1(eg33) strain BZ33). (a) We devised a genetic screen to identify mutations capable of suppressing the poor burrowing ability of dys-1 mutant. A 2-μl drop of the attractant 1:100 diacetyl diluted in ethanol was placed on one side of a 3% agar-filled pipette. After 24 hrs worms were injected 20 cm away from the attractant and allowed to burrow within it. After 2 hours most wild-type worms will have migrated more than 4 cm toward the attractant (top). During the same time course, dys-1 mutants fail to make progress toward the attractant (middle). We mutagenized dys-1(eg33) worms and selected animals whose dys-1 phenotype had been suppressed by newly acquired mutations (bottom). (b) Four strains of suppressor mutants were isolated (JPS515, JPS516, JPS517 and JPS519) which displayed an improved ability to burrow over their dys-1 background.
Isolation of muscular dystrophy suppressor mutants
Our suppressor screen produced four independently-derived mutants with an improved ability to burrow compared with the dys-1(eg33) mutant background strain (Fig 6b). For instance, although only 5% of dys-1 mutants could burrow further than 4 cm away from the start (dys-1 compared to WT: U = 0.00 P < 0.001), between 50 – 55% of the four suppressor mutants (JPS515, JPS516, JPS517 and JPS518) could burrow this distance (Fig. 6b, dys-1 compared to JPS515: U = 0.00, P = 0.006; JPS516: U = 1.5, P = 0.012; JPS517: U = 0.00 P = 0.006; and JPS518: U = 10, P < 0.001). Each of these strains was tested for their burrowing ability on at least three separate occasions producing the same result each time.
To gain insight into neuronal and/or muscular basis of the suppression, we performed additional analysis on their crawling behavior. We found that while one strain, JPS518, appeared hyperactive in its crawling velocity (Fig. 7a), and its rate of reversals while crawling (Fig. 7b), it displayed normal head-bend frequency during crawling (Fig. 7c). Interestingly, the remaining three suppressor strains performed all these crawling behaviors at wild-type levels.
Figure 7.
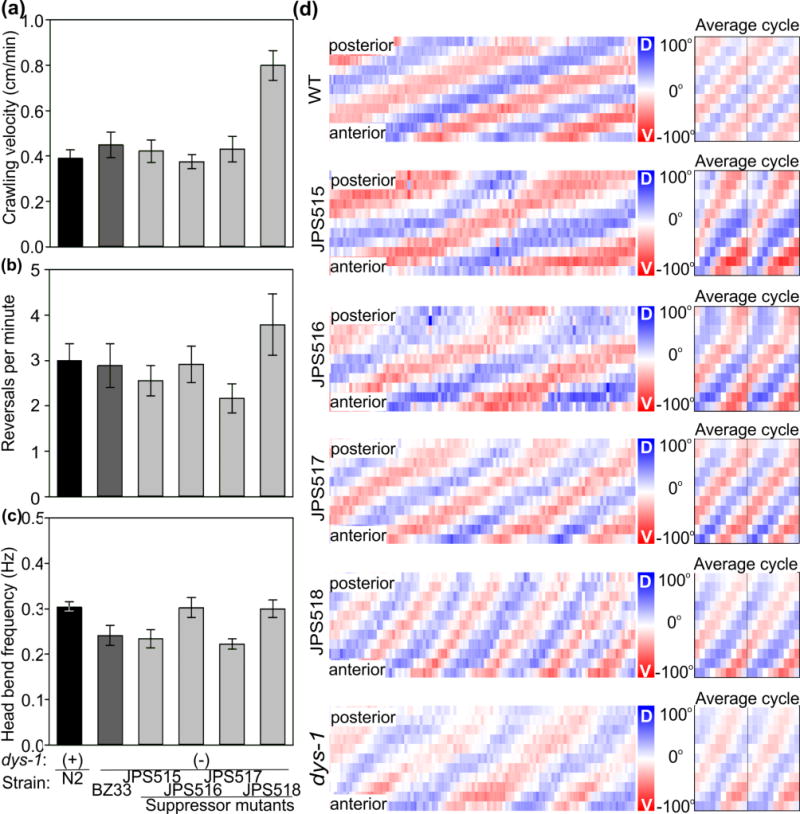
Behavioral characterization of suppressor mutants. Like the dys-1(eg33) mutant strain BZ33, most suppressor mutants carrying the dys-1 mutation displayed wild-type crawling velocity (a), reversal rates (b), and head-bend frequencies (c). (d) We next compared their detailed burrowing kinematics to those of wild-type (top), and dys-1 mutant worms (bottom) with curvature matrices. Suppressor mutants had kinematics reminiscent of both wild-type and dys-1 worms. All bars show the mean and s.e.m. of 100 animals (in five trials) for burrowing, and 15 animals for crawling (30 for dys-1(eg33) vxJPS518). Burrowing bouts of 10 seconds in duration are shown, alongside average phase plots (right). Anterior is down, and posterior is up.
To understand how suppressor mutations enabled proficient burrowing in the dys-1(eg33) mutant background, we analyzed their burrowing behavior in more detail. In general, we found that the burrowing kinematics for these suppressor strains was intermediate between that of wild-type and dys-1 mutant worms (Fig. 7d top and bottom respectively).
To begin to assess if improved burrowing ability correlated with improved muscle integrity, we crossed the JPS518 mutant with the dys-1(eg33) strain with labeled muscle mitochondria and nuclei to determine the extent of muscular degeneration caused by burrowing in this suppressor strain (Fig. 8). The JPS518 strain was tested first simply because it was the most proficient at mating among the different suppressor strains. We found that the behavioral rescue of the burrowing phenotype in the JPS518 strain was accompanied by a decrease in muscular degeneration. To quantify the extent of degeneration, we analyzed the number of gfp-labeled nuclei in muscles throughout the posterior half of worms (Supplemental Figure 4; 5–9 animals per group). In comparison to wild type (6.33 ± 0.08), the dys-1 mutant had a significantly smaller number of nuclei (4.66 ± 0.49) at the midbody. By contrast, the JPS518 suppressor strain had more muscle nuclei (5.66 ± 0.23) at the midbody than the dys-1 mutant. In addition, and although not investigated here, burrowing JPS518 worms also appeared to have brighter mitochondrial GFP label when compared with crawling animals (Fig. 8).
Figure 8.

Suppressor mutation that improved burrowing ability also improved muscle integrity of the dys-1 mutant. Muscle degeneration that is normally exacerbated by burrowing conditions in the dys-1 mutant background was suppressed with the suppressor mutant strain JPS518. Note the wild-type-like pattern of GFP-labeled muscle mitochondria and nuclei from confocal stack images in both crawling and burrowing-raised conditions. Scale bar for all representative images on bottom.
Discussion
Although the selection of C. elegans as a model organism was not made on the basis of its behavioral repertoire, decades of research have made it evident that this compact organism is capable of a wide array of interesting behaviors. Historically, crawling has been used as a behavioral readout to elucidate the normal function of muscle and neuronal genes as well as to model human neuromuscular diseases (Kaletta and Hengartner, 2006). While crawling is experimentally amenable, it is best suited for the assessment of neurological phenotypes where the timing or performance of a behavior is under neural control. The artificially facile media over which animals crawl, results in worms that are able to crawl even when severely challenged by mutation or transgene. As exemplified by our dys-1 results (Fig. 4), crawling is not an effective paradigm to distinguish subtle muscular phenotypes, where the musculature is challenged, as it is with neurological phenotypes. Using the burrowing assay developed here, we are now able to assign a strong motor phenotype to worms modeling muscular dystrophy, even when these mutant worms are capable of near normal crawling (Fig. 4).
The physical challenge posed by media of higher densities was evidenced by the lower frequency and variability observed for burrowing worms when compared with crawling or swimming ones (Fig. 2a). The increase in bend amplitude and the lack of posterior dampening of propagated bends further support the idea of an increased challenge to locomote in burrowing animals (Fig. 2d and Supplemental Figure 1). As the density of the media increased, worms altered their turning strategy (Fig. 3). Indeed, previous work on burrowing polychaetes showed that these annelids change their burrowing strategy with substrate density (Dorgan et al., 2008).
The increased muscle degeneration observed in dys-1 mutant worms under the burrowing treatment likely stems from induced damage to the muscle cells resulting from increased exertion, a phenomenon common in muscular dystrophy (Petrof et al., 1993, Sander et al., 2000; Sussman, 2002; Ozawa et al., 1999). By raising worms in forced burrowing conditions, research related to Muscular dystrophy in C. elegans will likely benefit from the more dramatic extent of cell death (few vs most muscle cells), an earlier onset (by several days), and behavioral correlate (poor crawling after being raised in burrowing conditions). This approach has an additional benefit over traditional ones where a sensitizing mutation is introduced to exacerbate the dys-1 phenotype (Giugia et al., 1999), because potential treatments, whether genetic or pharmacological, may be masked by the severity of the sensitizing mutation.
Muscle degeneration in dys-1 mutants was accompanied by a decrease in intensity of fluorescence of GFP-tagged mitochondria and nuclei (Fig. 5a). This may relate to a general decline in muscle protein production and/or increase in protein degradation. Alternatively, because the dys-1 mutant worms were defective in burrowing, they may have had less access to food at the other end of the pipette, and consequently been deprived of food. Starvation is known to induce organism-wide changes in protein processing, including autophagy (Kang et al., 2007). Thus, the dim GFP might also be explained by increased autophagy. Future work may compare the relative change in fluorescence in different tissues to resolve whether the dys-1 mutation leads to specific loss of muscle proteins.
Our suppressor screen resulted in four isolated strains that (with varying success) rescued the ability of dys-1 mutant worms to burrow (Fig. 6a). Further characterization of these strains may yield useful genetic loci for the treatment of the debilitating effects of muscular dystrophy, a fatal degenerative disease that affects one in 3,500 live male births (Davies and Nowak, 2006; Goldstein and McNally, 2010).
Aside from its translational advantages, burrowing is an important natural behavior common to free living and parasitic organisms, both terrestrial and aquatic. Understanding the neural and genetic basis of burrowing will shed light on a form of locomotion employed by most animal life on the planet.
Supplementary Material
Supplementary Figure 1: Dampening of bends. The maximal amplitude of body bends decreases in amplitude when propagating from neck to tail more for swimming and crawling than for burrowing. Quantification for bend dampening shown at bottom where ** represents p < 0.001 and bars s.e.m. N = 8 for each group.
Supplementary Figure 2: Lack of functional DYS-1 protein impairs burrowing but not the ability to tax toward a stimulus. (a) dys-1 mutants migrated across a 10cm agar plate towards a 2-μl diacetyl (1:100 dilution) droplet as well as wild-type animals (5 assays and >350 animals for each condition). (b) Two tested dys-1 alleles were similarly impaired at burrowing. To eliminate the possibility of a background mutation being responsible for their inability to burrow, we fed RNAi bacteria to wild-type animals which contained either an empty vector (RNAi ctrl) or dys-1 RNAi. Worms where dys-1 was selectively knocked down displayed a similar burrowing impairment as the mutant alleles.
Supplementary Figure 3: Dystrophin-independent disruption of the dystrophin complex impairs burrowing in C. elegans. (a) To test if disrupting other proteins involved in the dystrophin muscle complex could similarly impair burrowing in worms we assessed the burrowing ability of islo-1 worms. Similar to dys-1 mutants, islo-1 animals could crawl normally but were impaired at burrowing (b).
Supplementary Figure 4: dys-1 suppressor partially rescues muscle degeneration induced by burrowing. To quantify the partial rescue observed by dys-1-suppressors we counted the number of muscle nuclei observable in the mid-body (a), and tail (b) of worms. (c) Sample images from mid-body of worms in the three conditions. Arrowheads point at muscle nuclei. While degenerating nuclei are difficult to qualify, their dim and irregular shape is evident for dys-1 mutants. The disruption of the muscle fibers is also indirectly evidenced by areas devoid of mitochondrial GFP, or where GFP has become aggregated.
Video 1: Burrowing behavior of C. elegans. A wild-type worm is shown burrowing in a glass pipette containing 3% agar.
Video 2: C. elegans burrows using lateral bends. Worms burrowing at low densities (0.5% agar) engage in lateral bends as the one shown here. During lateral bending, a dorsoventral wave is altered to move at an orthogonal plane to the original plane of locomotion.
Video 3: Worms lacking dystrophin (dys-1) undergo periods of immobility while burrowing. A dys-1(eg33) mutant worm encounters immobile sisters as it burrows in 3% agar.
Acknowledgments
We are grateful to Dr. Hongkyun Kim for donated strains. Some strains were provided by the Caenorhabditis Genetic Center, which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440). This study was funded by The University of Texas at Austin Undergraduate Research Award to C.B. and grant (NS075541) from NIH NINDS to J. T. P-S.
Footnotes
Conflict of interest: The authors declare no competing financial interests.
Author contributions
C.B. Conducted burrowing experiments, suppression screen and contributed to the writing. A.G.V.-G. Contributed to all experiments, study design, and manuscript writing; J.C. performed imaging of musculature, contributed to writing; A.P. Contributed to burrowing experiments; G.H. Contributed to burrowing experiments; J.T.P.-S. Contributed to experiments, study design, and manuscript writing.
References
- Bargmann CI, Horvitz HR. Chemosensory neurons with overlapping functions direct chemotaxis to multiple chemicals in C. elegans. Neuron. 1991;7:729–742. doi: 10.1016/0896-6273(91)90276-6. [DOI] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croll NA. Components and patterns of in the behavior of the nematode Caenorhabditis elegans. J Zool. 1975;176:159–176. [Google Scholar]
- Culetto E, Sattelle DB. A role for Caenorhabditis elegans in understanding the function and interaction of human disease genes. Hum Mol Gen. 2000;9:869–877. doi: 10.1093/hmg/9.6.869. [DOI] [PubMed] [Google Scholar]
- Davies KE, Nowak KJ. Molecular mechanisms of muscular dystrophies: Old and new players. Nat Rev Mol Cell Biol. 2006;7:762–773. doi: 10.1038/nrm2024. [DOI] [PubMed] [Google Scholar]
- Deconinck AE, Rafael JA, Skinner JA, Brown SC, Potter AC, Metzinger L, Watt DJ, Dickson JG, Tinsley JM, Davies KE. Utrophin-dystrophin-deficient mice as a model for Duchenne muscular dystrophy. Cell. 1997;90:717–727. doi: 10.1016/s0092-8674(00)80532-2. [DOI] [PubMed] [Google Scholar]
- Dorgan KM. Worms as wedges: Effects of sediment mechanics on burrowing behavior. J Mar Res. 2008;66:219–254. [Google Scholar]
- Dougherty EC, Calhoun HG. Possible significance of free-living nematodes in genetic research. Nature. 1948;161:29–29. doi: 10.1038/161029a0. [DOI] [PubMed] [Google Scholar]
- Durbeej M, Campbell KP. Muscular dystrophies involving the dystrophin-glycoprotein complex: an overview of current mouse models. Curr Opin Genet Dev. 2002;12:349–361. doi: 10.1016/s0959-437x(02)00309-x. [DOI] [PubMed] [Google Scholar]
- Gieseler K, Grisoni K, Ségalat L. Genetic suppression of phenotypes arising from mutations in dystrophin-related genes in Caenorhabditis elegans. Cur Biol. 2000;10:1092–1097. doi: 10.1016/s0960-9822(00)00691-6. [DOI] [PubMed] [Google Scholar]
- Goldstein JA, McNally EM. Mechanisms of muscle weakness in muscular dystrophy. J Gen Physiol. 2010;136:29–34. doi: 10.1085/jgp.201010436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart AC. The C. elegans Research Community. WormBook. 2006 doi: 10.1895/wormbook.1.87.1. ed. Behavior, WormBook, ed. http://www.wormbook.org. [DOI]
- Harris HE, Epstein HF. Myosin and paramyosin of Caenorhabditis elegans: biochemical and structural properties of wild-type and mutant proteins. Cell. 1977;10:709–19. doi: 10.1016/0092-8674(77)90105-2. [DOI] [PubMed] [Google Scholar]
- Iino Y, Yoshida K. Parallel use of two behavioral mechanisms for chemotaxis in Caenorhabditis elegans. J Neurosci. 2009;29:5370–80. doi: 10.1523/JNEUROSCI.3633-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen EM, Mango SE. The art and design of genetic screens: Caenorhabditis elegans. Nat Rev Genet. 2002;3:356–369. doi: 10.1038/nrg794. [DOI] [PubMed] [Google Scholar]
- Kang C, You YJ, Avery L. Dual roles of autophagy in the survival of Caenorhabditis elegans during starvation. Genes Dev. 2007;21:2161–2171. doi: 10.1101/gad.1573107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaletta T, Hengartner MO. Finding function in novel targets: C. elegans as a model organism. Nat Rev Drug Disc. 2006;5:387–399. doi: 10.1038/nrd2031. [DOI] [PubMed] [Google Scholar]
- Kim H, Pierce-Shimomura JT, Oh HJ, Johnson BE, Goodman MB, McIntire SL. The dystrophin complex controls BK channel localization and muscle activity in Caenorhabditis elegans. PLoS Genet. 2009;5:e1000780. doi: 10.1371/journal.pgen.1000780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucherenko MM, Pantoja M, Yatsenko AS, Shcherbata HR, Fischer KA, Maksymiv DV, Chernyk YI, Ruohola-Baker H. Genetic modifier screens reveal new components that interact with the Drosophila dystroglycan complex. Plos ONE. 2008;3:e2418. doi: 10.1371/journal.pone.0002418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntire SL, Reimer RJ, Schuske K, Edwards RH, Jorgensen EM. Identification and characterization of the vesicular GABA transporter. Nature. 1997;389:870–6. doi: 10.1038/39908. [DOI] [PubMed] [Google Scholar]
- Michele DE, Barresi R, Kanagawa M, Saito F, Cohn RD, Satz JS, Dollar J, Nishino I, Kelley RI, Somer H, Straub V, Mathews KD, Moore SA, Campbell KP. Post-translational disruption of dystroglycan-ligand interactions in congenital muscular dystrophies. Nature. 2002;418:417–422. doi: 10.1038/nature00837. [DOI] [PubMed] [Google Scholar]
- Moore SA, Saito F, Chen J, Michelle DE, Henry MD, Messing A, Cohn RD, Ross-Barta SE, Westra S, Williamson RA, Hoshi T, Campbell KP. Deletion of brain dystroglycan recapitulates aspects of congenital muscular dystrophy. Nature. 2002;418:422–425. doi: 10.1038/nature00838. [DOI] [PubMed] [Google Scholar]
- Monaco AP, Neve RL, Colletti-Feener C, Bertelson CJ, Kurnit DM, Kunkel LM. Isolation of candidate cDNAs for portions of the Duchenne muscular dystrophy gene. Nature. 1986;323:646–650. doi: 10.1038/323646a0. [DOI] [PubMed] [Google Scholar]
- Oh KH, Kim H. Reduced IGF signaling prevents muscle cell death in a Caenorhabditis elegans model of muscular dystrophy. Proc Natl Acad Sci USA. 2013;110:19024–19029. doi: 10.1073/pnas.1308866110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa E, Hagiwara Y, Yoshida M. Creatine kinase, cell membrane and Duchenne muscular dystrophy. Mol Cell Biochem. 1999;190:143–151. [PubMed] [Google Scholar]
- Petrof BJ, Shrager JB, Stedman HH, Kelly AM, Sweeney HL. Dystrophin protects the sarcolemma from stresses developed during muscle contraction. Proc Natl Acad Sci USA. 1993;90:3710–3714. doi: 10.1073/pnas.90.8.3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce-Shimomura JT, Chen BL, Mun JJ, Ho R, Sarkis R, McIntire SL. Genetic analysis of crawling and swimming locomotory patterns in C. elegans. Proc Natl Acad Sci USA. 2008;105:20982–20987. doi: 10.1073/pnas.0810359105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce-Shimomura JT, Morse TM, Lockery SR. The fundamental role of pirouettes in Caenorhabditis elegans chemotaxis. J Neurosci. 1999;19:9557–69. doi: 10.1523/JNEUROSCI.19-21-09557.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander M, Chavoshan B, Harris SA, Iannaccone ST, Stull JT, Thomas GD, Victor RG. Functional muscle ischemia in neuronal nitric oxide synthase-deficient skeletal muscle of children with Duchenne muscular dystrophy. Proc Natl Acad Sci USA. 2000;97:13818–13823. doi: 10.1073/pnas.250379497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shcherbata HR, Yatsenko AS, Patterson L, Sood VD, Nudel U, Yaffe D, Baker D, Ruohola-Baker H. Dissecting muscle and neuronal disorders in a Drosophila model of muscular dystrophy. EMBO J. 2007;26:481–493. doi: 10.1038/sj.emboj.7601503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman M. Duchenne muscular dystrophy. J Am Acad Orthop Surgeons. 2002;10:138–151. doi: 10.5435/00124635-200203000-00009. [DOI] [PubMed] [Google Scholar]
- Timmons L, Fire A. Specific interference by ingested dsRNA. Nature. 1998;29:854. doi: 10.1038/27579. [DOI] [PubMed] [Google Scholar]
- Torres LF, Duchen LW. The mutant mdx: inherited myopathy in the mouse. Morphological studies of nerves, muscles and end-plates. Brain. 1987;110:269–299. doi: 10.1093/brain/110.2.269. [DOI] [PubMed] [Google Scholar]
- Vidal-Gadea AG, Davis S, Becker L, Pierce-Shimomura JT. Coordination of behavioral hierarchies during environmental transitions in Caenorhabditis elegans. Worm. 2012;1:5–11. doi: 10.4161/worm.19148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward S. Chemotaxis by the nematode Caenorhabditis elegans: identification of attractants and analysis of the response by use of mutants. Proc Natl Acad Sci USA. 1973;70:817–21. doi: 10.1073/pnas.70.3.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: Dampening of bends. The maximal amplitude of body bends decreases in amplitude when propagating from neck to tail more for swimming and crawling than for burrowing. Quantification for bend dampening shown at bottom where ** represents p < 0.001 and bars s.e.m. N = 8 for each group.
Supplementary Figure 2: Lack of functional DYS-1 protein impairs burrowing but not the ability to tax toward a stimulus. (a) dys-1 mutants migrated across a 10cm agar plate towards a 2-μl diacetyl (1:100 dilution) droplet as well as wild-type animals (5 assays and >350 animals for each condition). (b) Two tested dys-1 alleles were similarly impaired at burrowing. To eliminate the possibility of a background mutation being responsible for their inability to burrow, we fed RNAi bacteria to wild-type animals which contained either an empty vector (RNAi ctrl) or dys-1 RNAi. Worms where dys-1 was selectively knocked down displayed a similar burrowing impairment as the mutant alleles.
Supplementary Figure 3: Dystrophin-independent disruption of the dystrophin complex impairs burrowing in C. elegans. (a) To test if disrupting other proteins involved in the dystrophin muscle complex could similarly impair burrowing in worms we assessed the burrowing ability of islo-1 worms. Similar to dys-1 mutants, islo-1 animals could crawl normally but were impaired at burrowing (b).
Supplementary Figure 4: dys-1 suppressor partially rescues muscle degeneration induced by burrowing. To quantify the partial rescue observed by dys-1-suppressors we counted the number of muscle nuclei observable in the mid-body (a), and tail (b) of worms. (c) Sample images from mid-body of worms in the three conditions. Arrowheads point at muscle nuclei. While degenerating nuclei are difficult to qualify, their dim and irregular shape is evident for dys-1 mutants. The disruption of the muscle fibers is also indirectly evidenced by areas devoid of mitochondrial GFP, or where GFP has become aggregated.
Video 1: Burrowing behavior of C. elegans. A wild-type worm is shown burrowing in a glass pipette containing 3% agar.
Video 2: C. elegans burrows using lateral bends. Worms burrowing at low densities (0.5% agar) engage in lateral bends as the one shown here. During lateral bending, a dorsoventral wave is altered to move at an orthogonal plane to the original plane of locomotion.
Video 3: Worms lacking dystrophin (dys-1) undergo periods of immobility while burrowing. A dys-1(eg33) mutant worm encounters immobile sisters as it burrows in 3% agar.


