Abstract
Reduced sleep duration and quality appear to be endemic in modern society. Curtailment of the bedtime period to minimum tolerability is thought to be efficient and harmless by many. It has been known for several decades that sleep is a major modulator of hormonal release, glucose regulation and cardiovascular function. In particular, slow wave sleep (SWS), thought to be the most restorative sleep stage, is associated with decreased heart rate, blood pressure, sympathetic nervous activity and cerebral glucose utilization, compared with wakefulness. During SWS, the anabolic growth hormone is released while the stress hormone cortisol is inhibited. In recent years, laboratory and epidemiologic evidence have converged to indicate that sleep loss may be a novel risk factor for obesity and type 2 diabetes. The increased risk of obesity is possibly linked to the effect of sleep loss on hormones that play a major role in the central control of appetite and energy expenditure, such as leptin and ghrelin. Reduced leptin and increased ghrelin levels correlate with increases in subjective hunger when individuals are sleep restricted rather than well rested. Given the evidence, sleep curtailment appears to be an important, yet modifiable, risk factor for the metabolic syndrome, diabetes and obesity. The marked decrease in average sleep duration in the last 50 years coinciding with the increased prevalence of obesity, together with the observed adverse effects of recurrent partial sleep deprivation on metabolism and hormonal processes, may have important implications for public health.
Keywords: Sleep deprivation, Glucose metabolism, Diabetes, Appetite regulation, Leptin, Ghrelin, Obesity
1. Sleep patterns in society
For a variety of reasons, either by lifestyle choice, imposed by work or family demands, or due to physical or psychological problems, chronic sleep deprivation is increasingly common in our hectic modern society [1,2]. Societal changes, such as an increase in television viewing and internet use, have impacted sleep patterns, leading to chronic sleep deprivation in a substantial proportion of the population [1]. Over the past 50 years, sleep duration in adults and adolescents has decreased by 1.5–2 hours per night, and more than 30% of Americans between the ages of 30 and 64 report sleeping less than 6 hours per night [3]. In addition to societal impact, the aging of the population in Western countries is associated with a decrease in average sleep duration as older adults obtain on average 2 hours less sleep per night than younger adults, a deficit that is independent of the increased incidence of age-related disorders which can impact sleep patterns [4,5]. Furthermore, the quality of sleep declines with age, with a major reduction in the duration of slow wave sleep (SWS) and increased sleep fragmentation [4].
2. Hormone release is modulated by sleep
SWS or deep sleep occurs during stages 3 and 4 of non-rapid eye movement (REM) sleep and is thought to be the most restorative of all sleep stages. Most slow wave activity occurs in the first two sleep cycles (approximately the first 3 hours of sleep), and the total amount of SWS per night is drastically reduced with age. Several important physiological activities only occur during the SWS, including a reduction in heart rate, blood pressure, sympathetic nervous activity and an increase in vagal tone [6]. SWS is also associated with a decrease in brain glucose metabolism [7]. Additionally, SWS exerts major modulatory effects on endocrine release. The release of the hormones of the hypothalamamic–pituitary–adrenocortical (HPA) system is inhibited [8], whereas the release of growth hormone (GH) and prolactin is increased. Both GH and cortisol have important roles in glucose metabolism. Laboratory studies have shown that the levels of these metabolic hormones are adversely affected by acute total sleep deprivation [9]. Studies in normal sleepers have shown that nocturnal GH release is reduced in individuals who are totally sleep deprived, but subsequently increases during daytime recovery sleep (with the reverse observed for cortisol release) [2].
An analysis of data from a series of studies was undertaken to determine the chronology of age-related changes in sleep duration and sleep quality in 149 healthy men, and whether sleep changes were associated with hormonal alterations [4]. The study found that the mean percentage of SWS decreased from 18.9% during early adulthood (age 16–25 years) to 3.4% during midlife (age 36–50 years), but remained unchanged from midlife to late life (age 71–83 years). Also, a significant decrease in sleep duration was observed across the age groups: each 10-year increment in age was associated with a 28-minute decrease in sleep duration (P < 0.001). The reductions in SWS observed with age were associated with a significant decline in GH secretion both from early to midlife (P < 0.001) and from midlife to late life (P < 0.02), and reductions in GH secretion were significantly associated with reductions in SWS independent of age (P < 0.001) [4].
Although the full clinical impact of chronic sleep deprivation on metabolic hormone release is yet to be determined, evidence indicates that dysfunction of glucose metabolism, obesity and increased diabetes risk are all likely outcomes [9].
3. Effect of sleep deprivation on carbohydrate metabolism and diabetes risk
Sleep appears to play an important role in the control of blood glucose levels, and recurrent partial sleep deprivation has been shown to have detrimental effects on carbohydrate metabolism and endocrine function [9,10]. A sleep debt study compared glucose metabolism in 11 young men undergoing periods of enforced partial sleep deprivation (4 hours sleep per night), sleep extension (12 hours sleep per night) and “normal” sleep as a baseline (8 hours sleep per night). During the sleep-restriction period, the individuals had significantly impaired glucose tolerance (P < 0.04; measured using the intravenous glucose tolerance test [IVGTT]), and significant reductions in their acute insulin response to glucose (P = 0.05) and in glucose effectiveness (P < 0.0005), compared with those observed when they were fully rested [10]. Insulin sensitivity was also reduced (5.41 versus 6.73 × 104/min/μU/mL), but this was not statistically significant [10]. The disposition index, a product of the acute insulin response to glucose and insulin sensitivity [11] and a marker of diabetic risk used in genetic studies [12], was significantly lower following sleep restriction than when the individuals were fully rested (P = 0.0006) [10].
Another study in young healthy adults showed that suppression of SWS without any reduction in total sleep time resulted in decreased insulin sensitivity, reduced glucose tolerance and increased risk of type 2 diabetes, suggesting that a reduction in SWS (such as that seen in the elderly and in many obese individuals), independent of the overall duration of sleep, may be particularly important for normal glucose metabolism [13]. The mechanisms by which sleep deprivation impacts glucose tolerance are thought to be multifactorial, including decreased brain glucose utilization, alterations in the sympatho-vagal balance, increased evening cortisol and extended night-time GH secretion, and proinflammatory processes [14].
The impact of short sleep duration on the risk of diabetes has been shown in several epidemiological studies, with a significant increase in incidence of diabetes in individuals who have difficulty in maintaining sleep or who experience chronic short sleep duration [15–17]. The largest of these, the prospective 10-year Nurses Health Study in 70,026 women [15], showed that individuals who slept 5 hours per night or less had a significantly higher risk of being diagnosed with diabetes (odds ratio [OR] 1.57,95% CI: 1.28–1.92) compared with those who slept 8 hours per night, although this association was not significant after adjustment for obesity and other confounding factors (OR 1.18,95% CI: 0.96–1.44). However, the increase in the risk of symptomatic diabetes with ≤5 hours sleep per night versus 8 hours remained significant even after adjustment (OR 1.34, 95% CI: 1.04–1.72), suggesting that, although diabetes risk is increased by obesity (which appears to be more prevalent in short sleepers and, conversely, may result in poor sleep quality), insufficient sleep may be a risk factor for more severe diabetes [15].
Data from laboratory and epidemiological studies suggest that in addition to changes in glucose/carbohydrate metabolism, the relationship between sleep deprivation and diabetes risk may also involve upregulation of appetite and decreased energy expenditure, both of which can lead to obesity, itself a major risk factor for diabetes [14].
4. Sleep duration and appetite regulation
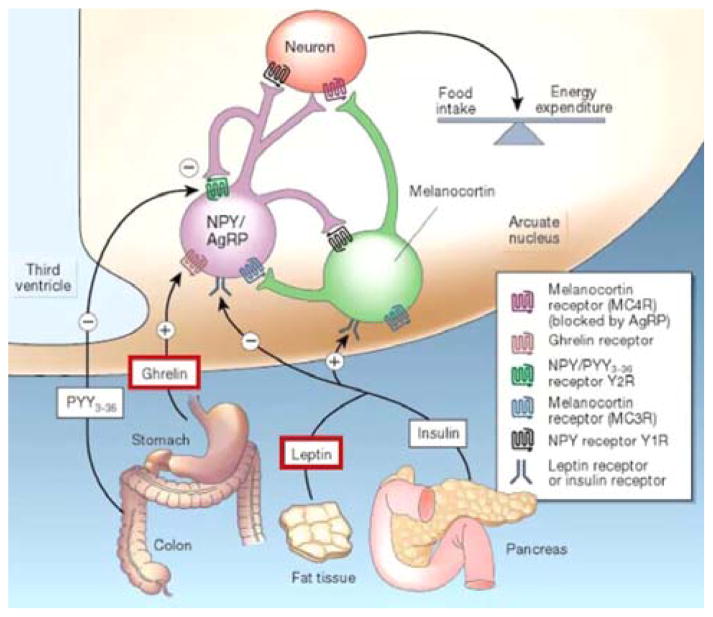
Food intake is controlled by the neuroendocrine system which is itself controlled by the central nervous system [18]. Long-term regulators of food intake include insulin and leptin, which are released in proportion to the amount of body fat. These hormones exert sustained inhibitory effects on food intake while increasing energy expenditure [18]. Ghrelin, on the other hand, is an appetite stimulating hormone released by cells in the stomach. In the non-pathological state, ghrelin levels rise rapidly before meals and fall equally rapidly after food intake (Figure 1). Both ghrelin and leptin are part of the orexin system, which integrates control of feeding, wakefulness and energy expenditure in the body, and they exert their influence on the central nervous system via receptors in the “appetite center” of the brain: the ventromedial and arcuate nuclei of the hypothalamus. Although the exact mechanisms are unclear, leptin and ghrelin are thought to act in parallel as opposing metabolic counterparts for body mass homeostasis [19].
Fig. 1.
Hormonal control of appetite and hunger [18]. NPY; neuropeptide Y; PYY; peptide YY, AgRP: agouti-related protein. Reprinted by permission from Macmillan Publishers Ltd: Nature, Schwartz MW et al. 2002 Aug 8;418(6898):595–7. Copyright © 2002.
Sleep duration plays an important role in the regulation of leptin and ghrelin levels in humans: several studies have shown that recurrent partial sleep deprivation and chronic short sleep are associated with a significant decrease in levels of leptin and increase levels of ghrelin (Figure 2A) [20–23]. In a randomized, cross-over study, 2 nights of short sleep (4 hours) were compared with 2 nights of long sleep (10 hours) on metabolic parameters. Results showed that a significant decrease in mean blood leptin levels (P = 0.041) occurred concomitantly with a significant increase in mean ghrelin levels (P = 0.038) after sleep restriction, compared with sleep extension, despite identical conditions of caloric intake [21]. Compared with sleep extension, sleep restriction was associated with significantly increased hunger (P < 0.01) and global appetite (P = 0.01; Figure 2B). Importantly, the increased hunger and appetite reported, especially for carbohydrate-rich foods, correlated with the increased ghrelin:leptin ratio (P = 0.014) [21].
Fig. 2.
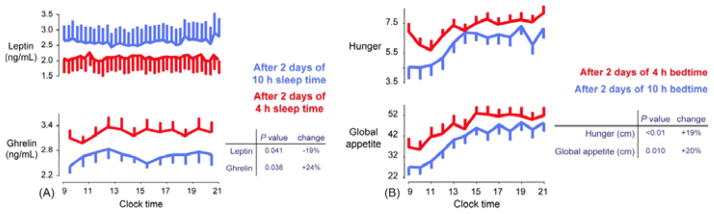
The effect of sleep deprivation on (A) leptin and ghrelin levels and (B) hunger and global appetite ratings[21]. ANNALS OF INTERNAL MEDICINE. ONLINE by Spiegel K, Tasali E, Penev P, Van Cauter E. Copyright © 2004 by American College of Physicians – Journals. Reproduced with permission of American College of Physicians – Journals in the format Journal via Copyright Clearance Center.
In another study, 24-hour hormonal and glucose profiles were sampled at frequent intervals in 11 individuals studied after 6 days of 4 hours in bed (sleep restriction) and after 6 days of 12 hours in bed (sleep extension) while calorie intake and activity levels were carefully controlled. Mean and peak levels, and rhythm amplitude of leptin concentration over 24 hours were all decreased (−19%, −26% and −20%, respectively) during sleep restriction compared with sleep extension [20]. The 26% reduction in peak leptin levels observed during sleep restriction is similar to a mean 22% reduction reported in healthy volunteers after 3 days of dietary restriction (70% of energy requirements) [24].
Similar changes in leptin and/or ghrelin have been observed in two large epidemiological studies [22,23]. In a study of 1,024 volunteers from the Wisconsin Sleep Cohort, a significant reduction in leptin levels (P = 0.01) and elevation of ghrelin levels (P = 0.008) was observed with 5 hours versus 8 hours sleep duration [22]. Given that the leptin and ghrelin changes are likely to increase appetite, this could explain the increase in body mass index (BMI) observed with chronic short sleep duration [22]. In the second study of 740 male and female participants from the Quebec Family Study it was shown that short sleep duration (5–6 hours per night) was associated with significantly lower leptin levels than were predicted by body fat mass alone (P < 0.01) [23].
5. Sleep duration and obesity
Over the past 50 years, the prevalence of obesity has increased at a rapid rate and most experts agree that reductions in physical activity and changes in food marketing practices (i.e., portion size) do not fully explain this epidemic. During the same time frame, a corresponding decrease in self-reported sleep hours has been reported (Figure 3), suggesting that the two may be linked [2]. Indeed, a number of population-based studies involving more than 500,000 adults [22,23,25–38] and 28,000 children [39–45] have identified short sleep duration to be an important, yet modifiable, risk factor for obesity. Moreover, these findings have been confirmed in prospective longitudinal studies in both European and American adults [31,46,47] and children [42,44]. A recent systematic review of both cross-sectional and longitudinal studies [48] concluded that “short sleep duration appears independently associated with weight gain, particularly in young age groups”.
Fig. 3.
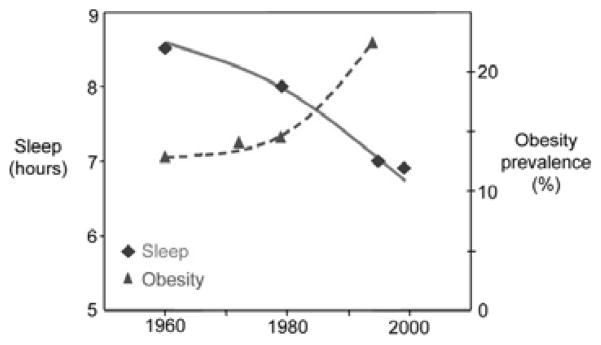
Prevalence of obesity and self-reported sleep in the USA [2]. Reprinted with permission from Medscape Neurology and Neurosurgery 2005. 7(1) http://www.medscape.com/viewarticle/502825 © 2005 Medscape.
The Quebec Family Study [23] was unique in also assessing adiposity and leptin levels and showed that both obesity and adiposity were reduced with 7–8 hours sleep compared with 5–6 hours. The adjusted OR for overweight/obesity (with 7–8 hours of sleep as reference) was 1.69 (95% CI: 1.15–2.39) for 5–6 hours of sleep and 1.38 (95% CI: 0.89–2.10) for 9–10 hours of sleep. Short sleep duration was also shown to be associated with reduced leptin levels after controlling for the degree of adiposity (as discussed above). The differences in obesity and adiposity between short sleepers and normal sleepers did not remain significant after adjustment for plasma leptin levels, implicating leptin as a potential mediator of the association between sleep duration and adiposity [23]. A recent meta-analysis of 30 cross-sectional studies investigated the relationship between sleep duration and obesity or BMI in adults and children (12 studies in children [n = 30,002] and 18 studies in adults [n = 604,509]) from around the world [49]. The risk of obesity was increased with short sleep duration in children (OR 1.89, 95% CI: 1.46–2.43; P < 0.0001) and in adults (OR 1.55, 95% CI: 1.43–1.68; P < 0.0001) [49]. The definition of short sleep duration varied among the studies, but was < 10 or ≤10 hours per night for children and < 5 or ≤5 hours average total sleep time per 24 hours for adults in the majority of studies included. Obesity was defined using national or international growth charts (BMI≥30 kg/m2 in adults). Studies varied in the degree of control for confounding factors such as age, gender, socioeconomic status, energy intake and expenditure, and comorbidity, particularly psychiatric disorders. As with other cross-sectional studies examining the relationship between sleep duration and obesity, the results of this meta-analysis, show an association but not causality between the two.
Although laboratory studies and prospective epidemio-logical studies suggest that short sleep duration may be a causal factor for obesity, the reverse direction of causality, i.e., that obesity can cause sleep disruption, is also possible, resulting in a vicious circle linking poor/short sleep and the risk of obesity. Further research is required to elucidate the mechanisms of the relationship.
6. Conclusion
Adequate sleep duration and quality are important for the normal functioning of daily metabolic and hormonal processes and appetite regulation. It is clear that chronic sleep deprivation has deleterious effects on carbohydrate metabolism and is associated with an increased risk of diabetes. Altered levels of hormones central to appetite regulation, such as leptin and ghrelin, occur in sleep-deprived individuals and, consistent with this neuroendocrine dysregulation of hunger and appetite, a large number of epidemiological studies have identified short sleep duration as a putative novel risk factor for weight gain and obesity. With the marked changes in sleep patterns that seem to have occurred in westernized countries over the last 50 years and an apparent reduction in average hours of sleep way beyond that predicted by aging of the population alone, it is probable that an increasing proportion of people suffer from chronic sleep deprivation. This has important implications for individual physical and psychological well being and serious consequences for society as a whole. Avoiding the build up of a chronic sleep debt through awareness, education and effective management of sleep disorders may be important to limit the rise in cardiometabolic dysfunction, diabetes and obesity that has occurred over recent years.
Acknowledgments
Work partly supported by NIH grants PO1 AG-11412, RO1 HL-075079 and P60 DK-20595 and by grant W81XWH-07–2–0071 of the US Department of Defence. We thank Matt Weitz, from Wolters Kluwer Health, who provided medical writing support on behalf of sanofi-aventis.
Footnotes
Disclosures
None.
References
- 1.Bonnet MH, Arand DL. We are chronically sleep deprived. Sleep. 1995 Dec;18(10):908–11. doi: 10.1093/sleep/18.10.908. [DOI] [PubMed] [Google Scholar]
- 2.Van Cauter E, Knutson K, Leproult R, Spiegel K. The impact of sleep deprivation on hormones and metabolism. Medscape Neurol Neurosurg. 2005;7(1) [Google Scholar]
- 3.National Health Interview Survey. QuickStats: Percentage of Adults Who Reported an Average of <6 Hours of Sleep per 24-Hour Period, by Sex and Age Group – United States, 1985 and 2004. MMWR Morb Mortal Wkly Rep. 2005;54(37):933. [Google Scholar]
- 4.Van Cauter E, Leproult R, Plat L. Age-related changes in slow wave sleep and REM sleep and relationship with growth hormone and cortisol levels in healthy men. J Am Med Assoc. 2000;284(7):861–8. doi: 10.1001/jama.284.7.861. [DOI] [PubMed] [Google Scholar]
- 5.Prinz PN. Sleep and sleep disorders in older adults. J Clin Neurophysiol. 1995 Mar;12(2):139–46. doi: 10.1097/00004691-199503000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Somers VK, Dyken ME, Mark AL, Abboud FM. Sympathetic-nerve activity during sleep in normal subjects. N Engl J Med. 1993 Feb 4;328(5):303–7. doi: 10.1056/NEJM199302043280502. [DOI] [PubMed] [Google Scholar]
- 7.Zoccoli G, Walker AM, Lenzi P, Franzini C. The cerebral circulation during sleep: regulation mechanisms and functional implications. Sleep Med Rev. 2002 Dec;6(6):443–55. doi: 10.1053/smrv.2001.0194. [DOI] [PubMed] [Google Scholar]
- 8.Friess E, Wiedemann K, Steiger A, Holsboer F. The hypothalamic–pituitary–adrenocortical system and sleep in man. Adv Neuroimmunol. 1995;5(2):111–25. doi: 10.1016/0960-5428(95)00003-k. [DOI] [PubMed] [Google Scholar]
- 9.Van Cauter E, Holmback U, Knutson K, Leproult R, Miller A, Nedeltcheva A, et al. Impact of sleep and sleep loss on neuroendocrine and metabolic function. Horm Res. 2007;67(Suppl 1):2–9. doi: 10.1159/000097543. [DOI] [PubMed] [Google Scholar]
- 10.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999 Oct 23;354(9188):1435–9. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 11.Bergman RN, Ader M, Huecking K, Van Citters G. Accurate assessment of beta-cell function: the hyperbolic correction. Diabetes. 2002 Feb;51(Suppl 1):S212–20. doi: 10.2337/diabetes.51.2007.s212. [DOI] [PubMed] [Google Scholar]
- 12.Palmer ND, Langefeld CD, Campbell JK, Williams AH, Saad M, Norris JM, et al. Genetic mapping of disposition index and acute insulin response loci on chromosome 11q. The Insulin Resistance Atherosclerosis Study (IRAS) Family Study. Diabetes. 2006 Apr;55(4):911–8. doi: 10.2337/diabetes.55.04.06.db05-0813. [DOI] [PubMed] [Google Scholar]
- 13.Tasali E, Leproult R, Ehrmann DA, Van Cauter E. Slow-wave sleep and the risk of type 2 diabetes in humans. Proc Natl Acad Sci USA. 2008 Jan 22;105(3):1044–9. doi: 10.1073/pnas.0706446105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knutson KL, Spiegel K, Penev P, Van Cauter E. The metabolic consequences of sleep deprivation. Sleep Med Rev. 2007 Jun;11(3):163–78. doi: 10.1016/j.smrv.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ayas NT, White DP, Al-Delaimy WK, Manson JE, Stampfer MJ, Speizer FE, et al. A prospective study of self-reported sleep duration and incident diabetes in women. Diabetes Care. 2003 Feb;26(2):380–4. doi: 10.2337/diacare.26.2.380. [DOI] [PubMed] [Google Scholar]
- 16.Mallon L, Broman JE, Hetta J. High incidence of diabetes in men with sleep complaints or short sleep duration: a 12-year follow-up study of a middle-aged population. Diabetes Care. 2005 Nov;28(11):2762–7. doi: 10.2337/diacare.28.11.2762. [DOI] [PubMed] [Google Scholar]
- 17.Yaggi HK, Araujo AB, McKinlay JB. Sleep duration as a risk factor for the development of type 2 diabetes. Diabetes Care. 2006 Mar;29(3):657–61. doi: 10.2337/diacare.29.03.06.dc05-0879. [DOI] [PubMed] [Google Scholar]
- 18.Schwartz MW, Morton GJ. Obesity: keeping hunger at bay. Nature. 2002 Aug 8;418(6898):595–7. doi: 10.1038/418595a. [DOI] [PubMed] [Google Scholar]
- 19.Cummings DE, Foster KE. Ghrelin-leptin tango in body-weight regulation. Gastroenterology. 2003 May;124(5):1532–5. doi: 10.1016/s0016-5085(03)00350-0. [DOI] [PubMed] [Google Scholar]
- 20.Spiegel K, Leproult R, L’Hermite-Baleriaux M, Copinschi G, Penev PD, Van Cauter E. Leptin levels are dependent on sleep duration: relationships with sympathovagal balance, carbohydrate regulation, cortisol, and thyrotropin. J Clin Endocrinol Metab. 2004 Nov;89(11):5762–71. doi: 10.1210/jc.2004-1003. [DOI] [PubMed] [Google Scholar]
- 21.Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004 Dec 7;141(11):846–50. doi: 10.7326/0003-4819-141-11-200412070-00008. [DOI] [PubMed] [Google Scholar]
- 22.Taheri S, Lin L, Austin D, Young T, Mignot E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004 Dec;1(3):e62. doi: 10.1371/journal.pmed.0010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chaput JP, Despres JP, Bouchard C, Tremblay A. Short sleep duration is associated with reduced leptin levels and increased adiposity: Results from the Quebec family study. Obesity (Silver Spring) 2007 Jan;15(1):253–61. doi: 10.1038/oby.2007.512. [DOI] [PubMed] [Google Scholar]
- 24.Chin-Chance C, Polonsky KS, Schoeller DA. Twenty-four-hour leptin levels respond to cumulative short-term energy imbalance and predict subsequent intake. J Clin Endocrinol Metab. 2000 Aug;85(8):2685–91. doi: 10.1210/jcem.85.8.6755. [DOI] [PubMed] [Google Scholar]
- 25.Vioque J, Torres A, Quiles J. Time spent watching television, sleep duration and obesity in adults living in Valencia, Spain. Int J Obes Relat Metab Disord. 2000 Dec;24(12):1683–8. doi: 10.1038/sj.ijo.0801434. [DOI] [PubMed] [Google Scholar]
- 26.Shigeta H, Shigeta M, Nakazawa A, Nakamura N, Yoshikawa T. Lifestyle, obesity, and insulin resistance. Diabetes Care. 2001 Mar;24(3):608. doi: 10.2337/diacare.24.3.608. [DOI] [PubMed] [Google Scholar]
- 27.Heslop P, Smith GD, Metcalfe C, Macleod J, Hart C. Sleep duration and mortality: The effect of short or long sleep duration on cardiovascular and all-cause mortality in working men and women. Sleep Med. 2002 Jul;3(4):305–14. doi: 10.1016/s1389-9457(02)00016-3. [DOI] [PubMed] [Google Scholar]
- 28.Kripke DF, Garfinkel L, Wingard DL, Klauber MR, Marler MR. Mortality associated with sleep duration and insomnia. Arch Gen Psychiatry. 2002 Feb;59(2):131–6. doi: 10.1001/archpsyc.59.2.131. [DOI] [PubMed] [Google Scholar]
- 29.Cournot M, Ruidavets JB, Marquie JC, Esquirol Y, Baracat B, Ferrieres J. Environmental factors associated with body mass index in a population of Southern France. Eur J Cardiovasc Prev Rehabil. 2004 Aug;11(4):291–7. doi: 10.1097/01.hjr.0000129738.22970.62. [DOI] [PubMed] [Google Scholar]
- 30.Bjorkelund C, Bondyr-Carlsson D, Lapidus L, Lissner L, Mansson J, Skoog I, et al. Sleep disturbances in midlife unrelated to 32-year diabetes incidence: the prospective population study of women in Gothenburg. Diabetes Care. 2005 Nov;28(11):2739–44. doi: 10.2337/diacare.28.11.2739. [DOI] [PubMed] [Google Scholar]
- 31.Gangwisch JE, Malaspina D, Boden-Albala B, Heymsfield SB. Inadequate sleep as a risk factor for obesity: analyses of the NHANES I. Sleep. 2005 Oct 1;28(10):1289–96. doi: 10.1093/sleep/28.10.1289. [DOI] [PubMed] [Google Scholar]
- 32.Ohayon MM, Vecchierini MF. Normative sleep data, cognitive function and daily living activities in older adults in the community. Sleep. 2005 Aug 1;28(8):981–9. [PubMed] [Google Scholar]
- 33.Singh M, Drake CL, Roehrs T, Hudgel DW, Roth T. The association between obesity and short sleep duration: a population-based study. J Clin Sleep Med. 2005 Oct 15;1(4):357–63. [PubMed] [Google Scholar]
- 34.Vorona RD, Winn MP, Babineau TW, Eng BP, Feldman HR, Ware JC. Overweight and obese patients in a primary care population report less sleep than patients with a normal body mass index. Arch Intern Med. 2005 Jan 10;165(1):25–30. doi: 10.1001/archinte.165.1.25. [DOI] [PubMed] [Google Scholar]
- 35.Gottlieb DJ, Punjabi NM, Newman AB, Resnick HE, Redline S, Baldwin CM, et al. Association of sleep time with diabetes mellitus and impaired glucose tolerance. Arch Intern Med. 2005 Apr 25;165(8):863–7. doi: 10.1001/archinte.165.8.863. [DOI] [PubMed] [Google Scholar]
- 36.Kohatsu ND, Tsai R, Young T, Vangilder R, Burmeister LF, Stromquist AM, et al. Sleep duration and body mass index in a rural population. Arch Intern Med. 2006 Sep 18;166(16):1701–5. doi: 10.1001/archinte.166.16.1701. [DOI] [PubMed] [Google Scholar]
- 37.Moreno CR, Louzada FM, Teixeira LR, Borges F, Lorenzi-Filho G. Short sleep is associated with obesity among truck drivers. Chronobiol Int. 2006;23(6):1295–303. doi: 10.1080/07420520601089521. [DOI] [PubMed] [Google Scholar]
- 38.Ko GT, Chan JC, Chan AW, Wong PT, Hui SS, Tong SD, et al. Association between sleeping hours, working hours and obesity in Hong Kong Chinese: the ‘better health for better Hong Kong’ health promotion campaign. Int J Obes (Lond) 2007 Feb;31(2):254–60. doi: 10.1038/sj.ijo.0803389. [DOI] [PubMed] [Google Scholar]
- 39.Locard E, Mamelle N, Billette A, Miginiac M, Munoz F, Rey S. Risk factors of obesity in a five year old population. Parental versus environmental factors. Int J Obes Relat Metab Disord. 1992 Oct;16(10):721–9. [PubMed] [Google Scholar]
- 40.Sekine M, Yamagami T, Handa K, Saito T, Nanri S, Kawaminami K, et al. A dose-response relationship between short sleeping hours and childhood obesity: results of the Toyama Birth Cohort Study. Child Care Health Dev. 2002 Mar;28(2):163–70. doi: 10.1046/j.1365-2214.2002.00260.x. [DOI] [PubMed] [Google Scholar]
- 41.von Kries R, Toschke AM, Wurmser H, Sauerwald T, Koletzko B. Reduced risk for overweight and obesity in 5- and 6-y-old children by duration of sleep - a cross-sectional study. Int J Obes Relat Metab Disord. 2002 May;26(5):710–6. doi: 10.1038/sj.ijo.0801980. [DOI] [PubMed] [Google Scholar]
- 42.Agras WS, Hammer LD, McNicholas F, Kraemer HC. Risk factors for childhood overweight: a prospective study from birth to 9.5 years. J Pediatr. 2004 Jul;145(1):20–5. doi: 10.1016/j.jpeds.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 43.Padez C, Mourao I, Moreira P, Rosado V. Prevalence and risk factors for overweight and obesity in Portuguese children. Acta Paediatr. 2005 Nov;94(11):1550–7. doi: 10.1080/08035250510042924. [DOI] [PubMed] [Google Scholar]
- 44.Reilly JJ, Armstrong J, Dorosty AR, Emmett PM, Ness A, Rogers I, et al. Early life risk factors for obesity in childhood: cohort study. Br Med J. 2005 Jun 11;330(7504):1357. doi: 10.1136/bmj.38470.670903.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chaput JP, Brunet M, Tremblay A. Relationship between short sleeping hours and childhood overweight/obesity: results from the ‘Quebec en Forme’ project. Int J Obes (Lond) 2006 Jul;30(7):1080–5. doi: 10.1038/sj.ijo.0803291. [DOI] [PubMed] [Google Scholar]
- 46.Hasler G, Buysse DJ, Klaghofer R, Gamma A, Ajdacic V, Eich D, et al. The association between short sleep duration and obesity in young adults: a 13-year prospective study. Sleep. 2004 Jun 15;27(4):661–6. doi: 10.1093/sleep/27.4.661. [DOI] [PubMed] [Google Scholar]
- 47.Patel SR, Malhotra A, White DP, Gottlieb DJ, Hu FB. Association between reduced sleep and weight gain in women. Am J Epidemiol. 2006 Nov 15;164(10):947–54. doi: 10.1093/aje/kwj280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Patel SR, Hu FB. Short sleep duration and weight gain: a systematic review. Obesity (Silver Spring) 2008 Mar;16(3):643–53. doi: 10.1038/oby.2007.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cappuccio FP, Taggart FM, Kandala NB, Currie A, Peile E, Stranges S, et al. Meta-analysis of short sleep duration and obesity in children and adults. Sleep. 2008 May 1;31(5):619–26. doi: 10.1093/sleep/31.5.619. [DOI] [PMC free article] [PubMed] [Google Scholar]