Abstract
HIV-1-associated neurocognitive disorders (HAND) affect almost 30-50% of infected individuals, even in the presence of successful control of virus replication by combined antiretroviral therapy (cART).HIV Tat protein, a nuclear trans-activator of viral gene transcription, that is secreted by infected cells and can be taken up by the neighboring cells, is present in various tissues despite the presence of cART, and has been shown to break down the integrity of the blood-brain barrier (BBB). This, in turn, leads to disruption of the neovascular unit, affecting functioning of the brain microvascular endothelial cells as well as astrocytes. Pericytes, yet another important constituent of the BBB, play a critical role in the maintenance of the integrity of the BBB. Loss of pericytes resulting in disruption of BBB has been observed in several pathologies including HAND. Furthermore, while PDGF-BB is essential for pericyte generation, paradoxically, high concentrations of PDGF-BB lead to loss of pericytes in tumor vessels. In this research highlight, we provide a brief review of our recently published finding, which have demonstrated a novel role of PDGF-BB in HIV-Tat mediated migration of pericytes, leading ultimately to loss of pericyte coverage from the endothelium, with a subsequent breach of the BBB. These findings underpin yet another mechanism by which BBB integrity is disrupted in HAND.
Keywords: Pericytes, PDGF-BB, HIV Tat, BBB
Human immunodeficiency virus (HIV), the causative agent of AIDS, is known to enter the CNS within days of infection; however, it is not until years later that the disease manifests in the brain. In the era of combined antiretroviral therapy (cART), although there is successful control of viremia and infected individuals continue to live longer, paradoxically, the lack of cART to enter the CNS and increased longevity of infected individuals, often leads to development of HIV-1-associated neurocognitive disorders (HAND) in almost 30-50% of infected individuals [1]. HAND is pathologically characterized by reversible synaptodendrtitic injury and inflammation in the CNS [2, 3]. One of the leading mechanism(s) underlying neuroinflammation associated with HAND involves breach of the blood-brain barrier (BBB) resulting in influx of inflammatory cells into the CNS with ensuing cognitive decline and neurological complications.
The cerebrovascular unit comprising of endothelial cells, astrocytes and pericytes, is a highly selective permeability barrier that is impermeable to toxic agents, ions and pathogens. This selective permeability is what maintains the CNS homeostasis with the brain being considered as an immunoprivileged organ [4]. The focus of our study is on the essential but understudied cells of the neurovascular unit, the pericytes that are contractile cells and uniquely positioned within the brain microvascular, playing an integral role in the development and maintenance of blood vessels [5]. Their role in HIV is emerging only recently. For example, pericytes have been demonstrated to be activated by inflammatory agents and can also be infected by HIV-1 [6], leading in turn, to transcytosis of HIV-1 virus across the BBB
Despite the presence of cART, early virus proteins such as HIV-1 Tat continue to lurk in tissues such as the lymph nodes and the CNS. This becomes problematic as HIV Tat is both neuroexcitatory as well as neurotoxic, and has been implicated in the pathogenesis of HAND [7, 8]. Our previous findings have demonstrated that exposure of human brain microvascular pericytes and the pericyte cell line -C3H/10T1/2 cells, to HIV-1 Tat101 resulted in increased expression of platelet-derived growth factor (PDGF)-BB that was concomitant with increased migration of these cells [9]. Tat-mediated increased expression of PDGF-BB is in agreement with previous reports demonstrating the same phenomenon in other cell types of the cerebrovascular unit, such as the endothelial cells and astrocytes [10, 11].
PDGF belongs to a family of growth factors that are comprised of four chains (A-D) and play key role in various cellular functions[12]. PDGF-BB plays critical role in pericyte functioning under both physiological as well as pathological conditions [13, 14], and has been shown to increase the migration of retinal microvascular pericytes [15]. Another interesting finding suggests that the PDGF content of the tumor milieu determines the fate of the pericytes [16]. For example, while PDGF is critical for the maintenance of pericytes, high concentrations of PDGF-BB, can in fact, lead to pericyte loss in tumor vessels. Based on this premise we hypothesized that Tat-mediated increased migration of pericytes could, in part, be a mechanism leading to loss of pericyte coverage in the HIV-1 infected brain. Using both in vitro and ex vivo approaches, we confirmed this hypothesis. Our findings demonstrated that HIV Tat101 significantly increased the migration of C3H/10T1/2 cells and Human Brain Microvascular Pericytes (HBVPs) as expected, and that this effect was abolished in cells treated with heated Tat101. Further validation of these findings was demonstrated ex vivo, wherein we observed that higher expression of PDGF-BB correlated with a concomitant reduction in the expression of NG2 positive pericytes in microvessels isolated from HIV-1 transgenic (Tg) 26 mice. Similar to the observation in HIV-1 Tg26 mice, reduced pericyte coverage was also observed in sections of frontal cortex from brains of individuals with HIV-encephalitis compared with the uninfected controls.
Since HIV Tat accumulates age-dependently in the older Tat Tg 26 mice, we reasoned that there would be increased pericyte loss in the microvessels isolated from older (>1 year) versus younger (< 2 months) mice. Intriguingly, there was increased expression of HIV Tat in the older compared to the younger Tg26 mice and this correlated with increased pericyte loss in the older animals. These findings thus support the role of Tat in mediating loss of pericyte coverage on the brain endothelium. In agreement with these studies, decreased pericyte coverage of BBB has also been reported in HIV-1 infected patients and in an animal model of chronic HIV-1 infection in humanized NSG mice by Hill et al [17].
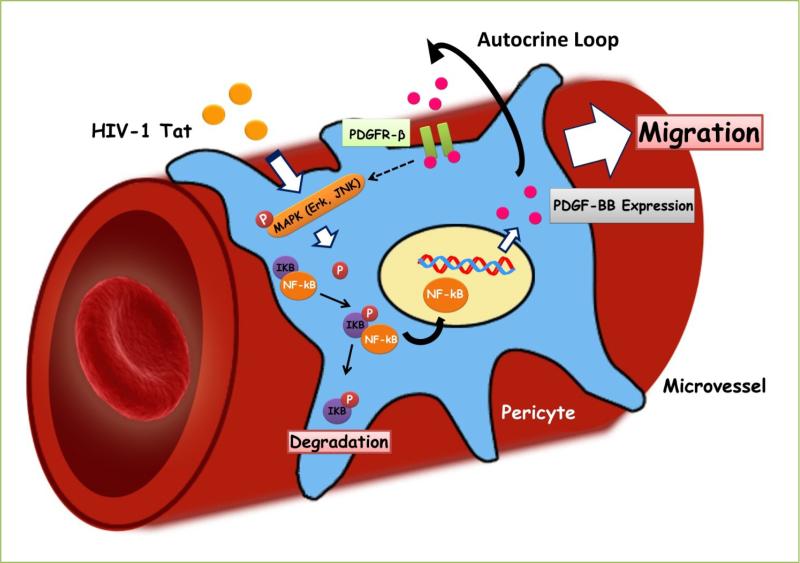
In our previous study, we also demonstrated a detailed molecular pathway of Tat101-mediated expression of PDGF-BB involved in pericyte migration in vitro (Fig. 1). Briefly, Tat-mediated induction of PDGF-BB expression in pericytes involved activation of ERK and JNK MAPK pathways, with the subsequent activation of NF-κB. Tat-mediated induction of PDGF-BB was further shown to engage and activate PDGFR-β signaling via autocrine regulation, ultimately leading to increased pericyte migration. PDGF-BB was implicated as the key player in Tat-mediated induction of pericyte loss. This brings to light the findings by Hosaka et al, reporting that the concentration of PDGF-BB is what determines the fate of pericytes [16]. Currently we do not have answers as to whether it is the concentration modulation of PDGF-BB by Tat that is mediating the pericyte loss, however, given that Tat-mediates autocrine regulation of PDGF, it can be envisioned that higher concentration of PDGF-BB generated by HIV Tat is driving the pericyte loss. These findings are currently ongoing in the authors’ laboratory.
Fig 1. Schematic diagram demonstrating signaling pathways involved in Tat101-mediated induction of PDGF-BB & pericyte migration.
Tat stimulates MAPK (Erk and JNK) pathways, which in turn, leads to nuclear translocation of NF-κB, with subsequent induction of PDGF-BB. Elevated PDGF-BB via autocrine PDGFR-β signaling, ultimately leads to increased pericyte migration and loss in microvessels within the brain.
In summary, mechanisms underlying HIV protein mediated loss of pericytes on the endothelium with breach of BBB are critical for future development of therapeutic strategies aimed at restoring BBB breach in the presence of HIV Tat.
Acknowledgments
We thank Novartis, Basel, Switzerland for providing us with STI-571. We also thank Dr. Young Han Lee (Konkuk University, Korea) for providing the dominant negative and constitutively active constructs of MEK. We acknowledge the help of Roy L. Sutliff (Emory University/Atlanta VA Medical Center, USA) for providing us with HIV Tg26 and WT mice. This work was supported by grants DA033150, DA033614, DA035203, DA036157 (SB).
Footnotes
To cite this article: Fang Niu, et al. HIV Tat 101-mediated loss of pericytes at the blood-brain barrier involves PDGF-BB. Ther Targets Neurol Dis 2015; 2: e471. doi: 10.14800/ttnd.471.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
References
- 1.Lindl KA, Marks DR, Kolson DL, Jordan-Sciutto KL. HIV-associated neurocognitive disorder: pathogenesis and therapeutic opportunities. J Neuroimmune Pharmacol. 2010;5:294–309. doi: 10.1007/s11481-010-9205-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ellis R, Langford D, Masliah E. HIV and antiretroviral therapy in the brain: neuronal injury and repair. Nat Rev Neurosci. 2007;8:33–44. doi: 10.1038/nrn2040. [DOI] [PubMed] [Google Scholar]
- 3.Bagashev A, Sawaya BE. Roles and functions of HIV-1 Tat protein in the CNS: an overview. Virol J. 2013;10:358. doi: 10.1186/1743-422X-10-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Winkler EA, Bell RD, Zlokovic BV. Central nervous system pericytes in health and disease. Nat Neurosci. 2011;14:1398–1405. doi: 10.1038/nn.2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bell RD, Winkler EA, Sagare AP, Singh I, LaRue B, Deane R, et al. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron. 2010;68:409–427. doi: 10.1016/j.neuron.2010.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakagawa S, Castro V, Toborek M. Infection of human pericytes by HIV-1 disrupts the integrity of the blood-brain barrier. J Cell Mol Med. 2012;16:2950–2957. doi: 10.1111/j.1582-4934.2012.01622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agrawal L, Louboutin JP, Reyes BA, Van Bockstaele EJ, Strayer DS. HIV-1 Tat neurotoxicity: a model of acute and chronic exposure, and neuroprotection by gene delivery of antioxidant enzymes. Neurobiol Dis. 2012;45:657–670. doi: 10.1016/j.nbd.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 8.Buscemi L, Ramonet D, Geiger JD. Human immunodeficiency virus type-1 protein Tat induces tumor necrosis factor-alpha-mediated neurotoxicity. Neurobiol Dis. 2007;26:661–670. doi: 10.1016/j.nbd.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Niu F, Yao H, Zhang W, Sutliff RL, Buch S. Tat 101-mediated enhancement of brain pericyte migration involves platelet-derived growth factor subunit B homodimer: implications for human immunodeficiency virus-associated neurocognitive disorders. J Neurosci. 2014;34:11812–11825. doi: 10.1523/JNEUROSCI.1139-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mermis J, Gu H, Xue B, Li F, Tawfik O, Buch S, et al. Hypoxia-inducible factor-1 alpha/platelet derived growth factor axis in HIV-associated pulmonary vascular remodeling. Respir Res. 2011;12:103. doi: 10.1186/1465-9921-12-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bethel-Brown C, Yao H, Hu G, Buch S. Platelet-derived growth factor (PDGF)-BB-mediated induction of monocyte chemoattractant protein 1 in human astrocytes: implications for HIV-associated neuroinflammation. J Neuroinflammation. 2012;9:262. doi: 10.1186/1742-2094-9-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Demoulin JB, Montano-Almendras CP. Platelet-derived growth factors and their receptors in normal and malignant hematopoiesis. American journal of blood research. 2012;2:44–56. [PMC free article] [PubMed] [Google Scholar]
- 13.Lindahl P, Johansson BR, Leveen P, Betsholtz C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science. 1997;277:242–245. doi: 10.1126/science.277.5323.242. [DOI] [PubMed] [Google Scholar]
- 14.McCarty MF, Somcio RJ, Stoeltzing O, Wey J, Fan F, Liu W, et al. Overexpression of PDGF-BB decreases colorectal and pancreatic cancer growth by increasing tumor pericyte content. J Clin Invest. 2007;117:2114–2122. doi: 10.1172/JCI31334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nadal JA, Scicli GM, Carbini LA, Scicli AG. Angiotensin II stimulates migration of retinal microvascular pericytes: involvement of TGF-beta and PDGF-BB. Am J Physiol Heart Circ Physiol. 2002;282:H739–748. doi: 10.1152/ajpheart.00656.2001. [DOI] [PubMed] [Google Scholar]
- 16.Hosaka K, Yang Y, Seki T, Nakamura M, Andersson P, Rouhi P, et al. Tumour PDGF-BB expression levels determine dual effects of anti-PDGF drugs on vascular remodelling and metastasis. Nat Commun. 2013;4:2129. doi: 10.1038/ncomms3129. [DOI] [PubMed] [Google Scholar]
- 17.Hill J, Rom S, Ramirez SH, Persidsky Y. Emerging roles of pericytes in the regulation of the neurovascular unit in health and disease. J Neuroimmune Pharmacol. 2014;9:591–605. doi: 10.1007/s11481-014-9557-x. [DOI] [PMC free article] [PubMed] [Google Scholar]