Abstract
t(14;18)-positive cells can not only be detected in follicular lymphoma (FL) patients but also in healthy individuals (HI). We used epidemiological data and blood samples of the population-based Study of Health in Pomerania (SHIP) to analyze associations of FL risk factors and t(14;18)-positive cells in HI. Buffy coat samples from 4152 study participants were tested by real-time PCR for t(14;18)-positive cells. Out of 3966 evaluable subjects, 1526 were t(14;18)-PCR positive (38.5%, median 3.9 t(14;18)-positive per million nucleated cells, range 0.6 – 9299). In multivariable analyses age and sex but not parameters of smoking exposure were significantly associated with t(14;18)-prevalence (logistic regression, p < 0.001). Multivariable analyses of t(14;18)-frequency showed a positive association with age but not with sex or smoking. These age and sex associations in HI require careful control in future studies of t(14;18) as a potential biomarker of lymphoma risk.
Keywords: Follicular Lymphoma, t(14;18), Lymphomagenesis, Healthy Individuals
Introduction
The t(14;18)(q32;q21)-translocation is the characteristic chromosomal aberration of the most common indolent non-Hodgkin lymphoma, follicular lymphoma (FL), and can be detected cytogenetically in about 90% of FL and 30-50% of diffuse large B cell lymphomas (DLBCL)[1-3]. This translocation juxtaposes the proto-oncogene BCL2 on chromosome 18 in cis with regulatory sequences of the immunoglobulin heavy chain (IgH) locus on chromosome 14 resulting in a constitutive overexpression of the anti-apoptotic BCL2 protein, which is believed to be one of the initiating events in the lymphomagenesis of FL [4;5]. Mice carrying an IGH-BCL2 fusion transgene show a three- to fourfold expansion of resting B cells manifesting as lymphatic hyperplasia [6;7]. About 10% of these IGH-BCL2 transgenic mice develop DLBCL, frequently exhibiting a re-arranged MYC [8]. This model suggests that the progression from benign follicular hyperplasia to malignant lymphoma is due to secondary changes and that the t(14;18)-translocation alone is not sufficient to transform a normal B cell into a malignant lymphoma cell.
In humans, circulating t(14;18)-positive cells can be detected not only in FL patients but also in healthy subjects without lymphoma. Using polymerase chain reaction (PCR) techniques with a sensitivity of ≤ 10-5, several groups have shown that t(14;18)-positive cells are detectable in 30-60% of healthy individuals (HI) at frequencies of 1-200 t(14;18)-positive cells per 106 peripheral blood mononuclear cells [9-14]. Previous epidemiological and molecular studies in subjects without lymphoma have suggested that these t(14;18)-positive cells might represent potential lymphoma precursor cells. This hypothesis is supported by associations of t(14;18) with known FL risk factors, including age, gender, pesticide exposure and tobacco smoking [10-12;15-17]. However, all of these studies were too small to simultaneously assess multiple risk factors and to verify the independent association of each of these factors with either t(14;18) prevalence or frequency. Therefore, we characterized a large set of 4152 subjects for t(14;18) prevalence and frequency. We used multivariable methods to evaluate simultaneously the associations of age, gender and smoking with t(14;18).
Material and Methods
Subjects/Study Population
The population-based Study of Health in Pomerania (SHIP) is a cross-sectional epidemiological survey carried out to estimate the prevalence of clinical diseases, subclinical disorders and risk factors, and to study their complex associations.
Objectives and design of this study have been published previously [18]. Briefly, the study took place in Western Pomerania (N=213057 inhabitants in 1996) located in the north-eastern part of Germany. In a two-stage design a stratified sample of 7008 men and women between 20-79 years of age (292 persons in each of twelve 5-year age strata for both genders) was randomly drawn using German population registries. Excluding migrated or deceased persons, the net sample comprised 6265 individuals invited to participate. A total of 4308 individuals (68.8%; 2192 females, 2116 males) agreed and written informed consent was obtained from each participant. The study was approved by the Ethics Committee of the Board of Physicians, Mecklenburg-Western Pomerania at the University of Greifswald. Each participant underwent comprehensive standardized medical and dental examinations. Data collection was completed by a computer-aided face-to-face health interview and a self-administered risk factor questionnaire. Venous blood samples were drawn according to standard procedure. Data collection started in October 1997 and finished in May 2001.
Smoking status and history were assessed in the computer-aided interview. Current smokers were defined as individuals who answered “yes” to the question “Do you currently smoke cigarettes?”. Ex-smokers were defined as persons who did not currently smoke but had done so in the past. Non-smokers were defined as persons who had never smoked at least one cigarette per day. The percentage of persons who smoked cigars or pipes was very low (1.9%). Persons who smoked cigars or pipe were not grouped separately but were regarded as smokers or former smokers, depending on their current tobacco use. Cumulative cigarette exposure was assessed as dose times duration in terms of pack years, defined as smoking 20 cigarettes per day for one year. Pack years were categorized as none, <10, 10-<20, 20-<30, 30-<40, and ≥40. Pipe and cigar smokers were not considered in the pack year analysis. Fifteen individuals were excluded from this evaluation that either had no interview, interrupted interviews with no data on smoking behaviors, or refused to answer the smoking questions.
Biosamples and t(14;18)-PCR
DNA was isolated from peripheral blood buffy coat fractions using the phenol-chloroform method and stored at -20°C. Real-time quantitative PCR was carried out for the t(14;18)-major breakpoint region (MBR) of BCL2 and for the wild type K-RAS reference gene (two copies per genome) as described previously [19]. Five replicates of 1 μg DNA, equivalent to testing a total of 7.5 × 105 nucleated cells, were used for the t(14;18) assay. The mean sensitivity of the t(14;18)-MBR PCR assay was estimated as one t(14;18)-positive cell in 5 × 105 nucleated cells (NC). The PCR mixture contained forward and reverse primers each at a concentration of 400 nM, a TaqMan probe at a concentration of 200 nM, the standard TaqMan Universal PCR Master Mix (Applied Biosystems, Weiterstadt, Germany) and 1 μg DNA in a total volume of 50 μl. After 2 min incubation at 50°C to allow for cleavage by Uracil-N-Glycosylase, AmpliTaq Gold was activated by incubation at 95°C for 10 min. Each PCR cycle consisted of 15 sec denaturation at 95°C, and 1 min of combined annealing/extension at 61°C. Standard curves were established for both the t(14;18)-MBR PCR and the K-RAS reference PCR by dilutions of DNA from Karpas 422 cells. All PCR experiments included appropriate positive and negative controls.
Buffy coat samples from 4152 individuals were received for testing in this study. For analyses of t(14;18) prevalence, a sample was regarded as positive if t(14;18) was detected in at least one of its DNA replicates and negative if t(14;18) was not detected. However, t(14;18)-negative samples containing <200,000 K-RAS genome equivalents were regarded as not informative and excluded from further consideration (n= 184). For analyses of t(14;18) frequency, the sum of t(14;18) copies from all five replicates was divided by the total number of tested cells (i.e., K-RAS copies divided by 2), multiplied by 106 and expressed as t(14;18)-positive cells per million NC.
Statistical Analyses
The statistical software packages SAS (SAS Institute Inc., Cary, NC, USA, V 9.1) and STATA (STATA/SE 10, Texas, USA) were used. Categorical data were expressed as percentages; continuous data were expressed as median and interquartile range (IQR). Differences between groups were tested using the chi-square test, Mann-Whitney U test, and Kruskal-Wallis test (for 3 and more group comparison). A p-value <0.05 was considered statistically significant.
We used multivariable logistic regression models to calculate odds ratios and 95% confidence intervals of t(14;18) prevalence for age, gender as well as for smoking status and smoking history). To analyze t(14;18) frequency with regard to age, gender and smoking, we fitted negative binomial regression models for persons with at least one copy of t(14;18). We also calculated zero-inflated negative binomial regression models including t(14;18) positive and negative subjects. Results from the latter models using data from all subjects irrespective of t(14;18) status showed in general similar associations as in models which were restricted to t(14;18)-positive subjects. Therefore and in order to allow the comparison of the results of the present study to previously reported data, we only show the t(14;18) frequency results from analyses restricted to t(14;18)-positive subjects.
To represent the wide numerical range of K-RAS genome equivalents (45,000 to 1,900,000) the number per subject was considered as a weighting factor in the multivariable analyses.
Role of the Funding Source
The funding source of the SHIP trial (German Federal Ministry of Education and Research (BMBF, grant 01ZZ96030, 01ZZ0701); the Ministry for Education, Research, and Cultural Affairs; and the Ministry for Social Affairs of the Federal State of Mecklenburg-West Pomerania) had no involvement in the study design, collection, analysis or interpretation of the data, writing of the report or in the decision to submit the paper for publication.
Results
The demographic characteristics of the study population are described in Table I. 1085 (27.4%) of the 3966 study subjects (both genders combined) were current smokers, 1022 (25.8%) former smokers and 1859 (46.9%) non-smokers.
Table I. Prevalence and Frequency of t(14;18) in SHIP-0.
| Characteristics | All [N (%)] | t(14;18)-Positive [N (%)] | p-value | Median t(14;18) Frequency§ per 106 NC of t(14;18)-Positive cells (IQR) | p-value |
|---|---|---|---|---|---|
| N | 3966 100) | 1526 (38.5) | 3.9 (2.0;8.1) | ||
|
| |||||
| Age [years] | |||||
| Median (IQR) | 50 (36;64) | 54 (40;65) | - | ||
|
| |||||
| Age Groups [years] | |||||
| 20-29 | 511 (12.9) | 123 (24.1) | 3.0 (1.7;4.6) | ||
| 30-39 | 706 (17.8) | 236 (33.4) | 3.7 (1.9;6.3) | ||
| 40-49 | 706 (17.8) | 265 (37.5) | 3.5 (2.1;6.5) | ||
| 50-59 | 721 (18.2) | 340 (47.2) | 4.1 (2.1;9.4) | ||
| 60-69 | 732 (18.5) | 318 (43.4) | 4.9 (2.5;9.7) | ||
| 70-81 | 590 (14.9) | 244 (41.4) | < 0.05† | 3.9 (1.9;8.6) | <0.001# |
|
| |||||
| Sex | |||||
|
| |||||
| Male | 1962 (49.5) | 850 (43.3) | 3.9 (2.1;8.0) | ||
| Female | 2004 (50.5) | 674 (33.7) | < 0.001$ | 3.9 (2.0;8.3) | 0.41* |
|
| |||||
| Smoking Status | |||||
|
| |||||
| Non-smoker | 1859 (46.9) | 678 (36.5) | 3.9 (2.0;8.3) | ||
| Ex-smoker | 1022 (25.8) | 430 (42.1) | 3.8 (2.1;8.1) | ||
| Current smoker | 1085 (27.4) | 418 (38.5) | 0.01$ | 3.9 (2.1;7.8) | 0.90# |
|
| |||||
| Pack Years | |||||
|
| |||||
| Median (IQR) | 1 (0;15) | 3 (0;18) | |||
|
| |||||
| Pack Year Categories | |||||
|
| |||||
| Missing | 162 (4.1) | 72 (44.4) | - | ||
| 0 | 1865 (47.0) | 680 (36.5) | 3.9 (2.0;8.3) | ||
| >0-<10 | 688 (17.3) | 238 (34.6) | 3.8 (2.1;7.1) | ||
| 10-<20 | 520 (13.1) | 204 (39.2) | 3.8 (1.9;7.3) | ||
| 20-<30 | 360 (9.1) | 154 (42.8) | 3.4 (2.0;7.2) | ||
| 30-<40 | 194 (4.9) | 85 (43.8) | 4.2 (2.3;8.5) | ||
| >=40 | 177 (4.5) | 93 (52.5) | < 0.05† | 5.0 (2.7;10.9) | 0.06# |
: Median t(14;18) frequency among t(14;18)-positive subjects
: Ptrend
: Chi-square test
: Kruskal-Wallis-test
Mann-Whitney-U test
IQR: interquartile range
Results of Bivariate Analyses of t(14;18) Prevalence and Age, Sex and Smoking
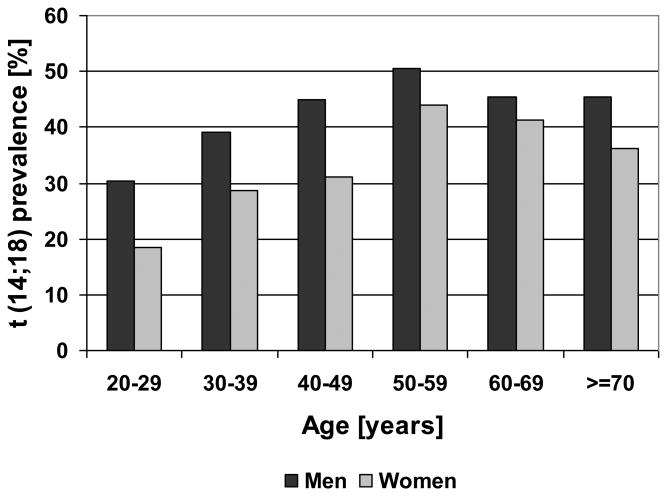
In a bivariate analysis t(14;18) prevalence in both sexes combined increased from 24.1% in the age group 20-29 years up to 47.2% in the age group 50-59 years (Table I) with no further increase above the age of 60. The percentage of t(14;18)-positive subjects was significantly higher in men than in women (43.3% vs. 33.7%, p < 0.001, Table I), with a consistently higher t(14;18) prevlance in men in all age groups (Figure 1A).
Figure 1. t(14;18)-Prevalence according to Age, Sex and Pack Years of Smoking.
t(14;18) prevalence according to age and sex (Figure 1A) and pack year categories of cigarette smoking (Figure 1B). PY: Pack years
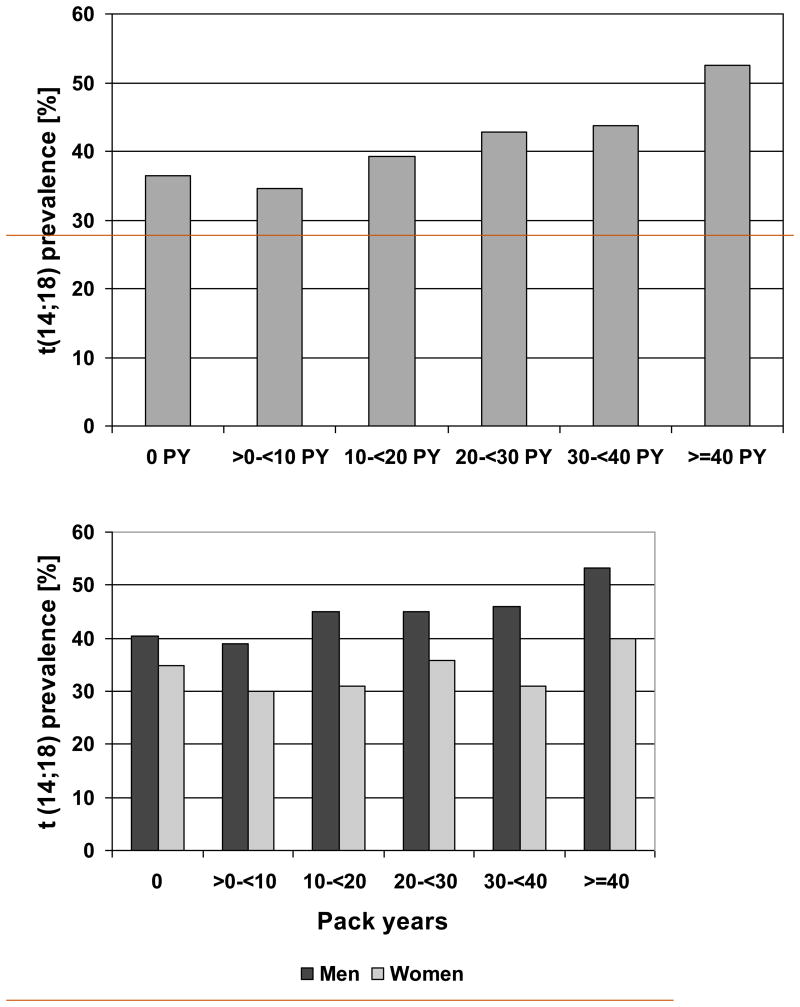
In a comparison of t(14;18) prevalence according to smoking status non-smokers had the lowest and former smokers the highest t(14;18) prevalence (Table I). However, if this analysis was done within age and sex groups there was no clear effect of smoking status on t(14;18) prevalence (Supplemental Table SI). When we analyzed the association of t(14;18) prevalence with lifelong smoking exposure quantified as cumulative pack years we observed a significant increase from 34.6% in the group of subjects with >0-10 pack years to 52.5% in subjects with at least 40 pack years (Table I). This significant increase is present only in men, but not in women (Figure 1B).
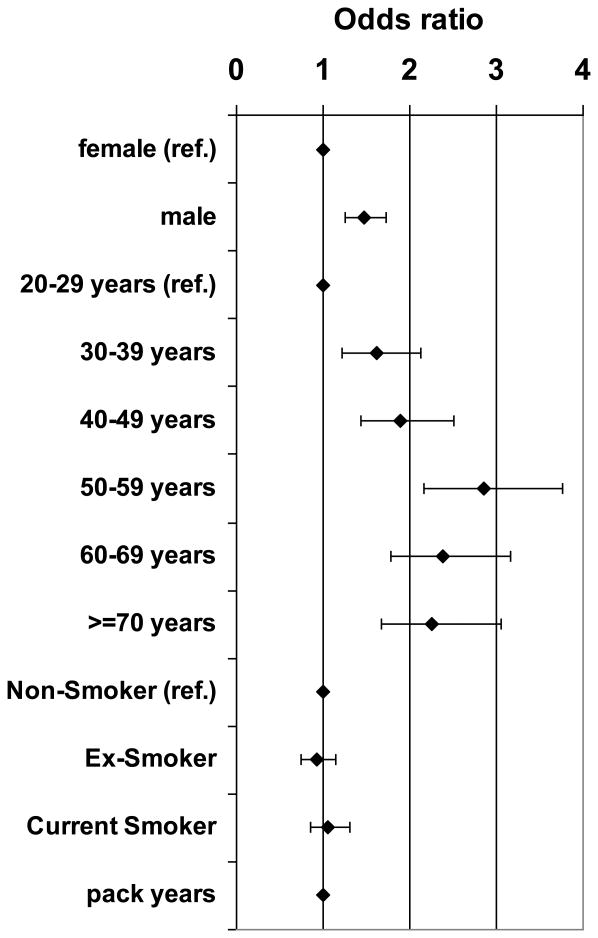
No Association of t(14;18) Prevalence and Exposure to Cigarette Smoking in Multivariable Models Adjusting for Age and Sex
Multivariable logistic regression models containing age, sex and different smoking parameters confirmed the association of age and sex and t(14;18) prevalence. In all models t(14;18) prevalence was higher in men than in women and increased with higher age (Figure 2, Supplemental Figure S1A-C and Table SII). In contrast, the increase in t(14;18) prevalence with increasing smoking exposure as observed in the bivariate analyses could not be confirmed in multivariable analysis adjusting for sex and age. In none of the models t(14;18) prevalence was associated with smoking, neither in analyses of pack years of cigarette smoking (Supplemental Figures S1A and S1B) or smoking status alone (Supplemental Figure 1C) nor in a combined analysis of pack years and smoking status (Figure 2).
Figure 2. Multivariable Analysis of t(14;18)-Prevalence and Age, Sex and Smoking.
Odds ratios (◆) and 95% confidence intervals for t(14;18) prevalence from a logistic regression model containing sex, age, smoking status and pack years of cigarette smoking as a continuous variable.
Results of Uni- and Bivariate Analyses of t(14;18) Frequency and Age, Sex and Smoking
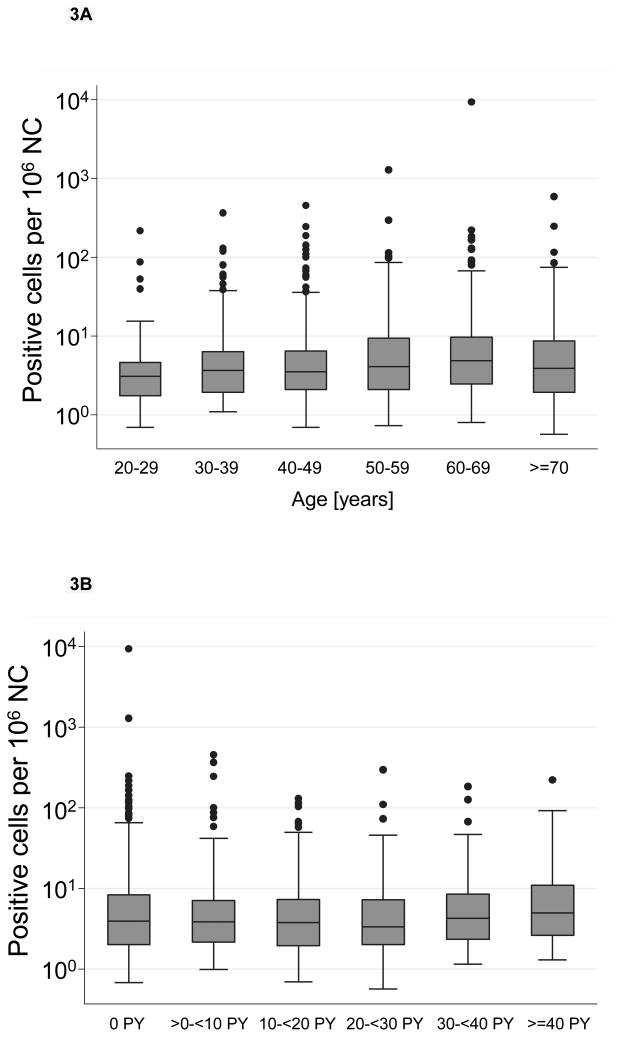
The analyses of the association of age, sex and smoking with the frequency of t(14;18)-positive cells were restricted to t(14;18)-positive subjects. The median t(14;18) frequency of all positive subjects was 3.9 per million NC, and the maximum observed was 9299 per million NC. Similar to the age-dependent rise in t(14;18) prevalence, there was a small but statistically significant increase in t(14;18) frequency with age (Table I, Figure 3A). Unlike the prevalence t(14;18) frequency did not differ significantly between men and women (Table I, Supplemental Figure S2A).
Figure 3. t(14;18) frequency according to Age and Pack Years of Smoking.
Univariate analysis of t(14;18)-frequency according to age (Figure 3A) and pack year categories of cigarette smoking (Figure 3B). The horizontal lines within the boxes denotes the median t(14;18)-frequency, the lower and upper bounds of the boxes represent the 25 and the 75 percentile, the I bars indicate upper (largest data value that is less than or equal to the 3rd quartile + 1.5 × interquartile range) and lower (smallest data value that is greater than or equal to the 1st quartile - 1.5 × interquartile range) adjacent limits, and • represents outliers.
In bivariate analyses cigarette smoking did not show a clear association with t(14;18) frequency. Comparing the median t(14;18) frequencies for non-smokers (3.9 per million NC), ex-smokers (3.8 per million NC) and current smokers (3.9 per million NC) we observed no differences (P= 0.9, Table I, Supplemental Figure S2B). Similarly, there was no association with the number of pack years (Table I and Figure 3B).
Multivariable Analyses Confirm that Age but Not Sex Nor Smoking Is Associated With t(14;18) Frequency in Subjects Without Lymphoma
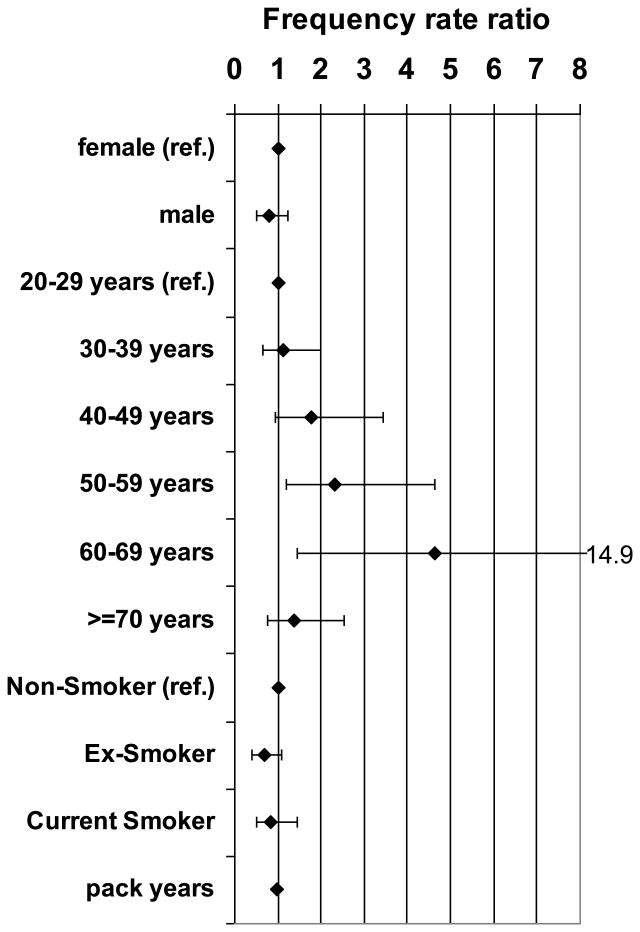
Multivariable models of age, sex and different measures of smoking exposure confirmed that only age was indeed positively associated with t(14;18) frequency whereas sex was not (relative frequency for men compared to women 0.77, P= 0.28; Figure 4, Supplemental Table SIII). Median t(14;18) frequency rose continuously with increasing age up to 60-69 years (Figure 4 and Supplemental Table SIII).
Figure 4. Multivariable Analysis of t(14;18) Frequency and Sex, Age and Smoking.
Frequency rate ratios (◆) and 95% confidence intervals from a multivariable negative binomial regression model of t(14;18) frequency in t(14;18)-positive subjects containing sex, age, smoking status and pack years of cigarette smoking as a continuous variable.
The results for smoking were inconclusive. Multivariable models containing age, sex and pack years either categorized or as a continuous variable failed to demonstrate a consistent association with t(14;18) frequency (Supplemental Figure S3A and S3B, Supplemental Table SIII). Compared to non-smokers (0 pack years) t(14;18) frequency was lower in all pack year categories. The model of age, sex and smoking status showed a lower t(14;18) frequency in both current smokers and ex-smokers compared to non-smokers. (Supplemental Figure 3C, Supplemental Table SIII). In a combined analysis of sex, age, smoking status and pack years the only parameter that was significantly associated with t(14;18) frequency was age whereas measures of smoking exposure were not associated with t(14;18) frequency (Figure 4, Supplemental Table SIII).
Discussion
The detection of t(14;18)-positive cells in individuals without lymphoma was first described in 1991 [9]. Since then a number of studies have analyzed the association of known FL risk factors with either prevalence and/or frequency of t(14;18)-positive cells in individuals without lymphoma. However, results were conflicting as to whether t(14;18) prevalence and frequency increases with age [10;12;16;17] or not [14;20-22]. Two studies reported a higher t(14;18) prevalence in males than in females [11;12], an observation that was not confirmed by other authors [14;20;22]. Baccarelli et al. found in bivariate analyses a higher prevalence in men then in women, but the difference was not statistically significant [23]. Similarly, the authors did not observe any differences in the frequency of t(14;18)-positive cells by sex.
Only two studies examined the association between smoking and t(14;18) prevalence and frequency in healthy individuals [11;21]. Rauzy et al. demonstrated an association between higher smoking exposure measured as pack years and t(14;18) prevalence among 39 bone marrow samples but found no association with smoking habits in peripheral blood samples from 137 additional healthy volunteers [21]. In a study of blood samples from 86 smokers and 36 non-smokers Bell et al. reported a statistically significant association between smoking exposure and t(14;18) prevalence and frequency, although the differences in t(14;18) frequencies between pack year groups were very small. To our knowledge the paper of Bell et al. has been the first (and so far only) analysis that examined different smoking exposure measures in combination with age, sex, and race. Sex and, unlike in our analysis, pack-years remained statistically significant whereas age showed no significant association in the adjusted model [11]. However, the small sample size required broad pack year categories increasing the chance of residual confounding.
Hence common limitations of most previous studies were small sample sizes (Liu: n= 84 [10], Ji: n= 132 [12], Rauzy: n= 185 [21], Summers: n= 481 [14], Schmitt: n= 204 [22], Roulland: n= 26 individuals [16]) and usually a focus on only one risk factor or exposure. Because of the small samples sizes most studies were not able to perform multivariable analyses and to adjust for confounding of different variables that might affect t(14;18) prevalence and frequency in healthy individuals.
To overcome these limitations of previous studies, we used peripheral blood buffy coat samples and epidemiological cross-sectional data of 4152 subjects from a population based cohort-study in north-eastern Germany (SHIP) to analyze factors associated with the prevalence and frequency of t(14;18)-positive cells in individuals without lymphoma. We did not find an association of smoking status or pack years and either t(14;18) prevalence or frequency in individuals without lymphoma. The observation of an increasing t(14;18) prevalence with increasing numbers of pack years in the initial bivariate analysis turned out to be completely due to confounding by age and sex as documented by multivariable analysis (Figure 2, Supplemental Figure S1A and S1B). Therefore we assume that positive associations of heavy smoking and t(14;18) frequency and prevalence as reported in much smaller studies [11;21] also resulted most likely from confounding and do not represent true associations.
This absence of an impact of smoking on t(14;18) prevalence and frequency in HI is in line with the evidence for associations of smoking with DLBCL and FL risk. Most epidemiologic studies did not observe an association between smoking status or a positive dose-response relationship of pack-years of smoking and NHL risk [24]. When analyzed according to histologic subtype, there seems to be a weak association with FL but no significant association for DLBCL [25-27].
Moreover, this lack of association is in accordance to results from studies that examined risk factors according to t(14;18) status of DLBCL and FL patients (detected either by FISH [28-30] or PCR [31]) which also found no consistent association between smoking and t(14;18)-positive NHL. Two of three studies that analyzed NHL cases according to the presence of a FISH-detectable t(14;18) found only an association between tobacco exposure and t(14;18)-negative NHL but no clear association between t(14;18)-positive NHL and tobacco use [28;29]. The study by Chang et al. did not find any association of smoking habits and t(14;18)-positive DLBCL and FL [30]. An earlier report from the FARM study that used PCR for the identification of t(14;18)-positive NHL [31] showed non-significant positive associations for cigarette smoking with t(14;18)-positive NHL (OR 1.7, CI 0.9–3.3), and odds ratios were around unity for all categories of high smoking exposure. These inconsistent results indicate that there is - if any - only a weak association between smoking and t(14;18)-positive NHL.
The results of the present study document clearly that t(14;18) prevalence and frequency in HI rise steadily with increasing age. The t(14;18) prevalence was highest in the age group 50-59 years and did not increase further in subjects older than 60 years. Among the prevalent subjects t(14;18) frequency increases up to the age group 60-69 years with a non-significant drop in the median frequency in subjects 70 years and older. Agopian et al. demonstrated in pesticide-exposed persons but also in some unexposed subjects that this increase in t(14;18) frequency is mainly due to a clonal expansion of one or two dominant t(14;18)-positive clones and not a result of the accumulation of an increasing number of distinct clones [32]. These authors hypothesized that clonal expansion results from an immunogenic effect of agricultural pesticide exposure and other environmental factors. Higher age by itself leads to an altered adaptive as well as innate immune response [33] and these alterations might – in addition to other factors – contribute to both an increased t(14;18) frequency and FL incidence with increasing age.
In the present study we observed a higher t(14;18) prevalence in men than in women (43.3% vs. 33.7%, ratio 1.3, p < 0.001) that parallels the higher incidence rates of FL and DLBCL in men (US SEER: Male-female incidence rate ratios among whites: 1.6 for DLBCL and 1.2 for FL)[34]. There was no difference in the frequency of t(14;18)-positive cells between men and women.
A potential weakness of our results is the lack of information on differential blood counts of the samples, therefore we were unable to adjust the t(14;18) results for the percentage of lymphocytes. However, using white blood count in a an alternative analysis based on absolute rather than relative t(14;18) frequency did not change the parameter estimates..
Up to now this report is the largest population-based cross-sectional study analyzing t(14;18) prevalence and frequency in subjects without lymphoma. Our data confirm that the detection of t(14;18)-positive cells is clearly associated with the known FL risk factors age and sex and that there is no association with smoking. Recent studies have demonstrated that t(14;18)-positive cells harbor additional molecular changes indicating that these cells could be bona fide lymphoma precursor cells [32], suggesting the potential utility of t(14;18) as a biomarker of lymphoma risk. It remains to be established by prospective studies in the future whether in some of these subjects t(14;18)-positive cells indeed transform into malignant lymphoma cells and which features of malignant progression (e.g. t(14;18) frequency, ongoing AID expression) allow an early identification of individuals at risk.
Supplementary Material
Supplemental Material for Online Publication: Additional Supplemental may be found in the online version of this article:
Table SI: t(14;18) prevalence according to age, sex and smoking status
Table SII: Logistic regression models analyzing the association of t(14;18) prevalence with age, sex and measures of cigarette smoking exposure
Table SIII: Negative binomial regression models of the association of t(14;18) frequency in t(14;18)-positive individuals with sex, age and measures of smoking
Figure S1: Multivariable logistic regression models of t(14;18) prevalence and sex, age and smoking (pack years continuous (S1A) or categorized (S1B) or smoking status (S1C))
Figure S2: Bivariate analyses of t(14 ;18) frequency according to sex (Figure S2A) and smoking status (Figure S2B).
Figure S3: Multivariable analysis of the association between t(14;18) frequency in t(14;18)-positive subjects and sex, age and measures of smoking (pack years continuous (S3A) or categorized (S3B) or smoking status (S3C)).
Acknowledgments
The authors greatly acknowledge the excellent technical assistance of Ute Pett. SHIP is part of the Community Medicine Net (http://www.medizin.uni-greifswald.de/icm) of the University of Greifswald, which is funded by grants from the German Federal Ministry of Education and Research (BMBF, grant 01ZZ96030, 01ZZ0701); the Ministry for Education, Research, and Cultural Affairs; and the Ministry for Social Affairs of the Federal State of Mecklenburg-West Pomerania.
C.H. received travel support from Roche Pharma AG.
Footnotes
Declaration of Interests: All other authors declare no potential financial or personal conflict of interest.
Previous Presentations: The data of this study have been presented in part at the Annual Meeting of the American Society of Hematology in 2009 in New Orleans and at the 11th International Conference on Malignant Lymphoma in Lugano, Switzerland, in June 2011.
References
- 1.Weiss LM, Warnke RA, Sklar J, Cleary ML. Molecular analysis of the t(14;18) chromosomal translocation in malignant lymphomas. N Engl J Med. 1987;317:1185–9. doi: 10.1056/NEJM198711053171904. [DOI] [PubMed] [Google Scholar]
- 2.Bende RJ, Smit LA, van Noesel CJ. Molecular pathways in follicular lymphoma. Leukemia. 2007 Jan;21:18–29. doi: 10.1038/sj.leu.2404426. [DOI] [PubMed] [Google Scholar]
- 3.Jaffe ES, Harris NL, Stein H, Vardiman JW. Pathology and genetics of tumours of haematopoietic and lymphoid tissues. Lyon, Frannce: IARC Press; 2008. [Google Scholar]
- 4.Graninger WB, Seto M, Boutain B, Goldman P, Korsmeyer SJ. Expression of Bcl-2 and Bcl-2-Ig fusion transcripts in normal and neoplastic cells. J Clin Invest. 1987;80:1512–5. doi: 10.1172/JCI113235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hockenbery D, Nuñez G, Milliman C, Schreiber RD, Korsmeyer SJ. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature. 1990;348:334–6. doi: 10.1038/348334a0. [DOI] [PubMed] [Google Scholar]
- 6.Vaux DL, Cory S, Adams JM. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature. 1988;335:440–2. doi: 10.1038/335440a0. [DOI] [PubMed] [Google Scholar]
- 7.McDonnell TJ, Deane N, Platt FM, et al. bcl-2-immunoglobulin transgenic mice demonstrate extended B cell survival and follicular lymphoproliferation. Cell. 1989;57:79–88. doi: 10.1016/0092-8674(89)90174-8. [DOI] [PubMed] [Google Scholar]
- 8.McDonnell TJ, Korsmeyer SJ. Progression from lymphoid hyperplasia to high-grade malignant lymphoma in mice transgenic for the t(14;18) Nature. 1991;349:254–6. doi: 10.1038/349254a0. [DOI] [PubMed] [Google Scholar]
- 9.Limpens J, De Jong D, Van Krieken JHJM, et al. Bcl-2/JH Rearrangements in benign lymphoid tissues with follicular hyperplasia. Oncogene. 1991;6:2271–6. [PubMed] [Google Scholar]
- 10.Liu Y, Hernandez AM, Shibata D, Cortopassi GA. BCL2 translocation frequency rises with age in humans. Proc Natl Acad Sci USA. 1994;91:8910–4. doi: 10.1073/pnas.91.19.8910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bell DA, Liu Y, Cortopassi GA. Occurrence of bcl-2 oncogene translocation with increased frequency in the peripheral blood of heavy smokers. J Natl Cancer Inst. 1995;87:223–4. doi: 10.1093/jnci/87.3.223. [DOI] [PubMed] [Google Scholar]
- 12.Ji W, Qu G, Ye P, Zhang XY, Halabi S, Ehrlich M. Frequent detection of bcl-2/JH translocations in human blood and organ samples by a quantitative polymerase chain reaction assay. Cancer Res. 1995;55:2876–82. [PubMed] [Google Scholar]
- 13.Dölken G, Illerhaus G, Hirt C, Mertelsmann R. BCL-2/JH-rearrangements in circulating B cells of healthy blood donors and patients with nonmalignant diseases. J Clin Oncol. 1996;14:1333–44. doi: 10.1200/JCO.1996.14.4.1333. [DOI] [PubMed] [Google Scholar]
- 14.Summers KE, Goff LK, Wilson AG, Gupta RK, Lister TA, Fitzgibbon J. Frequency of the Bcl-2/IgH rearrangement in normal individuals: implications for the monitoring of disease in patients with follicular lymphoma. J Clin Oncol. 2001;19:420–4. doi: 10.1200/JCO.2001.19.2.420. [DOI] [PubMed] [Google Scholar]
- 15.Roulland S, Lebailly P, Lecluse Y, Briand M, Pottier D, Gauduchon P. Characterization of the t(14;18) BCL2-IGH translocation in farmers occupationally exposed to pesticides. Cancer Res. 2004;64:2264–9. doi: 10.1158/0008-5472.can-03-3604. [DOI] [PubMed] [Google Scholar]
- 16.Roulland S, Lebailly P, Lecluse Y, Heutte N, Nadel B, Gauduchon P. Long-term clonal persistence and evolution of t(14;18)-bearing B cells in healthy individuals. Leukemia. 2006;20:158–62. doi: 10.1038/sj.leu.2404035. [DOI] [PubMed] [Google Scholar]
- 17.Schüler F, Dölken L, Hirt C, et al. Prevalence and frequency of circulating t(14;18)-MBR translocation carrying cells in healthy individuals. Int J Cancer. 2009;124:958–63. doi: 10.1002/ijc.23958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.John U, Greiner B, Hensel E, et al. Study of Health In Pomerania (SHIP): a health examination survey in an east German region: objectives and design. Soz Praventivmed. 2001;46:186–94. doi: 10.1007/BF01324255. [DOI] [PubMed] [Google Scholar]
- 19.Dölken L, Schüler F, Dölken G. Quantitative detection of t(14;18)-positive cells by real-time quantitative PCR using fluorogenic probes. BioTechniques. 1998;25:1058–64. doi: 10.2144/98256cr05. [DOI] [PubMed] [Google Scholar]
- 20.Fuscoe JC, Setzer RW, Collard DD, Moore MM. Quantification of t(14;18) in the lymphocytes of healthy adult humans as a possible biomarker for enviromental exposure to carcinogens. Carcinogenesis. 1996;17:1013–20. doi: 10.1093/carcin/17.5.1013. [DOI] [PubMed] [Google Scholar]
- 21.Rauzy O, Galoin S, Chale JJ, et al. Detection of t(14;18) carrying cells in bone marrow and peripheral blood from patients affected by non-lymphoid diseases. Mol Pathol. 1998;51:333–8. doi: 10.1136/mp.51.6.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmitt C, Balogh B, Grundt A, et al. The bcl-2/IgH rearrangement in a population of 204 healthy individuals: occurrence, age and gender distribution, breakpoints, and detection method validity. Leuk Res. 2006;30:745–50. doi: 10.1016/j.leukres.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 23.Baccarelli A, Hirt C, Pesatori AC, et al. t(14;18) translocations in lymphocytes of healthy dioxin-exposed individuals from Seveso, Italy. Carcinogenesis. 2006;27:2001–7. doi: 10.1093/carcin/bgl011. [DOI] [PubMed] [Google Scholar]
- 24.Alexander DD, Mink PJ, Adami HO, et al. The non-Hodgkin lymphomas: a review of the epidemiologic literature. Int J Cancer. 2007;120(Suppl 12):1–39. doi: 10.1002/ijc.22719. [DOI] [PubMed] [Google Scholar]
- 25.Morton LM, Hartge P, Holford TR, et al. Cigarette smoking and risk of non-Hodgkin lymphoma: a pooled analysis from the International Lymphoma Epidemiology Consortium (interlymph) Cancer Epidemiol Biomarkers Prev. 2005;14:925–33. doi: 10.1158/1055-9965.EPI-04-0693. [DOI] [PubMed] [Google Scholar]
- 26.Parker AS, Cerhan JR, Dick F, et al. Smoking and risk of non-Hodgkin lymphoma subtypes in a cohort of older women. Leuk Lymphoma. 2000;37:341–9. doi: 10.3109/10428190009089434. [DOI] [PubMed] [Google Scholar]
- 27.Stagnaro E, Tumino R, Parodi S, Crosignani P, Fontana A, Masala G, Miligi L, Nanni O, Ramazzotti V, Rodella S, Senoiri CA, Vigano C, et al. Non-Hodgkin's lymphoma and type of tobacco smoke. Cancer Epidemiol Biomarkers Prev. 2004;13:431–7. [PubMed] [Google Scholar]
- 28.Chiu BC, Dave BJ, Blair A, et al. Cigarette smoking, familial hematopoietic cancer, hair dye use, and risk of t(14;18)-defined subtypes of non-Hodgkin's lymphoma. Am J Epidemiol. 2007;165:652–9. doi: 10.1093/aje/kwk044. [DOI] [PubMed] [Google Scholar]
- 29.Chang CM, Schroeder JC, Olshan AF, et al. A case-control study of tobacco use and other non-occupational risk factors for lymphoma subtypes defined by t(14; 18) translocations and bcl-2 expression. Cancer Causes Control. 2010;21:1147–54. doi: 10.1007/s10552-010-9531-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang CM, Wang SS, Dave BJ, et al. Risk factors for non-Hodgkin lymphoma subtypes defined by histology and t(14;18) in a population-based case-control study. Int J Cancer. 2011;129:938–47. doi: 10.1002/ijc.25717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schroeder JC, Olshan AF, Baric R, et al. A case-control study of tobacco use and other non-occupational risk factors for t(14;18) subtypes of non-Hodgkin's lymphoma (United States) Cancer Causes Control. 2002;13:159–68. doi: 10.1023/a:1014397920185. [DOI] [PubMed] [Google Scholar]
- 32.Agopian J, Navarro JM, Gac AC, et al. Agricultural pesticide exposure and the molecular connection to lymphomagenesis. J Exp Med. 2009;206:1473–83. doi: 10.1084/jem.20082842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cramer DW, Finn OJ. Epidemiologic perspective on immune-surveillance in cancer. Curr Opin Immunol. 2011;23:265–71. doi: 10.1016/j.coi.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morton LM, Wang SS, Devesa SS, Hartge P, Weisenburger DD, Linet MS. Lymphoma incidence patterns by WHO subtype in the United States, 1992-2001. Blood. 2006;107:265–76. doi: 10.1182/blood-2005-06-2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Online Publication: Additional Supplemental may be found in the online version of this article:
Table SI: t(14;18) prevalence according to age, sex and smoking status
Table SII: Logistic regression models analyzing the association of t(14;18) prevalence with age, sex and measures of cigarette smoking exposure
Table SIII: Negative binomial regression models of the association of t(14;18) frequency in t(14;18)-positive individuals with sex, age and measures of smoking
Figure S1: Multivariable logistic regression models of t(14;18) prevalence and sex, age and smoking (pack years continuous (S1A) or categorized (S1B) or smoking status (S1C))
Figure S2: Bivariate analyses of t(14 ;18) frequency according to sex (Figure S2A) and smoking status (Figure S2B).
Figure S3: Multivariable analysis of the association between t(14;18) frequency in t(14;18)-positive subjects and sex, age and measures of smoking (pack years continuous (S3A) or categorized (S3B) or smoking status (S3C)).