Abstract
MicroRNA-21 (miR-21) is recognized as an oncomir and shows up-regulation in many types of human malignancy. The aim of this study was to investigate the association of miR-21 expression associated with HPV infection in normal and abnormal cervical tissues. Cervical tissue samples with different cytological or histopathological grades were investigated for HPV by PCR and for miR-21 and programmed cell death, protein 4 (PDCD4) expression using quantitative real-time PCR (qRT-PCR). Laser capture microdissection (LCM) of stromal and epithelial tissues and in situ hybridization (ISH) using locked nucleic acid (LNA) probes were performed on a subset of fixed specimens. Cell line experiments were conducted on fibroblasts stimulated in culture media from HeLa cells, which were then assessed for miR-21, PDCD4, IL-6 and α-SMA expression by qRT-PCR. Twenty normal cervical cell, 12 cervicitis, 14 cervical intraepithelial neoplastic I (CIN I), 22 CIN II-III and 43 cervical squamous cell carcinoma (SCC) specimens were investigated. miR-21 levels were significantly lower in normal than in abnormal tissues. The expression of miR-21 in HPV negative normal cytology was significantly lower than in HPV positive samples in abnormal tissue and SCC. The miR-21 expression was significantly higher in HPV negative cervicitis than HPV negative normal cells. LCM and ISH data showed that miR-21 is primarily expressed in the tumor-associated stromal cell microenvironment. Fibroblasts treated with HeLa cell culture media showed up-regulated expression of miR-21, which correlated with increased expression of α-SMA and IL-6 and with down-regulation of PDCD4. These results demonstrate that miR-21 is associated with HPV infection and involved in cervical lesions as well as cervicitis and its up-regulation in tumor-stroma might be involved in the inflammation process and cervical cancer progression.
Introduction
Cervical cancer is the second most common malignancy in women worldwide. High-risk human papillomavirus (HR-HPV) infection is recognized as the most important risk factor. Chronic over-expression of the HPV E6 and E7 oncogenes promote tumor progression by inducing genetic and epigenetic instability [1–3].
Epigenetic instability is impacted by microRNAs (miRNA or miR-). miR- dysregulation is associated with a wide variety of human malignancies. Through 3’-UTR binding of the target-mRNA, miRs repress gene translation [4]. Several miRs are reported to be involved in cervical cancer such as miRs-21, -23b, -34a, -143, -146a, -218 and miR-182; these miRs play crucial roles in cervical cell proliferation, differentiation and apoptosis [5–13].
miR-21 is a regulator of gene expression at the post-transcriptional level, and is increased in many types of human malignancies including colon [14], pancreatic [15], breast, prostate [16], oral [17], ovarian [18] and cervical [7–9, 19] cancers. miR-21 is known to promote cell proliferation [7] and initiate inflammation-associated carcinogenesis via nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB) and interleukin-6 (IL-6) signaling pathways in colon and cervical cancer cells [20, 21]. The genes of programmed cell death, protein 4 (PDCD4) [7, 19] and phosphatase and tensin homolog (PTEN), are known as the targets of miR-21 [16]. The regulatory region of miR-21 gene consists of several binding sites for transcription factors such as activator protein 1 (AP-1), and signal transducer and activator of transcription 3 (STAT3) [20].
miR-21 expression is up-regulated in cervical cancer cell lines, especially in HPV16-positive CaSki cells and HPV18-positive HeLa cells [8, 9]. The up-regulation of miR-21 promotes cell proliferation and down-regulation of PDCD4 [7]. Moreover, miR-21 qRT-PCR expression levels correlate with the histological staging of cervical cancer, and so might be used as a screening marker [19]. High miR-21 levels have been reported in the tumor-stromal compartment of colorectal cancers: miR-21 expression is associated with the progression and short disease-free survival in patients with stage II colon cancer [14, 22]. The importance of tumor-stromal fibroblast expression patterns in tumor progression is well recognized; for example, stromal fibroblasts promote tumor growth and angiogenesis in pancreatic cancer [23] and invasive human breast carcinomas [24]. The aberrant expression of miR-21 in tumor-stromal cells may therefore be associated with carcinogenesis [25].
Fibroblasts are the major component cell type in connective tissues. They are involved in the production of growth factors, chemokines and the extracellular matrix and differentiate to perform altered functions in response to cytokines and other protein signals. IL-6 is a pleiotropic cytokine that is involved in several stages of tumor development and can mediate epithelial-stromal interactions [26]. IL-6 up-regulation has been implicated as a significant element in cervical cancer pathogenesis by a number of studies [27–33]. IL-6 has been frequently detected in the stromal region of cervical cancer tissues containing a large number of fibroblasts [21, 34]. Additionally, IL-6 positive cells in the stromal cells show positive staining for the fibroblast marker α-smooth muscle actin (α-SMA) [26]. The α-SMA together with IL-6 and transforming growth factor-β (TGF-β) are involved in (myofibroblast differentiation by up-regulation of miR-21 by interaction with PDCD4 in the tumor-stroma [25].
To date, there have been few studies of miR-21 in cervical cancer [7–9, 19]; the aim of the present study was the investigation of miR-21 in cervical carcinogenesis in relation to HPV infection and apoptotic functioning. miR-21 expression in normal cervical cells and fresh cervical tissues with different histopathological grades was evaluated using quantitative real-time PCR (qRT-PCR). The localization of miR-21 expression was investigated in formalin-fixed, paraffin-embedded (FFPE) sections by qRT-PCR comparison of cervical epithelium and stromal region RNA extracts isolated by laser capture microdissection (LCM) by qRT-PCR and by in situ hybridization (ISH). HPV status in fresh biopsies was demonstrated by PCR. Additionally, the induction of miR-21 in fibroblasts was investigated by treatment of the amniotic fibroblasts with culture medium from HeLa cells (HeLa CM) and TGF-β1 following: α-SMA, miR-21, PDCD4 and IL-6 levels were measured by qRT-PCR.
Materials and Methods
Patients and tissues
Cervical samples including normal cervical cells and cervical tissues were collected from women who underwent routine cervical cancer investigation by Papanicolaou (Pap) smear testing in combination with colposcopy at Srinagarind Hospital and Khon Kaen Hospital, Khon Kaen, Thailand and provided their written informed consent to participate in previous projects approved by the Human Research Ethics Committee of Khon Kaen University and Khon Kaen Hospital. This work used leftover specimens from the previous projects and was approved by the Human Research Ethics Committee of Khon Kaen University (HE541168). The requirement for written informed consent was waived by the committee for reasons including the use of archival specimens collected prior to study initiation. The specimens were grouped according to the histological diagnosis reviewed by the pathologist. Samples used in this study consisted of normal cytology diagnosed by Pap smear (20 samples), fresh cervical tissues obtained at colposcopy (91 samples) and FFPE cervical tissues (70 samples). The fresh cervical tissues (91 samples) were classified histopathologically into cervicitis (12 cases), cervical intraepithelial neoplasia-I (CIN I) (14 cases), CIN II-III (22 cases) and cervical squamous cell carcinoma (SCC) (43 cases). The FFPE cervical tissues consisted of cervititis (n = 26), CIN I (n = 11), CIN II-III (n = 21) and SCC (n = 12).
RNA and DNA extraction
RNA and DNA were extracted from normal cervical cells, cervical tissues and FFPE samples (cervical lesions and stromal region) using the Trizol reagent kit (Ambion, Life Technology, Carlsbad, CA, USA) according to manufacturer’s instructions. For RNA extraction from FFPE, tissues were deparaffinized with xylene and rehydrated with serial ethanol and air-dried before experiments. Cells and tissues samples were mixed with Trizol reagent, homogenized and incubated for 5 min at 15–30°C. Chloroform (0.2 ml) was added to the homogenized solution (per 1 ml) and then the sample tubes were shaken for 2–3 min at 15–30°C and centrifuged at 12,000x g for 15 min at 2–8°C. The aqueous phase was transferred to a fresh tube and the organic phase was collected for DNA isolation. For precipitation of the RNA, isopropyl alcohol (0.5 ml) was added to 1 ml Trizol aqueous phase, mixed, incubated for 10 min at 15–30°C and centrifuged at 12,000x g for 10 min at 2–8°C. The supernatant was removed and with 1 ml of 75% ethanol per 1 ml Trizol, was added to wash RNA pellet by vortexing and centrifugation at 7,500x g for 5 min at 2–8°C. Finally, RNA pellets were dried for 10 min at room temperature (RT). RNA was eluted with 30 μl of RNase-free water, incubated for 10 min at 55°C and kept at -70°C until used.
The organic phase from the previous step was used for isolation of DNA according to the manufacturer’s instructions. The organic phase was mixed with 0.3 ml ethanol per 1 ml Trizol and DNA was mixed and precipitated by incubation for 15–30°C for 2–3 min and centrifuged at 4,000x g for 5 min at 2–8°C. The phenol-ethanol supernatant was removed. Then, 1 ml 0.1 M sodium citrate was added for washing DNA. The DNA pellet was kept in the washing solution for 30 min at 15–30°C and centrifuged at 4,000x g for 5 min at 2–8°C. Next, the DNA pellet was air dried for 15 min, 50 μl of 8 mM NaOH added and stored at -20°C until used.
HPV DNA detection by polymerase chain reaction (PCR)
The detection of HPV DNA from normal cervical cells, fresh cervical biopsies, and FFPE samples were processed with PCR using GP5+/6+ primers for detection of Late gene 1 (L1) and PCO4/GH2O primers for detection of the β-globin gene (Table 1). The PCR master mix included; 1x PCR buffer, 3.5 mM MgCl2, 0.2 mM dNTP, 0.4 mM GP5+ primer, 0.4 mM GP6+ primer [35], Taq DNA polymerase (Thermo scientific, Pittsburgh, PA, USA), DNA template 50–100 ng and distilled water to 25 μl. The PCR condition was as follows; pre-denaturation 5 min at 94°C and 40 cycles of 1 min denaturation at 94°C, 1 min annealing at 42°C, 1 min extension at 72°C and then 4 min final extension at 72°C. The positive cases of HPV DNA were identified by the detection of an L1 PCR product of approximately 150 bp size. The β-globin gene as an internal control was approximately 268 bp sizes [36]. The PCR product was detected using 2.0% agarose gel electrophoresis in 0.5x TAE buffer at 100 V for 27 min.
Table 1. Primer sequences.
| Gene | Primer sequence | Amplicon (bp) | Ref. |
|---|---|---|---|
| HPV L1 | Forward: 5’-TTTGTTACTGTGGTAGATAC TAC-3’ | 150 | 35 |
| Reverse: 5’-GAAAAATAAACTGTAAATCATATTC-3' | |||
| β-globin | Forward: 5′-GAAGAGCCAAGGACAGGTAC-3’ | 268 | 36 |
| Reverse: 5′-CAACTTCATCCACGTTCACC-3’ | |||
| PDCD4 | Forward: 5’-GATTAACTGTGCCAACCAGTCCAAAG-3’ | 150 | 37 |
| Reverse: 5’-CATCCACCTCCTCCACATCATACAC-3’ | |||
| IL-6 | Forward: 5’-CTTCGGTCCAGTTGCCTTCT-3’ | 86 | |
| Reverse: 5’-TGGAATCTTCTCCTGGGGGT-3’ | |||
| α-SMA | Forward: 5’-AGGTAACGAGTCAGAGCTTTGGC-3’ | 199 | 25 |
| Reverse: 5’-CTCTCTGTCCACCTTCCAGCAG-3’ | |||
| GAPDH | Forward: 5’-TCATCAGCAATGCCTCCTGCA-3’ | 117 | 38 |
| Reverse: 5’-TGGGTGGCAGTGATGGCA-3’ |
Quantitative real-time RT-PCR analysis
TaqMan MicroRNA Reverse transcription reactions and TaqMan MicroRNA quantitative polymerase chain reactions (qPCR) were performed to detect miR-21 and an endogenous control, RNA U6 small nuclear (RNU6B) expression using the MicroRNA TaqMan Reverse Transcription Kit and the TaqMan MicroRNA Assays (Applied BioSystems, Carlsbad, CA, USA) according to manufacturer’s instructions. The miRNA detection conditions were: 95°C for 10 min and 40 cycles of 95°C for 15 s, 60°C for 1 min. The miRNA expression levels were calculated as the cycle threshold (-delta CT) of miR-21 and normalized with an endogenous control. The extracted RNA from the HeLa cell line was used as a positive control. Reverse transcription (RT) reactions and quantitative polymerase chain reactions (qPCR) were performed using the Super Script VILO Synthesis Kit and the SsoAdvanced SYBR Green Supermix (Bio-Rad, Hercules, CA, USA). The amount of mRNA targeting gene PDCD4 [37], signal transducer IL-6 and fibroblast differentiation marker α-SMA were detected (Table 1). The expression of mRNA was normalized to the level of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) [38] and calculated as the cycle threshold (2-delta delta CT) of the mRNA detection. The conditions were: 95°C for 1 min and 40 cycles of 95°C for 10 s, 60°C for 1 min.
MicroRNA in situ hybridization
Six μm-thick paraffin sections were mounted on Superfrost glass slides and de-paraffinized. The steps of in situ hybridization were as follows: Pre-hybridization step; the slides were treated with 15 μg/ml proteinase-K for 7.5 min at 37°C, then immersed into 3% hydrogen peroxide (H2O2)/phosphate buffered saline (PBS) for peroxidase blocking for 15 min and dehydrated in new ethanol solutions. Hybridization step; 50–100 μl hybridization mix 50 nM double-DIG LNA microRNA-21 probe (positive control test: 1 nM LNA U6 snRNA probe; negative control test 50 nM Scramble probe) (Exqion, Vedbeak, Denmark) was applied onto the slides. The slides were incubated overnight at 56°C (StatSpin ThermoBrite oven, Abbott Molecular Abbott Park, IL, USA), then washed twice with 5x SSC buffer for 5 min at RT; slides were then treated by 5 min stringent wash steps at 56°C in 1x SSC buffer twice followed by 0.2x SSC buffer once. Then, the slides were immersed into 0.2x SSC buffer for 5 min at RT, and incubated with blocking solution (Roche, Mannheim, Germany) containing 2% sheep serum in a humidifying chamber at RT for 15 min. Sheep anti-DIG-horseradish peroxidase (HRP) conjugate (Roche, Mannheim, Germany) was applied diluted at 1:50 for 15 min at RT. Digoxogenin-labeled tyramide [39] was then applied at 1:50 dilution for 30 min at RT, followed by the incubation with sheep anti-DIG-HRP conjugate at 1:100 for 30 min at RT. The hybridization signal was demonstrated using the HRP substrate 3-amino-9-ethylcarbazole (AEC) (Dako, Carpinteria, CA, USA) for 5 min, incubated at RT and counterstained with hematoxylin for 5 min at RT. Finally, the slides were mounted directly with 1–2 drops of aqua mount (Polysciences, Warrington, PA, USA) and air-dried. The image analysis was performed using a Leica microscope (Leica, Herlev, Denmark) fitted with 20x-60x objectives.
Laser capture microdissection (LCM)
Two or three sections of 3–10 μm-thick FFPE cervical tissues were mounted onto a slide under RNase-free conditions. The procedures were as follows: a deparaffinization step by serial immersion through xylene and graded ethanol washes followed by staining with hematoxylin and eosin; tissue fraction step by selection of 20–100 x 103 μm2 (4–6 areas) of tumor stromal or cervical epithelium/cancer cells isolated into separate cups using the PALM Carl Zeiss MicroImaging Laser Capture Microdissection system (Carl Zeiss Microscopy, Jena, Germany) and used for total RNA extraction using a Trizol reagent kit (Ambion Life Technology, Carlsbad, CA, USA) as described above.
Treatment of fibroblasts
The HPV18-positive HeLa cell line was cultured in DMEM medium (Gibco, life technology, Grand Island, NY, USA) for 48 h. Human primary fibroblasts were sequestered from leftover amniotic fluid cell culture taken by amniocentesis and used for evaluation of prenatal diagnosis of chromosomal abnormalities. The fibroblasts were taken anonymously following laboratory report without any identifying information and without a link to a specific participant or donor. The fibroblasts were subcultured and used after growing more than 95% confluent under microscope. The procedures were approved by the Human Research Ethics Committee of Khon Kaen University (HE571157). The requirement for written informed consent was waived by the committee for reasons including the use of leftover specimens without any identifying information and without a link to a specific participant or donor. These cells were cultured at 37°C in a 5% CO2 atmosphere and were treated with culture media (CM) from HeLa cell line. The fibroblast differentiation was evaluated using α-SMA as the differentiated marker by qRT-PCR. The expression of miR-21 and the functional target gene PDCD4 were measured using qRT-PCR at 24–48 h after treatment with CM and 2 ng/ml recombinant human TGF-β1 (Gibco/Invitrogen, Carlsbad, CA, USA) [25]. In addition, IL-6 that acts as signal transducer of miR-21 and during myofibroblast conversion was examined using qRT-PCR. To confirm the expression of miR-21 in fibroblasts, exosome was isolated from 100 ml HeLa CM and fetal bovine serum (FBS) by ultracentifugation twice at 70,000x g for 30 min as well as form HeLa cell lysate. The exosome pellet was determined for CD63 (exosome marker) and cytochrome C (cellular marker) by western blot using anti-CD63 antibody (clone ab8219, Abcam, Cambridge, England) and anti-cytochrome C antibody (clone ab110325, Abcam, Cambridge, England). The miRNA in the isolated exosome was detected by qRT-PCR.
Statistical analysis
Data were expressed as mean ± S.D. The results were analyzed with one-way ANOVA or Kruskal-Wallis test for differences among groups of multi-data. The differences between two groups were evaluated by the Student’s t-test or Mann-Whitney U test. The relative expressions of miR-21 in cervical tissues were calculated by the equation:-delta CT = —(CTmiR-21-CTU6). The relative expression of RNA in the in vitro study was calculated as the 2-delta delta CT, after normalizing with the housekeeping gene (GAPDH) and relative to the untreated control. Statistical significance was considered a P-value <0.05 using SPSS software.
Results
HPV infection and miR-21 expression in cervical tissues
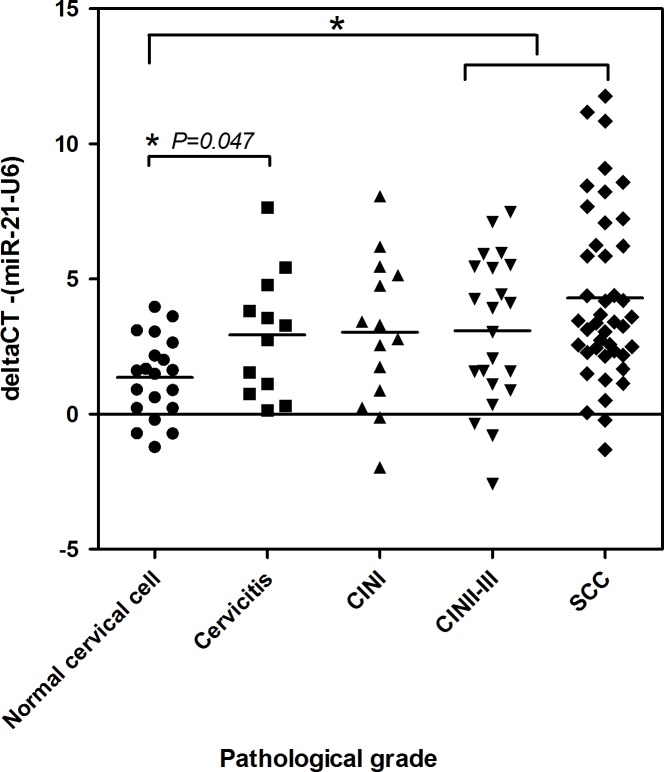
A total of 111 fresh specimens were available for miR-21 expression analysis. These specimens included 20 normal cervical cell, 12 cervicitis, 14 CIN I, 22 CIN II-III, and 43 SCC samples. The expression levels of miR-21 calculated as ∆CT were lower in normal cervical cells (Mean = 1.35±S.D. = 1.49) than in CIN I (3.03±2.75), CIN II-III (3.09±2.73) and SCC (4.30±3.15) (P = 0.052, P = 0.014 and P = 0.000, respectively) (Fig 1).
Fig 1. The miR-21 expression levels of fresh cervical biopsy samples in relation to histological grading.
miR-21 expression levels were measured by qRT-PCR. Significant differences between pathological grades and miR-21 expression (-delta CT) were found at P = 0.005. The expression levels of miR-21 were significantly lower in normal cervical cells than in cervicitis, CIN II-III and SCC (*Student’s T-Test, P = 0.025, P = 0.014 and P = 0.000).
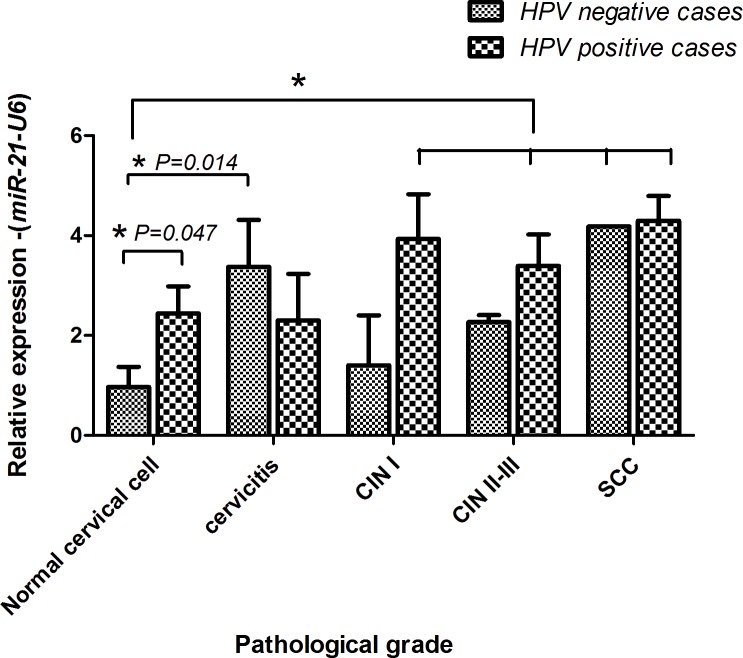
HPV DNA was detected in 40% (8/20 cases) of normal cervical cells, 50% (5/12 cases) of cervicitis, 64.2% (9/14 cases) of CIN I, 72.7% (16/22 cases) of CIN II-III and 79% (34/43 cases) of SCC. The expression of miR-21 in normal cervical cells was significantly higher in HPV positive cases (1.93±1.52) than HPV negative cases (0.97±1.40, P = 0.047). In addition, the expression of miR-21 in HPV negative normal cytology (0.97±1.40) was significantly lower than HPV positive samples in worsening histopathological grades (Kruskal-Wallis Test, P = 0.003); CIN I (3.93±2.68, P = 0.011); CIN II-III (3.39±2.50, P = 0.003) and SCC (4.37±3.24, P = 0.000) (Fig 2). HPV positive cases of CINs and SCC showed higher levels of miR-21 than HPV negative cases, but not significantly (Fig 2). A significant down-regulation of PDCD4, which is the targeting gene of miR-21, was found and associated with the up-regulation of miR-21 status (P = 0.000).
Fig 2. The characteristics of miR-21 expression in cervicitis, CIN I, CIN II-III and SCC.
The level of transcriptional change was examined by qRT-PCR. HPV positive rates and miR-21 expression levels in relation to cytological stages. (*: significant difference from control at P<0.05)
miR-21 expression in cervicitis
The expression of miR-21 was up-regulated in cervicitis (2.93±2.29) and significantly higher than normal cervical cells (1.35±1.49, P = 0.025) (Fig 1). Interestingly, the level of miR-21 was higher in HPV negative cervicitis (3.37±2.49) than HPV negative normal cervical cells (0.97±1.39, P = 0.014) whereas no differences were found between the HPV positive cases of normal cervical cells and cervicitis cases (Fig 2).
ISH detection for the miR-21 in the cervical cancer tissues
To understand the up-regulation of miR-21 expression in cervical cancer tissues, 5 cases of FFPE SCC samples were sectioned and miR-21 expression was detected by ISH. The results showed that the miR-21 expression was predominantly detected in tumor associated stroma of SCC. In the cancer tissues, positive signals were seen mostly at the invasive front near the stroma (Fig 3A and 3B). Fig 3C shows staining of tumor and stromal nuclei for RNU6. Fig 3D shows negative staining with a scramble probe.
Fig 3. The localization of miR-21 in invasive cervical cancer tissues.
A and B: tissue sections from cervical cancer samples were incubated with DIG-labeled locked nuclei acid (LNA) probe to miR-21. Strong signals were observed in the stroma. The signal was also seen at the rim of cancer cells adjacent to the stroma (Bar: 100 μm). C: RNU6B staining in cervical cancer and stromal nuclei (Bar: 50 μm). D: cervical cancer negative control staining using a ‘scramble’ LNA probe (Bar: 50 μm).
Localization of miR-21 expression in FFPE cervical tissues
To confirm ISH observations, the expression of miR-21 in the cancer cells and the stroma were separately measured in all 70 FFPE cervical tissue samples including cervicitis tissues using qRT-PCR. The results showed that the miR-21 was up-regulated in the tumor-associated stroma of CIN II-III and SCC, corresponding with the histopathological grades. The results showed that the expression level of miR-21 in tumor (-3.19±5.75) was less than in tumor-stroma (3.00±1.4, P = 0.045) (Table 2).
Table 2. Comparative expressions of miR-21 in the epithelial and stromal regions of 70 FFPE cervical tissues.
| miR-21 (-∆CT) N = 70 | Cervicitis | CIN I | CIN II-III | SCC |
|---|---|---|---|---|
| N = 26 | N = 11 | N = 21 | N = 12 | |
| Epithelium | -0.40±4.02 | -0.39±6.64 | -0.35±3.05 | -3.19±5.75 |
| Stroma | 1.68±1.83 | -1.60±4.10 | 1.75±1.16 | 3.00±1.44 |
| P-value | 0.236 | 0.892 | 0.244 | 0.045 |
Cervical intraepithelial neoplasia I (CIN I), Cervical intraepithelial neoplasia II and III (CIN II-III), cervical squamous cell carcinoma (SCC). A significant difference was found between epithelium and stroma of SCC (P<0.05).
miR-21 expression in the activated fibroblasts
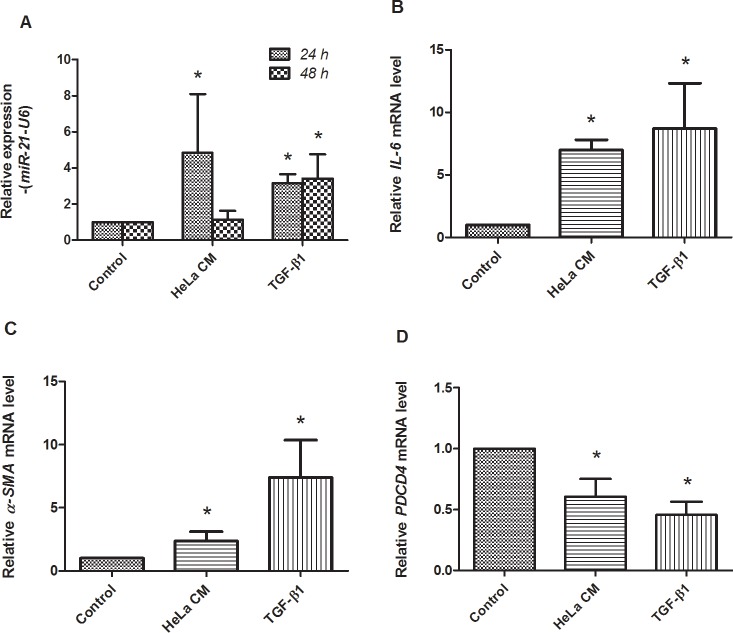
Culture media from HPV positive HeLa cells (HeLa CM) is involved in the activation of miR-21 over-expression in fibroblasts [25]. To investigate the role of HPV infection on the up-regulation of miR-21 in the stromal cells, fibroblasts were treated with HeLa CM and TGF-β1 was used as the positive control. The levels of miR-21 expression were measured by qRT-PCR at 24–48 h. The results are shown in Fig 4. After 24-h treatment with HeLa CM and TGF-β1, the miR-21 levels were significantly 4.85- and 3.15-fold higher than untreated controls (P = 0.029 and 0.029) (Fig 4A). A decrease in miR-21 expression was found in fibroblasts at 48 h after HeLa CM treatment. The IL-6 expression as a signal transducer of miR-21 [26] was also determined in the treated fibroblasts. Fig 4B shows the levels of IL-6 mRNA expression that are significantly up-regulated by 6.99 and 8.71 fold (P = 0.002 and 0.024) in fibroblasts treated with HeLa CM and TGF-β1, for 48 h, compared to the untreated controls. These results demonstrated that the up-regulation of miR-21 was associated with IL-6 expression. In addition, α-SMA, a myofibroblast differentiation marker was also up-regulated by 2.35 and 7.40 folds (P = 0.002 and P = 0.010) at 48 h in fibroblasts treated with HeLa CM and TGF-β1 (Fig 4B and 4C). The myofibroblasts, which are known to express miR-21 and drive tumorigenesis [25], are induced by numerous cytokines including TGF-β. To confirm the expression of miR-21 in the treated fibroblasts, the levels of PDCD4 expression were investigated. The results showed that PDCD4 expression was significantly down-regulated as shown in Fig 4D. Fig 5 shows morphology of the treated fibroblasts at 24 and 48 h after treatment. These data demonstrated the up-regulation of miR-21 in myofibroblasts that may be associated with HPV infected cervical cells. This result supported that miR-21 was detected in HeLa cells but not in exosome extracted from HeLa CM and fetal bovine serum (FBS). In addition, we also detected miR-21 expression in fibroblasts treated with culture media from HeLa cultured in exosome-depleted FBS media (10% exosome-free FBS in DMEM) and the result was quite similar to the experiment using culture media from HeLa cultured in conventional cell culture media (data not shown). This result confirmed that miR-21 was not from HeLa CM but was expressed in the HeLa CM-treated fibroblasts.
Fig 4. The characteristics of stromal fibroblasts after treatment with culture media from HeLa cells (HeLa CM) and TGF-β1.
The level of transcriptional change was examined by real-time RT-PCR. A: the expression of miR-21 was significantly up-regulated at 24 and 48 h after HeLa CM or TGF-β1 treatment. B and C: the expression of IL-6 and alpha-SMA was significantly up-regulated at 48 h after HeLa CM or TGF-β1 treatment. D: PDCD4 was significantly down-regulated at 48 h after HeLa CM or TGF-β1 treatment. (*: significant differences from control at P<0.05)
Fig 5. The morphology of fibroblasts treated with culture media from HeLa cells.
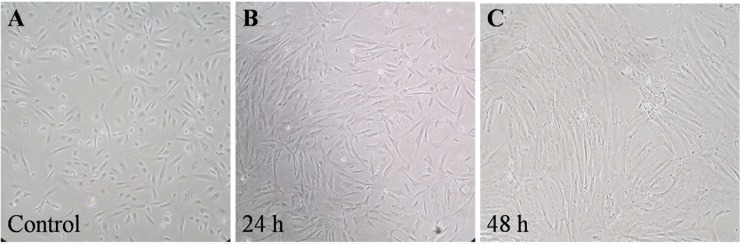
HeLa cells were cultured in exosome-depleted media. The treated cells were elongated.
Discussion
miR-21 is a target of considerable interest because of its up-regulation demonstrated by qRT-PCR in a variety of human cancers including cervical tumors [7–9, 14, 16, 19, 25]. In this study, miR-21 expression in normal, cervicitis, CIN and SCC was investigated by combining qRT-PCR, microdissection and in situ hybridization. HPV infections were also investigated. The relationship of miR-21 to α-SMA, IL-6 and PDCD4 expression was examined in a fibroblast cell differentiation model.
In this study, prevalence of HPV in normal was quite high (40%) that may be affected from a small sample size, sampling method, specimen collection and HPV detection technique. The cervical cell samples were taken from women who underwent cervical cancer screening and collected in ThinPrep test vials. HPV detection was performed by PCR using Q5 High-Fidelity DNA Polymerase which is ideal for difficult amplicons.
The results showed the up-regulation of miR-21 corresponded with histological grades when compared with HPV negative normal cytology; this finding is similar to a study by Deftereos et al. who found that miR-21 expression had a significant correlation with histological changes ranging from normal to a worsening histological diagnosis of cervical cancer [19]. In these experiments, the expression of miR-21 was shown to be significantly associated with HPV infection. In addition, the studies found that the expression of miR-21 was significantly up-regulated in the transition from normal to cervicitis corresponding with down-regulation of PDCD4. This may represent an important event in the role of miR-21 in carcinogenesis and an inflammatory response such as: First, by down-regulation of molecule PDCD4 [40] resulting in suppression of the inflammation process via transcriptional factor NF-kB and activated anti-inflammatory cytokine, interleukin 10 (IL-10) [41, 42] leading to immune evasion and cervical cancer progression. Second, by down regulated PTEN and activated Akt signaling pathways that increased NF-kB activity leading to inflammation as described by Lliopoulos et al., 2010 who studied colon cancer and suggested that epigenetic switch of untransformed cells to transformed cells links the inflammation to cancer [20]. The present studies demonstrate that the induction of miR-21 might be involved in HPV infection, and the inflammation process of cervicitis.
The microdissection/qRT-PCR and ISH data show for the first time that miR-21 expression in cervical tumors may occur primarily in the tumor-associated stromal microenvironment rather than directly in tumor cells themselves (Fig 3). This finding accords with recent colorectal cancer studies (that included ISH), which have found miR-21 up-regulation in the stromal compartment rather than in tumor cells, and associated with poor disease free survival [14]. The tumor-stromal cells of cervical cancer that expressed miR-21 generally showed fibroblast-like morphology. Most cancer associated fibroblasts constitute a heterogeneous cell population and differentiate to cells called myofibroblasts which promote tumor progression via production of growth promoting factors [23]. Fibroblasts may act on tumor cells through expression of growth factors, such as TGF-β1, and thereby contribute to the survival and proliferation of tumor cells. TGF-β1 activated the Erk-MAP kinase pathway leading to activation of AP-1 expression that acts as a putative activator of miR-21 promoter in stromal fibroblasts and in cancer cells of an advanced stage [25, 40, 43]. By qRT-PCR, this present study demonstrated that expression of miR-21 was up-regulated in fibroblasts treated with HeLa CM or TGF-β1 (Fig 4). However, a decrease of miR-21 was shown in fibroblasts treated with HeLa CM for 48 h. This result is similar to the result of the previous study [25]. It might be that there was a limited amount of TGF- β1 in HeLa CM compared with culture media supplemented with TGF-β1. Treatment with HeLa CM and TGF-β1 also showed significant up-regulation of α-SMA. This result suggested differentiation of fibroblasts to myofibroblasts (Fig 4). miR-21 might have a potential role in gene regulation during promotion of fibroblast differentiation. These findings suggest that up-regulation of miR-21 may contribute to the development of carcinogenesis.
The results of the current study also showed the over-expression of IL-6 mRNA in fibroblasts after treatment with HeLa CM and TGF-β1 (Fig 4). IL-6 mRNA was up-regulated during fibroblast differentiation observed by α-SMA detection. This suggested an involvement of the IL-6 signaling pathway in up-regulation of miR-21 in the stroma. Iglesias et al. showed that cervical fibroblasts constitutively secreted IL-6 in significantly greater amounts than normal cervical epithelial cells [44]. Kinoshita et al. reported that IL-6 mediates epithelial-stromal interactions and promotes gastric tumorigenesis [26]. Tartour et al. also showed that IL-6 protein detected by immunohistochemistry (IHC) was only found in the cervical stromal cells [34]. The results in this study confirm that up-regulation of miR-21 expression is mostly found in cervical stromal lesion. Wei et al. showed that IL-6 protein was expressed in most basal and parabasal cells of normal epithelium and invasive squamous cell carcinoma of the cervix by IHC [27]. The present study found the up-regulation of miR-21 in both stroma and the rim of cervical cancer cells near the stroma. These findings support the idea that fibroblasts are a significant source of miR-21 expression and IL-6 production, and HPV positive cervical cancer cells play a crucial role in the induction of IL-6 mRNA expression and up-regulation of miR-21 in stromal fibroblasts.
In these studies it was found that the expression of miR-21 was associated with HPV-infection in cervical lesions and cervicitis. In addition, the studies herein showed the expression of miR-21 in tumor-stromal of SCC and an association with fibroblast differentiation and warrants further studies.
Acknowledgments
We would like to thank Assist. Prof. Onanong Kritpetcharat, Mr. Piyawut Swangphon and Mr. Sulav Acharya for their help and suggestions. We would like to acknowledge Prof. James A. Will and Prof. Yukifumi Nawa for editing the manuscript via Publication Clinic KKU, Thailand.
Data Availability
All relevant data are within the paper.
Funding Statement
Financial support was from the Thailand Research Fund through the Royal Golden Jubilee Ph.D. Program (Grant No. PHD/0212/2550) to student S.B. and advisor C.P. The research was supported by Faculty of Medicine, Khon Kaen University (Grant No. I54225 and I57210); Khon Kaen University, Thailand (Grant No. 551601, 564101 and 573001) and Department of Pathology and Laboratory Medicine, University of Vermont, Burlington, VT, USA. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Lehoux M, D’Abramo CM, Archambault J. Molecular mechanisms of human papillomavirus-induced carcinogenesis. Public Health Genomics. 2009; 12: 268–280. 10.1159/000214918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Moody CA, Laimins LA. Human papillomavirus oncoproteins: pathways to transformation. Nat Rev Cancer. 2010; 10: 550–560. 10.1038/nrc2886 [DOI] [PubMed] [Google Scholar]
- 3. Jiménez-Wences H, Peralta-Zaragoza O, Fernández-Tilapa G. Human papilloma virus, DNA methylation and microRNA expression in cervical cancer (Review). Oncol Rep. 2014; 31: 2467–2476. 10.3892/or.2014.3142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Medina PP, Slack FJ. microRNAs and cancer: an overview. Cell Cycle. 2008; 7: 2485–2492 [DOI] [PubMed] [Google Scholar]
- 5. Lee JW, Choi CH, Choi JJ, Park YA, Kim SJ, Hwang SY, et al. Altered MicroRNA expression in cervical carcinomas. Clin Cancer Res. 2008; 14: 2535–2542. 10.1158/1078-0432 [DOI] [PubMed] [Google Scholar]
- 6. Rao Q, Shen Q, Zhou H, Peng Y, Li J, Lin Z. Aberrant microRNA expression in human cervical carcinomas. Med Oncol. 2012; 29: 1242–1248. 10.1007/s12032-011-9830-2 [DOI] [PubMed] [Google Scholar]
- 7. Yao Q, Xu H, Zhang QQ, Zhou H, Qu LH. MicroRNA-21 promotes cell proliferation and down-regulates the expression of programmed cell death 4 (PDCD4) in HeLa cervical carcinoma cells. Biochem Biophys Res Commun. 2009; 388: 539–542. 10.1016/j.bbrc.2009.08.044 [DOI] [PubMed] [Google Scholar]
- 8. Wang X, Tang S, Le SY, Lu R, Rader JS, Meyers C, et al. Aberrant expression of oncogenic and tumor-suppressive microRNAs in cervical cancer is required for cancer cell growth. PLoS One. 2008; 3: e2557 10.1371/journal.pone.0002557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lui WO, Pourmand N, Patterson BK, Fire A. Patterns of known and novel small RNAs in human cervical cancer. Cancer Res. 2007; 67: 6031–6043. [DOI] [PubMed] [Google Scholar]
- 10. Tang T, Wong HK, Gu W, Yu MY, To KF, Wang CC, et al. MicroRNA-182 plays an onco-miRNA role in cervical cancer. Gynecol Oncol. 2013; 129: 199–208. 10.1016/j.ygyno.2012.12.043 [DOI] [PubMed] [Google Scholar]
- 11. Wang X, Wang HK, McCoy JP, Banerjee NS, Rader JS, Broker TR, et al. Oncogenic HPV infection interrupts the expression of tumor-suppressive miR-34a through viral oncoprotein E6. RNA. 2009; 15: 637–647. 10.1261/rna.1442309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Au Yeung CL, Tsang TY, Yau PL, Kwok TT. Human papillomavirus type 16 E6 Induces cervical cancer cell migration through the p53/microRNA-23b/urokinase-type plasminogen activator pathway. Oncogene. 2011; 30: 2401–2410. 10.1038/onc.2010.613 [DOI] [PubMed] [Google Scholar]
- 13. Yue C, Wang M, Ding B, Wang W, Fu S, Zhou D, et al. Polymorphism of the pre-miR-146a is associated with risk of cervical cancer in a Chinese population. Gynecol Oncol. 2011; 122: 33–37. 10.1016/j.ygyno.2011.03.032 [DOI] [PubMed] [Google Scholar]
- 14. Nielsen BS, Jørgensen S, Fog JU, Søkilde R, Christensen IJ, Hansen U, et al. High levels of microRNA-21 in the stroma of colorectal cancers predict short disease-free survival in stage II colon cancer patients. Clin Exp Metastasis. 2011; 28: 27–38. 10.1007/s10585-010-9355-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hwang JH, Voortman J, Giovannetti E, Steinberg SM, Leon LG, Kim YT, et al. Identification of microRNA-21 as a biomarker for chemoresistance and clinical outcome following adjuvant therapy in resectable pancreatic cancer. PLoS One. 2011; 5: e10630 10.1371/journal.pone.0010630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Folini M, Gandellini P, Longoni N, Profumo V, Callari M, Pennati M, et al. miR-21: an oncomir on strike in prostate cancer. Mol Cancer. 2010; 9: 12 10.1186/1476-4598-9-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Reis PP, Tomenson M, Cervigne NK, Machado J, Jurisica I, Pintilie M, et al. Programmed cell death 4 loss increases tumor cell invasion and is regulated by miR-21 in oral squamous cell carcinoma. Mol Cancer. 2010; 9: 238 10.1186/1476-4598-9-238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chan JK, Blansit K, Kiet T, Sherman A, Wong G, Earle C, et al. The inhibition of miR-21 promotes apoptosis and chemosensitivity in ovarian cancer. Gynecol Oncol. 2014; 132: 739–744. 10.1016/j.ygyno.2014.01.034 [DOI] [PubMed] [Google Scholar]
- 19. Deftereos G, Corrie SR, Feng Q, Morihara J, Stern J, Hawes SE, et al. Expression of mir-21 and mir-143 in cervical specimens ranging from histologically normal through to invasive cervical cancer. PLoS One. 2011; 6: e28423 10.1371/journal.pone.0028423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Iliopoulos D, Jaeger SA, Hirsch HA, Bulyk ML, Struhl K. STAT3 activation of miR-21 and miR-181b-1 via PTEN and CYLD are part of the epigenetic switch linking inflammation to cancer. Mol Cell. 2010; 39: 493–506. 10.1016/j.molcel.2010.07.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ren C, Cheng X, Lu B, Yang G. Activation of interleukin-6/signal transducer and activator of transcription 3 by human papillomavirus early proteins 6 induces fibroblast senescence to promote cervical tumourigenesis through autocrine and paracrine pathways in tumour microenvironment. Eur J Cancer. 2013; 49: 3889–3899. 10.1016/j.ejca.2013.07.140 [DOI] [PubMed] [Google Scholar]
- 22. Wang P, Zou F, Zhang X, Li H, Dulak A, Tomko RJ Jr, et al. microRNA-21 negatively regulates Cdc25A and cell cycle progression in colon cancer cells. Cancer Res. 2009; 69: 8157–8165. 10.1158/0008-5472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hwang RF, Moore T, Arumugam T, Ramachandran V, Amos KD, Rivera A, et al. Cancer-associated stromal fibroblasts promote pancreatic tumor progression. Cancer Res. 2008; 68: 918–926. 10.1158/0008-5472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005; 121: 335–348. [DOI] [PubMed] [Google Scholar]
- 25. Yao Q, Cao S, Li C, Mengesha A, Kong B, Wei M. Micro-RNA-21 regulates TGF-β-induced myofibroblast differentiation by targeting PDCD4 in tumor-stroma interaction. Int J Cancer. 2011; 128: 1783–1792. 10.1002/ijc.25506 [DOI] [PubMed] [Google Scholar]
- 26. Kinoshita H, Hirata Y, Nakagawa H, Sakamoto K, Hayakawa Y, Takahashi R, et al. Interleukin-6 mediates epithelial-stromal interactions and promotes gastric tumorigenesis. PLoS One. 2013; 8: e60914 10.1371/journal.pone.0060914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wei LH, Kuo ML, Chen CA, Cheng WF, Cheng SP, Hsieh FJ, et al. Interleukin-6 in cervical cancer: the relationship with vascular endothelial growth factor. Gynecol Oncol. 2001; 82: 49–56 [DOI] [PubMed] [Google Scholar]
- 28. Tjiong MY, van der Vange N, ten Kate FJ, Tjong-A-Hung SP, ter Schegget J, Burger MP, et al. Increased IL-6 and IL-8 levels in cervicovaginal secretions of patients with cervical cancer. Gynecol Oncol. 1999; 73: 285–291 [DOI] [PubMed] [Google Scholar]
- 29. Bauknecht T, Randelzhofer B, Schmitt B, Ban Z, Hernando JJ. Response to IL-6 of HPV-18 cervical carcinoma cell lines. Virology. 1999; 258: 344–354 [DOI] [PubMed] [Google Scholar]
- 30. Kyo S, Kanaya T, Takakura M, Inoue M. A case of cervical cancer with aggressive tumor growth: possible autocrine growth stimulation by G-CSF and Il-6. Gynecol Oncol. 2000; 78: 383–387 [DOI] [PubMed] [Google Scholar]
- 31. Castrilli G, Tatone D, Diodoro MG, Rosini S, Piantelli M, Musiani P. Interleukin 1alpha and interleukin 6 promote the in vitro growth of both normal and neoplastic human cervical epithelial cells. Br J Cancer. 1997; 75: 855–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wei LH, Kuo ML, Chen CA, Chou CH, Lai KB, Lee CN, et al. Interleukin-6 promotes cervical tumor growth by VEGF-dependent angiogenesis via a STAT3 pathway. Oncogene. 2003; 22: 1517–1527. [DOI] [PubMed] [Google Scholar]
- 33. Abdelwahab SI, Abdul AB, Zain ZN, Hadi AH. Zerumbone inhibits interleukin-6 and induces apoptosis and cell cycle arrest in ovarian and cervical cancer cells. Int Immunopharmacol. 2012; 12: 594–602. 10.1016/j.intimp.2012.01.014 [DOI] [PubMed] [Google Scholar]
- 34. Tartour E, Gey A, Sastre-Garau X, Pannetier C, Mosseri V, Kourilsky P, et al. Analysis of interleukin 6 gene expression in cervical neoplasia using a quantitative polymerase chain reaction assay: evidence for enhanced interleukin 6 gene expression in invasive carcinoma. Cancer Res.1994; 54: 6243–6248. [PubMed] [Google Scholar]
- 35. de Roda Husman AM, Walboomers JM, van den Brule AJ, Meijer CJ, Snijders PJ. The use of general primers GP5 and GP6 elongated at their 3' ends with adjacent highly conserved sequences improves human papillomavirus detection by PCR. J Gen Virol. 1995; 76: 1057–1062. [DOI] [PubMed] [Google Scholar]
- 36. Bon MA, van Oeveren-Dybicz A, van den Bergh FA. Genotyping of HLA-B27 by real-time PCR without hybridization probes. Clin Chem. 2000; 46: 1000–1002. [PubMed] [Google Scholar]
- 37. Liu X, Zhang Z, Sun L, Chai N, Tang S, Jin J, et al. MicroRNA-499-5p promotes cellular invasion and tumor metastasis in colorectal cancer by targeting FOXO4 and PDCD4. Carcinogenesis. 2011; 32: 1798–1805. 10.1093/carcin/bgr213 [DOI] [PubMed] [Google Scholar]
- 38. Kenngott RA, Vermehren M, Sauer U, Ebach K, Sinowatz F. Cellular expression and localization of estrogen receptor α and progesterone receptor mRNA in the bovine oviduct combining laser-assisted microdissection, quantitative PCR, and in situ hybridization. J Histochem Cytochem. 2011; 59: 312–327. 10.1369/0022155410397995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hopman AH, Ramaekers FC, Speel EJ. Rapid synthesis of biotin-, digoxigenin-, trinitrophenyl-, and fluorochrome-labeled tyramides and their application for in situ hybridization using CARD amplification. J Histochem Cytochem. 1998; 46: 771–777. [DOI] [PubMed] [Google Scholar]
- 40. Asangani IA, Rasheed SA, Nikolova DA, Leupold JH, Colburn NH, Post S, et al. MicroRNA-21 (miR-21) post-transcriptionally downregulates tumor suppressor Pdcd4 and stimulates invasion, intravasation and metastasis in colorectal cancer. Oncogene. 2008; 27: 2128–2136. [DOI] [PubMed] [Google Scholar]
- 41. Sheedy FJ, Palsson-McDermott E, Hennessy EJ, Martin C, O'Leary JJ, Ruan Q, et al. Negative regulation of TLR4 via targeting of the proinflammatory tumor suppressor PDCD4 by the microRNA miR-21 . Nat Immunol. 2010; 11: 141–147. 10.1038/ni.1828 [DOI] [PubMed] [Google Scholar]
- 42. Yang HS, Jansen AP, Nair R, Shibahara K, Verma AK, Cmarik JL, et al. A novel transformation suppressor, Pdcd4, inhibits AP-1 transactivation but not NF-kappaB or ODC transactivation. Oncogene. 2001; 20: 669–676. 10.1038/sj.onc.1204137 [DOI] [PubMed] [Google Scholar]
- 43. Shen L, Ling M, Li Y, Xu Y, Zhou Y, Ye J, et al. Feedback regulations of miR-21 and MAPKs via Pdcd4 and Spry1 are involved in arsenite-induced cell malignant transformation. PLoS One. 2013; 8: e57652 10.1371/journal.pone.0057652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Iglesias M, Plowman GD, Woodworth CD. Interleukin-6 and interleukin-6 soluble receptor regulate proliferation of normal, human papillomavirus-immortalized, and carcinoma-derived cervical cells in vitro. Am J Pathol. 1995; 146: 944–952. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.