Abstract
Brain metastases are common in patients with advanced breast cancer (BC), causing considerable morbidity and mortality. Eribulin is a microtubule dynamics inhibitor approved for treating certain patients with metastatic BC, previously treated with an anthracycline and a taxane. In the 301 phase 3 study in 1102 women with advanced BC, eribulin and capecitabine treatments did not differ for co-primary endpoints (overall survival [OS]: 15.9 vs 14.5 months, P = 0.056; progression-free survival [PFS]: 4.1 vs 4.2 months, P = 0.30). Here, we report outcomes for six patients (eribulin, n = 3; capecitabine, n = 3) who had received treatment for brain metastases from BC (BCBM) at baseline. All eribulin-treated patients experienced brain lesion shrinkage at some point during treatment, compared with one capecitabine-treated patient. Fewer patients in study 301 developed new BCBM with eribulin (13/544, 2.4%) compared with capecitabine (25/546, 4.6%). Eribulin does not cross the healthy blood–brain barrier (BBB), but could have the potential to do so after cranial radiation therapy. Capecitabine may cross the BBB and has demonstrated activity in BCBM. Data from these patients and previous cases suggest that further investigation of eribulin for BCBM may be warranted.
Keywords: eribulin, advanced breast cancer, brain metastases, case series, capecitabine
Introduction
Eribulin (eribulin mesylate: Halaven®, Eisai Co., Ltd.) is a microtubule dynamics inhibitor1,2 in clinical use for certain patients with metastatic breast cancer (BC).3 There is a range of treatment options recommended for advanced BC after first or further lines of therapy4; however, the challenge remains to extend survival and maintain quality of life once recurrence or progression occurs.4 In the phase 3 Eisai Metastatic Breast Cancer Study Assessing Physician’s Choice Versus Eribulin (EMBRACE) study, eribulin improved survival in women with metastatic or locally advanced BC previously treated with an anthracycline and a taxane, compared with those who received treatment of physician’s choice.5 The 301 phase 3 study compared eribulin with capecitabine in women with advanced BC; the co-primary endpoints were overall survival (OS) and progression-free survival (PFS) and 1102 patients were randomized (intention-to-treat population [ITT]).6 Median OS was 15.9 months for patients who received eribulin and 14.5 months for those treated with capecitabine (hazard ratio [HR] = 0.88; 95% confidence interval [CI], 0.77–1.00; P = 0.056). Median PFS was 4.1 and 4.2 months with eribulin and capecitabine, respectively (HR = 1.08; 95% CI, 0.93–1.25; P = 0.30). In a pooled analysis of the two phase 3 studies, eribulin demonstrated a significant survival benefit in certain patient subgroups, including women with human epidermal growth factor receptor 2 (HER2)-negative disease and triple-negative BC.7
Brain metastases develop in approximately 10–16% of patients with BC8–10 and are associated with poor survival.11 Recently, a case report of eribulin treatment for advanced BC showed improvement in brain metastases.12 A 57-year-old woman with hormone-receptor-negative and HER2-positive BC and brain metastases received eribulin and experienced a decrease in the size of brain lesions, which persisted for four months. In an earlier case, a woman with advanced triple-negative BC had brain metastases identified during treatment with eribulin after four previous lines of chemotherapy.13 She received whole brain radiation therapy and three cycles of eribulin; however, lung metastases progressed and new liver metastases were identified, with death occurring 2.7 months after diagnosis of brain metastases. Previous studies of eribulin have not reported efficacy or safety in patients with brain metastases,5,14,15 and it is important to evaluate outcomes with eribulin in this highly relevant patient population.
Capecitabine is a fluoropyrimidine prodrug that is metabolized to 5-fluorouracil (5-FU) by enzymes in the liver and in tumors.16 It is used as first-, second-, or third-line therapy (as monotherapy or in combination regimens) to treat metastatic BC.4 Capecitabine has demonstrated some activity against brain metastases arising from BC,17–19 and lapatinib plus capecitabine has shown efficacy against brain metastases in patients with HER2-positive BC.20,21
Here, we report observations from a series of six patients with advanced BC and brain metastases in the 301 phase 3 study (ClinicalTrials.gov identifier: NCT00337103).6 We also assessed the proportion of patients who developed central nervous system (CNS) metastases during the study to determine whether there was any indication as to whether eribulin had a protective effect.
Methods
The 301 study methodology is detailed elsewhere,6 and only a brief description is provided here. Women were included if they had a confirmed diagnosis of locally advanced or metastatic BC and had received no more than three previous chemotherapy regimens (no more than two previous chemotherapy regimens for advanced or metastatic disease), including prior therapy with an anthracycline and a taxane. Baseline tumor assessment could occur up to 28 days before the start of study treatment. Patients with brain metastases were excluded from the study, unless they had completed local therapy and had discontinued the use of corticosteroids for at least four weeks before starting study treatment. Any symptoms due to brain metastases were to have been stable for at least four weeks before starting study treatment. In addition, radiographic stability (absence of progression) was established by comparing a contrast-enhanced brain scan performed during screening with a scan performed at least four weeks earlier.
Patients were randomized (1:1), after stratification for geographical region and HER2 status, to receive eribulin mesylate 1.4 mg/m2 (equivalent to 1.23 mg/m2 eribulin as free base) intravenously over 2.5 minutes on days 1 and 8 or capecitabine 1.25 g/m2 orally twice daily on days 1–14, both in 21-day cycles. Patients received study medication until disease progression, unacceptable toxicity, or patient/investigator request to discontinue. Tumor response was determined according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.0.22 The ITT population comprised all randomized patients; the safety population, used for all analyses of adverse events and reported here, comprised all randomized patients who received ≥1 dose of study medication.
The 301 study was funded by Eisai and was conducted in accordance with the World Medical Association (WMA) Declaration of Helsinki (WMA General Assembly, Tokyo, 2004), guidelines of the International Conference for Harmonisation/Good Clinical Practice (CPMP/ICH/135/95), and local ethical and legal requirements. Approval for the study was obtained from independent ethics committees and regulatory authorities in participating countries. All patients provided written informed consent.
Interpretation of the data for this case series was performed by AYC; additional statistical analyses were performed and funded by Eisai. Eisai provided funding for writing and submission of this manuscript by Oxford PharmaGenesis, under the guidance of AYC. The decision to submit to this journal was agreed by the authors.
Results
Patients with brain metastases at baseline
Seven patients in the study (0.6% of the safety population; n = 1090) had brain metastases at baseline; three were randomized to the eribulin arm (n = 544) and four to the capecitabine arm (n = 546). One patient in the capecitabine arm received only three days of capecitabine treatment and was lost to follow-up, so is not described in this case series. Baseline characteristics for the remaining six patients are provided in Table 1. The responses according to RECIST shown here refer only to patients’ brain metastases unless otherwise stated.
Table 1.
Baseline characteristics of six patients who had BC and brain metastases at baseline.
| AGE, YEARS* | DIAGNOSIS, TNM STAG-ING AT DIAGNOSIS AND RECEPTOR STATUS | SITE OF METASTASES* | PREVIOUS AGENTS FOR LOCALLY ADVANCED OR METASTATIC DISEASE | BEST RESPONSE | |
|---|---|---|---|---|---|
| Treated with eribulin | |||||
| Case E1 | 42 | Ductal adenocarcinoma T3b/NX/M1 HER2−, ER−, PrR− |
Brain, lung, lymph nodes | 1. Cyclophosphamide, doxorubicin 2. Paclitaxel plus gemcitabine |
PR PD |
| Case E2 | 61 | Ductal adenocarcinoma T4/N1/M1 HER2−, ER+, PrR+ |
Bone, brain, liver, lung, spleen | 1. Cyclophosphamide, docetaxel, doxorubicin 2. Anastrozole 3. Exemestane 4. Fulvestrant 5. Cisplatin plus docetaxel 6. Further cisplatin plus docetaxel |
PR NK NK NK SD SD |
| Case E3 | 49 | Ductal adenocarcinoma T1/N0/M0 HER2+, ER+, PrR+ |
Bone, brain, liver, lung | 1. Letrozole 2. Fulvestrant 3. Docetaxel plus epirubicin |
CR PD PR |
| Treated with capecitabine | |||||
| Case C1 | 39 | Ductal adenocarcinoma T2/N1/M1 HER2−, ER−, PrR+ |
Bone, brain, liver | 1. Goserelin, epirubicin, paclitaxel, and docetaxel 2. Docetaxel plus doxorubicin |
NK PD |
| Case C2 | 35 | Ductal adenocarcinoma T4/N3/M0 HER2−, ER−, PrR− |
Brain, liver, lung, lymph nodes | 1. Cyclophosphamide plus doxorubicin, followed by docetaxel 2. Paclitaxel plus gemcitabine |
PD PD |
| Case C3 | 45 | Ductal adenocarcinoma T1/N1/M0 HER2+, ER+, PrR− |
Bone, brain, lung | 1. Paclitaxel, bevacizumab 2. Trastuzumab, goserelin 3. Cisplatin plus gemcitabine |
PR SD SD |
Note:
At screening for study.
Abbreviations: CR, complete response; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; NK, not known; PD, progressive disease; PR, partial response; PrR, progesterone receptor; SD, stable disease; TNM, tumor, node, metastasis.
Patients treated with eribulin
Case E1
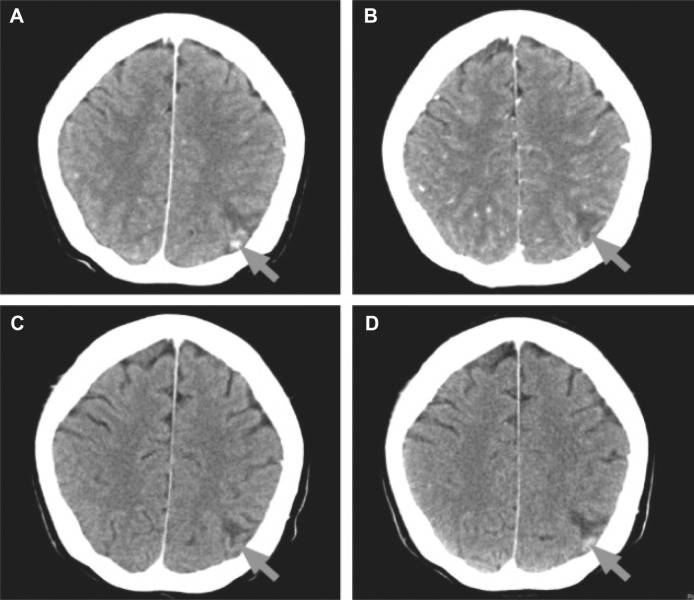
This patient had triple-negative BC at diagnosis. At study baseline, she had brain metastases in the left parietal cortex on computed tomography (CT) scan (Fig. 1A), for which she had previously received radiation therapy with a partial response (PR) about one month before initiation of eribulin. During the study, the patient received six cycles of eribulin with PR as the best response overall. After cycle 2, there was complete resolution of the brain lesion (Fig. 1B) and a 45% reduction from baseline in the sum of the longest diameter of all target lesions. This response in the brain lesion was maintained at cycle 4, when it remained completely resolved on imaging (Fig. 1C). Disease progressed after cycle 6, with relapse of the brain lesion (Fig. 1D), and a 125% increase in all RECIST-evaluated lesions compared with the previous smallest value and a 24% increase with baseline. The patient died five months after her last study dose of eribulin. During treatment, two grade 3 adverse events occurred (leukopenia and sensory neuropathy); no grade 4 adverse events were recorded.
Figure 1.
Computed tomography brain scans from a patient with triple-negative BC treated with eribulin (Case E1): (A) baseline, (B) after cycle 2 (resolution of brain lesion), (C) after cycle 4 (brain lesion remains resolved), and (D) after cycle 6 (relapse of brain lesion). Arrows indicate area with brain lesion.
Case E2
At diagnosis, this patient had estrogen-receptor (ER)-positive, progesterone-receptor (PrR)-positive, and HER2-negative BC. At baseline, she had multiple brain metastases (detected on magnetic resonance imaging [MRI]), and radiation therapy to the brain had been administered approximately five months before initiation of eribulin. The patient received six cycles of eribulin, with stable disease (SD) as the best response. MRI of the brain after cycles 2 and 4 showed a decrease in the size of brain lesions, but the sum of the longest diameter of all lesions had decreased by less than 30% compared with the baseline value. Brain and non-CNS metastases increased in size and number after cycle 6, and the patient stopped eribulin treatment; she died three months later. No grade 3 or 4 adverse events occurred during eribulin treatment.
Case E3
At diagnosis, this patient had ER-positive, PrR-positive, and HER2-positive BC, and at study baseline, she had brain lesions in the right parieto-occipital region identified by CT scan. She had received radiation therapy to the brain about two months before initiation of eribulin and had received six cycles of eribulin, with SD as the best outcome. No change in the size of the brain lesions was seen after cycle 2, but a reduction in size was observed after cycle 4. By the end of cycle 6, no further change in size was detected. The patient had clinical progression after cycle 6, with deterioration in her performance status, and she died three weeks after the last eribulin dose. During treatment, she experienced five grade 3 adverse events: myoclonic seizures, peripheral motor neuropathy, hyperglycemia, neutropenia, and asthenia.
Patients treated with capecitabine
Case C1
This patient had ER-negative, PrR-positive, and HER2-negative BC at diagnosis. She had received radiation therapy for brain metastases approximately four months before entering the study. She received two cycles of capecitabine before being withdrawn from the study due to progressive disease (PD). A spiral CT scan during cycle 2 showed that the size of the brain lesions had increased. The patient died four months after the last study dose. No grade 3 or 4 adverse events occurred during this short period of treatment.
Case C2
The patient had triple-negative BC and brain metastases in the right cerebellum on CT scan at baseline. She had received radiation therapy to the brain three months before she was randomized to study treatment and received 2.5 cycles of capecitabine before withdrawal due to PD (occurrence of new bone metastases). However, brain metastases apparent at baseline were absent on CT scan after cycle 2. This complete response in the brain was not confirmed because no repeat CT scan was performed. The patient died six weeks after receiving her last study dose of capecitabine. Oral mucositis was the only grade 3 adverse event.
Case C3
This patient had ER-positive, PrR-negative, and HER2-positive BC. She had received radiation therapy to the brain for metastases seven months before study entry and had experienced a PR in these brain lesions. At baseline, MRI showed progressive diffuse brain lesions. The patient was able to receive 11 cycles of capecitabine, with SD as her best response before progression of lung metastases, leading to withdrawal from the study. MRI showed no change in the size of brain metastases throughout the study. She died 21 months after withdrawal, having received multiple additional lines of chemotherapy. During capecitabine treatment, there were four grade 3 adverse events: hypocalcemia, hypokalemia, hand–foot syndrome, and diarrhea.
Development of CNS metastases during the study
During the study, 291/544 patients (53.5%; safety population) treated with eribulin developed new metastases at any site, compared with 316/546 (57.9%) patients treated with capecitabine (P = 0.145; χ2 test). Median OS for patients with new metastases was 15.8 and 13.1 months for those treated with eribulin and capecitabine, respectively. For the total safety population, new CNS metastases (brain and/or spinal cord metastases) occurred in 13 (2.4%) eribulin-treated patients and 25 (4.6%) capecitabine-treated patients (P = 0.068; Fisher’s exact test). Median OS for these patients was similar for both treatment arms: 7.5 versus 7.1 months with eribulin or capecitabine, respectively.
Discussion
For patients with BC, the development of brain metastases is associated with increased morbidity and mortality.9,11 In the 301 study, for the three patients with stable brain metastases at baseline who were treated with eribulin, a reduction in the size of brain lesions was observed at some point during treatment. For two of these individuals, the reduction was maintained at the next assessment two cycles later. In contrast, these findings were not observed in patients treated with capecitabine. One individual had an increase in the size of brain metastases at the first assessment after baseline screening, one patient showed no change throughout her treatment, and one individual had brain lesions at baseline that were not detected after her second treatment cycle. The hormone-receptor status of the BC tumors in these women was heterogeneous for both treatment arms.
Furthermore, while relatively few patients developed new CNS metastases during the 301 study, these occurred in more women treated with capecitabine than with eribulin (4.6 vs 2.4%, respectively). The lower proportion of patients developing CNS metastases in the eribulin arm compared with the capecitabine arm may suggest a protective effect of eribulin. Although limited, these data could offer a signal of potential beneficial effects of eribulin with respect to bone metastases arising from BC.
Study 301 compared eribulin and capecitabine treatment for 1102 women (ITT population) with pretreated advanced BC. Co-primary endpoints were OS and PFS, and the outcomes for this population were not different between study arms.6 This was in contrast to findings from the phase 3 EMBRACE study, in which eribulin treatment resulted in a significant OS benefit compared with physicians’ choice of treatment.5 However, outcomes for the EMBRACE study have not yet been analyzed by site of metastases, and data were not recorded for individual non-target lesions (which included brain lesions).
Matsuoka and colleagues recently reported on the effects of eribulin in a 57-year-old woman with brain metastases arising from BC.12 This patient received radiation therapy for her brain metastases and then treatment with lapatinib, an epidermal growth factor receptor (HER1/EGFR/ErbB1) and HER2 tyrosine kinase inhibitor, and capecitabine. After three months, she experienced progression of brain and liver metastases and was treated with eribulin. After one month, brain lesions decreased significantly in size, and this decrease was maintained for at least four months. One other similar case has been reported, with no apparent benefit on brain metastases from three cycles of eribulin.13
The rationale for the potential positive effects of eribulin on brain metastases requires explanation because preclinical evidence suggests that the agent does not cross the blood–brain barrier (BBB) to a significant extent.23–25 Currently, there are no clinical data that evaluate eribulin levels in the tissue from brain metastases arising from BC. It is known, however, that radiation therapy to the brain compromises the BBB,26 reducing expression of the efflux transporter P-glycoprotein.27 Evidence suggests that this effect may last for as long as several years after post-radiation therapy.28 All three eribulin-treated patients in the current study had received radiation therapy to the brain. Therefore, it is possible that eribulin could have entered brain tissue in therapeutic quantities, although in case E1, we cannot rule out the possibility that the therapeutic effect on brain metastases may have been due to radiation alone.
There is evidence that both 5-FU and capecitabine can cross the BBB.21 In patients with brain metastases arising from HER2-negative BC, a range of patient cases and other small studies have demonstrated a benefit with capecitabine treatment.17,29,30 For individuals with advanced HER2-positive BC, lapatinib plus capecitabine has shown some efficacy against brain metastases,31 and both agents have been shown to cross the BBB in patients with brain metastases arising from HER2-positive BC.21
Our data, taken together with the previously reported case study, suggest that eribulin may have a potentially beneficial effect on existing brain metastases and a protective effect against the development of new brain metastases. Further investigation is warranted.
Acknowledgments
Catriona Scott of Oxford PharmaGenesis provided editorial support to the authors, funded by Eisai.
Footnotes
ACADEMIC EDITOR: Goberdhan P. Dimri, Editor in Chief
FUNDING: Study 301 was funded by Eisai. For this case series, Eisai provided funding for statistical analyses, interpretation, writing, and submission. The authors confirm that the funder had no influence over the study design, content of the article, or selection of this journal.
COMPETING INTERESTS: AYC is an Eisai advisory board member. XXY discloses no competing interests.
Paper subject to independent expert blind peer review by minimum of two reviewers. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Investigator on the 301 study and treated one of the six patients in this case series: AYC. Conceived this analysis: AYC. Analyzed the study data on patients with brain metastases from the Johns Hopkins Singapore International Medical Center and the other 301 study centers (additional analyses were provided by and funded by Eisai): AYC and XXY. Contributed to the development of the structure and arguments for the paper: AYC and XXY. Contributed to the manuscript, made critical revisions, and approved the final version: AYC and XXY.
REFERENCES
- 1.Okouneva T, Azarenko O, Wilson L, Littlefield BA, Jordan MA. Inhibition of centromere dynamics by eribulin (E7389) during mitotic metaphase. Mol Cancer Ther. 2008;7(7):2003–2011. doi: 10.1158/1535-7163.MCT-08-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith JA, Wilson L, Azarenko O, et al. Eribulin binds at microtubule ends to a single site on tubulin to suppress dynamic instability. Biochemistry. 2010;49(6):1331–1337. doi: 10.1021/bi901810u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drugs@FDA HALAVEN® (Eribulin mesylate): Highlights of prescribing information. 2014. [Accessed May 6, 2014]. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/201532s006lbl.pdf.
- 4.National Comprehensive Cancer Network NCCN Clinical practice guidelines in oncology breast cancer Version 3. 2014. [Accessed November 26, 2014]. Available at: http://www.nccn.org/professionals/physician_gls/PDF/breast.pdf.
- 5.Cortes J, O’Shaughnessy J, Loesch D, et al. EMBRACE (Eisai Metastatic Breast Cancer Study Assessing Physician’s Choice Versus E7389) investigators Eribulin monotherapy versus treatment of physician’s choice in patients with metastatic breast cancer (EMBRACE): a phase 3 open-label randomised study. Lancet. 2011;377(9769):914–923. doi: 10.1016/S0140-6736(11)60070-6. [DOI] [PubMed] [Google Scholar]
- 6.Kaufman PA, Awada A, Twelves C, et al. Phase III open-label randomized study of eribulin mesylate versus capecitabine in patients with locally advanced or metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol. 2015;33(6):594–601. doi: 10.1200/JCO.2013.52.4892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Twelves C, Cortes J, Vahdat L, et al. Efficacy of eribulin in women with metastatic breast cancer: a pooled analysis of two phase 3 studies. Breast Cancer Res Treat. 2014;148(3):553–561. doi: 10.1007/s10549-014-3144-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dawood S, Gonzalez-Angulo AM. Progress in the biological understanding and management of breast cancer-associated central nervous system metastases. Oncologist. 2013;18(6):675–684. doi: 10.1634/theoncologist.2012-0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin NU, Winer EP. Brain metastases: the HER2 paradigm. Clin Cancer Res. 2007;13(6):1648–1655. doi: 10.1158/1078-0432.CCR-06-2478. [DOI] [PubMed] [Google Scholar]
- 10.Minisini AM, Moroso S, Gerratana L, et al. Risk factors and survival outcomes in patients with brain metastases from breast cancer. Clin Exp Metastasis. 2013;30(8):951–956. doi: 10.1007/s10585-013-9594-5. [DOI] [PubMed] [Google Scholar]
- 11.Lu J, Steeg PS, Price JE, et al. Breast cancer metastasis: challenges and opportunities. Cancer Res. 2009;69(12):4951–4953. doi: 10.1158/0008-5472.CAN-09-0099. [DOI] [PubMed] [Google Scholar]
- 12.Matsuoka H, Tsurutani J, Tanizaki J, et al. Regression of brain metastases from breast cancer with eribulin: a case report. BMC Res Notes. 2013;6:541. doi: 10.1186/1756-0500-6-541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nieder C, Aandahl G, Dalhaug A. A case of brain metastases from breast cancer treated with whole-brain radiotherapy and eribulin mesylate. Case Rep Oncol Med. 2012;2012:537183. doi: 10.1155/2012/537183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cortes J, Vahdat L, Blum JL, et al. Phase II study of the halichondrin B analog eribulin mesylate in patients with locally advanced or metastatic breast cancer previously treated with an anthracycline, a taxane, and capecitabine. J Clin Oncol. 2010;28(25):3922–3928. doi: 10.1200/JCO.2009.25.8467. [DOI] [PubMed] [Google Scholar]
- 15.Vahdat LT, Pruitt B, Fabian CJ, et al. Phase II study of eribulin mesylate, a halichondrin B analog, in patients with metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol. 2009;27(18):2954–2961. doi: 10.1200/JCO.2008.17.7618. [DOI] [PubMed] [Google Scholar]
- 16.Blum JL. The role of capecitabine, an oral, enzymatically activated fluoropyrimidine, in the treatment of metastatic breast cancer. Oncologist. 2001;6(1):56–64. doi: 10.1634/theoncologist.6-1-56. [DOI] [PubMed] [Google Scholar]
- 17.Ekenel M, Hormigo AM, Peak S, Deangelis LM, Abrey LE. Capecitabine therapy of central nervous system metastases from breast cancer. J Neurooncol. 2007;85(2):223–227. doi: 10.1007/s11060-007-9409-0. [DOI] [PubMed] [Google Scholar]
- 18.Wang ML, Yung WK, Royce ME, Schomer DF, Theriault RL. Capecitabine for 5-fluorouracil-resistant brain metastases from breast cancer. Am J Clin Oncol. 2001;24(4):421–424. doi: 10.1097/00000421-200108000-00026. [DOI] [PubMed] [Google Scholar]
- 19.Rivera E, Meyers C, Groves M, et al. Phase I study of capecitabine in combination with temozolomide in the treatment of patients with brain metastases from breast carcinoma. Cancer. 2006;107(6):1348–1354. doi: 10.1002/cncr.22127. [DOI] [PubMed] [Google Scholar]
- 20.Lin NU, Diéras V, Paul D, et al. Multicenter phase II study of lapatinib in patients with brain metastases from HER2-positive breast cancer. Clin Cancer Res. 2009;15(4):1452–1459. doi: 10.1158/1078-0432.CCR-08-1080. [DOI] [PubMed] [Google Scholar]
- 21.Morikawa A, Peereboom DM, Thorsheim HR, et al. Capecitabine and lapatinib uptake in surgically resected brain metastases from metastatic breast cancer patients: a prospective study. Neuro Oncol. 2014;17(2):289–295. doi: 10.1093/neuonc/nou141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 23.Taur JS, DesJardins CS, Schuck EL, Wong YN. Interactions between the chemotherapeutic agent eribulin mesylate (E7389) and P-glycoprotein in CF-1 abcb1a-deficient mice and Caco-2 cells. Xenobiotica. 2011;41(4):320–326. doi: 10.3109/00498254.2010.542256. [DOI] [PubMed] [Google Scholar]
- 24.Yu MJ, Zheng W, Tendyke K. Atom-based enumeration: new eribulin analogues with low susceptibility to P-glycoprotein-mediated drug efflux. Bioorg Med Chem Lett. 2012;22(24):7363–7366. doi: 10.1016/j.bmcl.2012.10.077. [DOI] [PubMed] [Google Scholar]
- 25.Narayan S, Carlson EM, Cheng H, et al. Novel second generation analogs of eribulin. Part III: blood–brain barrier permeability and in vivo activity in a brain tumor model. Bioorg Med Chem Lett. 2011;21(6):1639–1643. doi: 10.1016/j.bmcl.2011.01.096. [DOI] [PubMed] [Google Scholar]
- 26.Qin DX, Zheng R, Tang J, Li JX, Hu YH. Influence of radiation on the blood–brain barrier and optimum time of chemotherapy. Int J Radiat Oncol Biol Phys. 1990;19(6):1507–1510. doi: 10.1016/0360-3016(90)90364-p. [DOI] [PubMed] [Google Scholar]
- 27.Bart J, Nagengast WB, Coppes RP, et al. Irradiation of rat brain reduces P-glycoprotein expression and function. Br J Cancer. 2007;97(3):322–326. doi: 10.1038/sj.bjc.6603864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Vulpen M, Kal HB, Taphoorn MJ, El-Sharouni SY. Changes in blood–brain barrier permeability induced by radiotherapy: implications for timing of chemotherapy? (review) Oncol Rep. 2002;9(4):683–688. [PubMed] [Google Scholar]
- 29.Fabi A, Vidiri A, Ferretti G, et al. Dramatic regression of multiple brain metastases from breast cancer with capecitabine: another arrow at the bow? Cancer Invest. 2006;24(4):466–468. doi: 10.1080/07357900600705805. [DOI] [PubMed] [Google Scholar]
- 30.Tham YL, Hinckley L, Teh BS, Elledge R. Long-term clinical response in leptomeningeal metastases from breast cancer treated with capecitabine monotherapy: a case report. Clin Breast Cancer. 2006;7(2):164–166. doi: 10.3816/CBC.2006.n.028. [DOI] [PubMed] [Google Scholar]
- 31.Bachelot T, Romieu G, Campone M, et al. Lapatinib plus capecitabine in patients with previously untreated brain metastases from HER2-positive metastatic breast cancer (LANDSCAPE): a single-group phase 2 study. Lancet Oncol. 2013;14(1):64–71. doi: 10.1016/S1470-2045(12)70432-1. [DOI] [PubMed] [Google Scholar]