Abstract
Background
Evidence about the association between Bisphenol A (BPA) and the risk of recurrent miscarriage (RM) in human being is still limited.
Objective
We evaluated the association of urinary BPA concentrations with RM in human being.
Methods
A hospital-based 1:2 matched case-control study on RM was carried out in Suzhou and Kunshan in Jiangsu Province in China between August 2008 and November 2011. Total urinary BPA concentrations in 264 eligible urine samples (102 RM patients and 162 controls) were measured using liquid chromatography-tandem mass spectrometry (LC-MS/MS). The Wilcoxon test and conditional logistic regression were used to estimate the differences between the groups and odds ratios (OR) with 95% confidence intervals (CI), respectively.
Results
The median ± IQR (interquartile range) (P 75-P 25) values of non-creatinine-adjusted total urinary BPA levels in the RM patients and the controls were 1.66±3.69ng/ml and 0.58±1.07ng/ml, respectively (0.98±2.67μg/g Cr (creatinine) and 0.40±0.77μg/g Cr. The adjusted BPA level was significantly higher in the RM patients than in the controls (Wilcoxon test, Z = 4.476, P<0.001). Higher level of urinary BPA was significantly associated with an increased risk of RM (P-trend <0.001). Compared to the groups with urinary BPA levels less than 0.16μg/g Cr, the women with levels of 0.40–0.93μg/g Cr and 0.93μg/g Cr or above had a significantly higher risk of RM (OR = 3.91, 95%CI: 1.23–12.45 and OR = 9.34, 95%CI: 3.06–28.44) that persisted after adjusting for confounding factors. The time from recently RM date to recruitment does not significantly influence the urinary BPA level (P = 0.090).
Conclusion
Exposure to BPA may be associated with RM risk.
Introduction
Recurrent miscarriage (RM) has traditionally been defined as three consecutive unexplained terminations of pregnancy before 20 weeks of gestation or expulsions of a fetus weighing<500g [1]. The prevalence of RM has been reported to be as high as 0.5–3% [2, 3]. There is a tendency to expand this definition to include women who have experienced only two miscarriages, particularly in studies focusing on the aetiology or risk factors of RM [1, 4]. The causes of RM are very complicated. The identifiable factors of RM may include parental chromosomal anomalies, uterine pathology, a prothrombotic state, endocrine disorders, immunological factors, and infections [5–10]. However, the causes of RM remain unexplained for approximately half of the women who experience RM, despite thorough investigations [4].
Currently, endocrine-disrupting chemicals (EDCs), including bisphenol A (BPA), polychlorinated biphenyls (PCBs), 1,1-dichloro-2,2-bis(p-chlorophenyl) ethylene (DDE) and hexachlorobenzene (HCB), are the most prominent potential causes of unexplained RM [11, 12]. BPA, a monomer used in the production of polycarbonates and epoxy resins and as an antioxidant in PVC plastics, is one of the most widely used industrial compounds worldwide. In general, polycarbonates are used to manufacture plastic food containers and the epoxy resins that coat the inner surfaces of food and beverage cans. PVC is used in a variety of products, including materials that may come into contact with food, such as plastic film used for packaging food. The migration of BPA from polycarbonate plastics, surfaces of epoxy-coated cans, and PVC products into food and food stimulants has been reported, especially when heated [13–15]. Humans may be exposed to BPA in food, beverages, dust, etc. BPA is a well-known endocrine disruptor with estrogenic and anti-androgen activities that can cause a variety of adverse effects in both animals and humans. Evidence from large experimental studies suggests that exposure to BPA has adverse effects on reproductive development in animals [16–21], and recently there two epidemiologic studies on the association between BPA exposure and miscarriage risk [22, 23], but human studies on the BPA associated with RM risk are still limited. Urinary BPA may be associated with lower semen quality, increased sperm DNA damage [24], and self-reported sexual dysfunction in males [25]. Bloom and colleagues assessed BPA in couples undergoing in vitro fertilization (IVF) and measured indicators of embryo quality. The study showed that male BPA exposure may affect embryo quality by influencing the early embryo cleavage rate during IVF [26]. Several studies found that serum BPA levels were associated with endometrial hyperplasia and ovarian dysfunction in females [27–29]. To date, only one study from Japan has reported findings about the relationship between serum BPA levels and RM [11].
We measured the total urinary BPA level (conjugated and free) to quantify the amount of human exposure to BPA. Although BPA has a short half-life in biological tissues [30, 31], urinary BPA is still considered to be the preferred indicator to assess biological exposure [32].
Suzhou and neighboring Kunshan cities are located in the lower reaches of the Yangtze River––the most industrialized areas in eastern China. After 30 years of rapid economic development and marked life style changes, environment challenges in this area on public health such as plasticizer use and other endocrine disrupting chemicals should be examined. The aim of the present study is to investigate the association between urinary BPA levels and unexplained RM, a relationship that has not yet been reported.
Methods
Subjects and recruitment
First, between August 2008 and November 2011, we initially registered 121 patients aged 20–40 years who sought treatment and self-reported history of RM with repeated (2–6 RMs), consecutive unexplained terminations of pregnancy before 20 weeks of gestation from the outpatients of obstetric and gynaecological clinics of three hospitals––the Maternal and Child Health Center in Kunshan City, the First People's Hospital, and the Second People's Hospital affiliated with Soochow University. The time from the nearest RM date for the patients to recruitment were various from 2 days to 352 days. The patients were asked to answer questionnaire, provide morning spot urine and blood samples less than two weeks after the recruitment. They also completed regular medical examination (B-mode ultrasound, autoimmune disorder test, and abnormal coagulate function test) to exclude other known causes of RM.
When the study was completed, 19 cases were excluded for the exclusion criteria including reproductive system malformation (uterus bicornis, uterus septus, uterine leiomyoma) (8), autoimmune disorders (anticardiolipin antibody- and antinuclear antibody-positive) (6) and abnormal coagulate function (5). We have not excluded the patients who suffered chromosome abnormalities and endocrine disorders. In each hospital, there are more than 20 pregnant women performing the routine prenatal examination in every working day, who were registered their name, age and gestational age, etc. Once acquired one eligible RM case, based on the prenatal examination name list, we tried to match 2 controls of pregnant women aged 20–40 years with no history of miscarriage in the same hospital with the following criteria: age (±2 years), gestational age (±1 week), and living in the same district. We excluded the controls who had a history of pregnancy complications, miscarriage, still birth or pre-eclampsia, and none had given birth to a small for gestational age infant. Totally 60 cases were successfully matched 2 controls, however 42 cases only had 1 match.
The controls were also asked to provide information on demographic characteristics, lifestyle, obstetric history, and other RM risk factors by a structured questionnaire and morning spot urine and blood samples. To avoid contamination from plastic equipment, glass containers were used in the sampling and experimental processes. The containers were soaked in nitric acid solution for at least 12h, washed with deionised water, and baked at 70°C for at least 10h. The urine samples were stored at -80°C before LC-MS/MS analysis. Written informed consent was obtained from each participant. The study was approved by the ethics committee of Soochow University.
Analysis of Urinary BPA
LC-MS/MS was used to measure total BPA (conjugated and free) in the urine samples from the participants, using selective reaction monitoring (SRM). After pre-treatment, the samples (25μl) were separated by a BR-C18 column (5μm, 100×2.1mm; Sepax, USA) using a Thermo Finnigan high performance liquid chromatography system combined with a TSQ Quantum Ultra triple quadrupole tandem mass spectrometer (Thermo Electron, Waltham, MA, USA) equipped with an electrospray ionization probe (Thermo Electron). Gradient elution with a 0.3% ammonia solution (solvent A) and methanol (solvent B) was carried out using the following procedure: 40% B for 0.5min, gradually increased to 95% B within 2min, solvent composition held at 95% B for 1.5min and equilibrated at 40% B for 1.5min. The total chromatographic separation time was 5.5min. The flow rate was 300μl/min. To record spectral data, a vaporizer temperature of 300°C and a TurboIonSpray voltage of -3.0kV in the negative ionization mode were applied. Nitrogen served as the sheath gas, the auxiliary gas, and the ion sweep gas; the values of these gases were set to 50, 10, and 1 arbitrary unit(s), respectively. The mass spectrometer was operated in the selected reaction monitoring (SRM) mode with a signal time segment. The scan width for SRM was 0.01m/z, scan time was 0.1s, and the peak width settings (FWHM) for both Q1 and Q3 were 0.7.
The pre-treatment process was a modified version of the methods used by Matsumoto [33]. Urine (500μl) and 50μl d16-BPA 200ng/ml (internal standard) were buffered with 350μl of 1.0 M sodium acetate buffer (pH5.0) and hydrolysed enzymatically with β-glucuronidase/sulfatase(110,000U/ml/4,000U/ml, Sigma Chemical Co., USA) at 37°C overnight in a shaking water bath [34]. After hydrolysis, the hydrolysate was extracted once with 5ml of ethyl acetate. After centrifugation, the supernatant was transferred to a new glass tube and evaporated with N2 gas. The residue was dissolved with 500μl of 30/70 (v/v) methanol/water solution, filtered using a microporous membrane filter and injected into the LC-MS/MS system described above.
The standard curve of this method was y = 0.1304+0.6801x. Satisfactory linearity was obtained (r>0.999). The limit of detection (LOD) of urinary BPA was 0.20ng/ml, similar other studies [35, 36]. Recoveries of BPA ranged from 105.0% to 109.5%. The repeatability of the method was evaluated by analysing six replicates of four samples spiked with standard BPA. Expressed as the relative standard deviation (RSD) within each day and between days, the values were calculated as 3.16%-4.22% and 5.22%-5.30%, respectively. Fig 1 showed the chromatograms of standard, case and control urine samples, which had concentrations of 1ng/ml, 0.7ng/ml and 0.61ng/ml, respectively. BPA levels were measured by comparing the retention time to the retention time of the main peak (3.6min) in the chromatogram of standard BPA. To measure BPA concentration, a mean blank value of 0.4ng/ml had been determined and was subtracted from each measured value. In addition, the BPA value was adjusted for the urinary creatinine concentration to correct for the urine volume. Creatinine was detected using the method of measuring enzymatic creatinine in an automatic biochemical instrument (Hitachi 7020). The concentration of urinary BPA was expressed in micrograms of BPA per gram of creatinine.
Fig 1. Chromatogram of BPA standard (1ng/ml) (a), one RM patient’s urine sample (0.7ng/ml) (b) and one control patient’s urine sample (0.61ng/ml) (c).
Statistical analysis
We imputed the missing levels by the value of the LOD divided by the square root of 2 if the geometric standard deviation (GSD) was less than 3; otherwise, we imputed by the LOD divided by 2 [35, 37]. We used the Wilcoxon method to compare the difference in urinary BPA levels between RM patients and controls or between other different groups because the urinary BPA levels of the patients in the two groups were not normally distributed. Univariate and multivariate conditional logistic regression were used to calculate crude and adjusted ORs (95%CI), respectively, to estimate the association between urinary BPA and RM. Potential confounders include age, occupation, years of education, BMI and passive smoking during pregnancy in our study. Statistical power was calculated using SAS Proc power. On the basis of prevalence of urinary BPA ≤0.16 μg/g Cr in 25% of general women population and a ratio of two control subjects to 1 case, 102 cases and 162 control subjects has 92.7% power to detect an odds ratio of 2.5. The significance level was set at P<0.05 for all tests. SAS 9.2 software (SAS Institute, Inc., Cary, NC) was used for all analyses.
Results
Characteristics of subjects
The mean age ± SD of the RM patients was 28.04±3.68 years, and the mean age of the controls was 28.36±3.75 years. On average, the women in the case group had experienced 2.47 previous miscarriages (2 to 6 RMs), some consecutively and some not consecutively but at least 2 consecutive. There were no significant differences in age or occupation between the case and control groups (P>0.05). However, BMI on average was significantly lower among the controls than among the RM patients (P<0.001). The distributions of BMI and the years of education of the two groups were significantly different (P = 0.047 and P = 0.001, respectively) (Table 1).
Table 1. Demographic characteristics of the RM patients and the controls*.
| RM patients(102) | Controls(162) | P-values | |
|---|---|---|---|
| Age | 28.36±3.75 | 28.04±3.68 | 0.351 |
| No.of RM | 2.47±0.73 | - | |
| BMI | 21.18±2.29 | 20.19±2.37 | <0.001 |
| n (%) | n (%) | ||
| Age | |||
| <25 | 18(17.65) | 25(15.43) | 0.256 |
| 25~29 | 43(42.16) | 85(52.47) | |
| >29 | 41(40.20) | 52(32.10) | |
| BMI (kg/m 2 ) | |||
| <18.5 | 11(10.78) | 36(22.22) | 0.047 |
| 18.5~ | 78(76.47) | 116(71.60) | |
| 24~ | 12(11.76) | 9(5.56) | |
| ≥28 | 1(0.98) | 1(0.62) | |
| Years of education | |||
| ≤6 | 23(22.55) | 25(15.43) | 0.001 |
| 7~9 | 24(23.53) | 58(35.80) | |
| 10~12 | 23(22.55) | 56(34.57) | |
| >12 | 32(31.37) | 23(14.20) | |
| Occupation | |||
| Workers | 15(14.71) | 26(16.05) | 0.593 |
| Business/services | 31(30.39) | 57(35.19) | |
| Professionals | 24(23.53) | 25(15.43) | |
| Unemployed | 23(22.55) | 39(24.07) | |
| Other | 9(8.82) | 4(9.26) |
*The values for age, number of RM and BMI are expressed as the mean ± SD. BMI = body mass index (kg/m2).
Urinary BPA concentration in RM patients and controls
Fig 2 shows the distributions of non-creatinine-adjusted and creatinine-adjusted urinary BPA in the RM patients and the controls. No significant differences in the detection rate of urinary BPA were found between the RM patients (85.29%) and the controls (82.10%) (P = 0.498). Without adjusting for creatinine, the median ± IQR value of total urinary BPA levels in the RM patients was 1.66±3.69ng/ml, significantly higher than the level measured in the controls (0.58±1.07ng/ml) (P<0.001). After adjusting for creatinine, the median ± quartile range urinary BPA level among the RM patients was 0.98±2.67μg/g Cr, significantly higher than the level among the controls (0.40±0.77μg/g Cr) (P<0.001). Significant differences can be observed between cases and controls in the subgroups defined by age, BMI, years of education, occupation and passive smoking during pregnancy (P<0.05). However, for the same RM patients group or the same controls group, we did not find that the age, BMI, years of education, occupation and passive smoking status significantly were associated with the creatinine-adjusted BPA level (all P>0.05) (Table 2).
Fig 2. Distribution of non-creatinine-adjusted (a) and creatinine-adjusted (b) urinary BPA concentration in the RM patients and the controls.
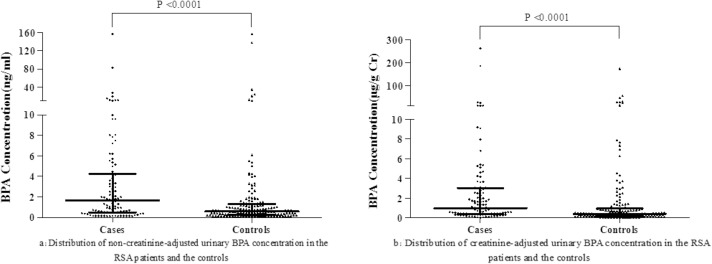
Table 2. Creatinine-adjusted urinary BPA concentration in the RM patients and the controls (μg/g Cr).
| RM patients(102) | Controls(162) | P-values | |||
|---|---|---|---|---|---|
| n(Detection rate)* | Median (P 25-P 75) (μg/g Cr) | n(Detection rate) * | Median(P 25-P 75) (μg/g Cr) | ||
| Age Age(years) | |||||
| <25 | 18(88.89) | 0.55(0.32–4.17) | 25(88.00) | 0.47(0.26–1.44) | 0.242 |
| 25~29 | 43(86.05) | 1.74(0.24–4.22) | 85(84.71) | 0.40(0.16–0.97) | 0.001 |
| >29 | 41(82.93) | 0.85(0.43–1.65) | 52(75.00) | 0.37(0.14–0.73) | 0.001 |
| P-values | 0.216 | 0.311 | |||
| BMI | |||||
| <18.5 | 11(81.82) | 0.51(0.19–3.61) | 36(75.00) | 0.37(0.14–0.82) | 0.156 |
| 18.5~ | 78(85.90) | 1.19(0.35–3.02) | 116(85.34) | 0.46(0.17–0.97) | <0.001 |
| 24~31 | 13(84.62) | 0.95(0.44–1.66) | 10(70.00) | 0.21(0.16–0.52) | 0.017 |
| P-values | 0.899 | 0.557 | |||
| Years of education | |||||
| ≤6 | 23(91.30) | 1.12(0.42–2.49) | 25(88.00) | 0.46(0.26–0.88) | 0.041 |
| 7~9 | 24(83.33) | 1.63(0.33–3.89) | 58(79.31) | 0.39(0.14–0.73) | 0.002 |
| 10~12 | 23(86.96) | 1.33(0.44–5.34) | 56(85.71) | 0.52(0.21–1.60) | 0.050 |
| >12 | 32(81.25) | 0.72(0.29–2.13) | 23(73.91) | 0.30(0.13–0.57) | 0.015 |
| P-values | 0.491 | 0.136 | |||
| Occupation | |||||
| Workers | 15(93.33) | 0.55(0.41–5.25) | 26(69.23) | 0.38(0.16–0.75) | 0.021 |
| Business/services | 31(90.32) | 1.33(0.34–3.61) | 57(84.21) | 0.50(0.14–0.97) | 0.014 |
| Professionals | 24(83.33) | 0.90(0.31–2.19) | 25(76.00) | 0.38(0.14–0.90) | 0.027 |
| Unemployed | 23(73.91) | 0.54(0.32–2.49) | 39(94.87) | 0.40(0.21–0.73) | 0.080 |
| Other | 9(88.89) | 1.66(0.60–4.69) | 15(73.33) | 0.32(0.17–2.26) | 0.190 |
| P-values | 0.767 | 0.970 | |||
| Passive Smoking | |||||
| No | 76(82.89) | 1.23(0.40–3.05) | 128(82.81) | 0.39(0.16–0.80) | <0.001 |
| Yes | 26(92.31) | 0.55(0.34–2.80) | 34(79.41) | 0.42(0.17–1.55) | 0.236 |
| P-values | 0.348 | 0.438 | |||
| Total | 102(85.29) | 0.98(0.35–3.02) | 162(82.10) | 0.40(0.16–0.93) | <0.001 |
| 1.66(0.49–4.18) # | 0.58(0.24–1.31) # | <0.001 | |||
* n = detection of total number.
# The median ± IQR values of non-creatinine-adjusted total urinary BPA levels in the RM patients and the controls
Association between urinary BPA level and RM
In the model, BPA levels were categorized into four groups: ≤P 25, P 25-≤P 50, P 50-P 75, >P 75 of the control group BPA concentration. After adjusting for occupation, years of education, BMI, passive smoking during pregnancy, higher urinary BPA levels were associated with an increased risk of RM (P-trend<0.001). Compared to the group with urinary BPA level≤0.16μg/g Cr, the RM odds ratios for the groups with BPA levels of 0.16–0.40μg/g Cr, 0.40–0.93μg/g Cr and >0.93μg/g Cr were 2.90 (95%CI: 0.93–9.03), 3.91(95%CI:1.23–12.45), and 9.34 (95%CI: 3.06–28.44), respectively (Table 3). In addition, though significant difference for creatinine-adjusted BPA level was found between three kinds of women with 0, 2 times and >2 times RM history (P<0.001), no dose-response relationship existed between the RM times and BPA levels (Table 4).
Table 3. Association between urinary BPA level and RM.
| Urinary BPA Concentration (μg/g Cr) | RM patients (%) | Controls(%) | OR(95%CI) | OR* (95%CI) | P-values |
|---|---|---|---|---|---|
| ≤0.16 | 7(6.86) | 41(25.31) | 1.0 | 1.0 | |
| 0.16~ | 20(19.61) | 40(24.69) | 3.10(1.07–9.01) | 2.90(0.93–9.03) | 0.067 |
| 0.40~ | 22(21.57) | 41(25.31) | 3.71(1.27–10.85) | 3.91(1.23–12.45) | 0.021 |
| >0.93 | 53(51.96) | 40(24.69) | 9.64(3.35–27.74) | 9.34(3.06–28.44) | <0.001 |
| Trend test | <0.001 | ||||
| age | 2.25(0.97–1.62) | 0.085 | |||
| occupation, | 1.03(0.82–1.29) | 0.791 | |||
| years of education | 1.22(0.89–1.68) | 0.213 | |||
| BMI | 1.23(1.05–1.44) | 0.010 | |||
| passive smoking | 2.98(0.73–12.21) | 0.130 |
*Adjusted for age, occupation, years of education, BMI, passive smoking during pregnancy
Table 4. Distribution of urinary BPA levels in controls, two and three or more RM patients.
| n(Detection rate)* | Median(P25-P75) (ng/ml) | Median(P 25-P 75) † (μg/g Cr) | F-value | P-value # | |
|---|---|---|---|---|---|
| Controls | 162(82.10) | 0.58(0.24–1.31) b | 0.40(0.16–0.93) b | 12.46 | <0.001 |
| Two RMs | 64(90.63) | 2.01(0.57–5.26) a | 1.35(0.43–3.35) a | ||
| ≥Three RMs | 38(76.32) | 0.95(0.22–2.38) b | 0.60(0.32–1.97) a |
* n = detection of total number
† Adjustment for creatinine-adjusted BPA
# for creatinine-adjusted BPA
a, b: In the method of SNK analysis, there was not statistically significant difference with the same letters (a, b) (P>0.05); There was statistically significant difference with the different letters (a, b) (P <0.05).
Association between time from recently RM date to recruitment and urinary BPA level
There was no significant difference for the median creatinine-adjusted urinary BPA concentration (μg/g Cr) among the different groups of time from recently RM date to recruitment (Wilcoxon χ2 = 6.48, P = 0.090) (Table 5). No significant rank correlation was found between the duration from RM date to recruitment and urinary BPA level (Spearman correlation coefficient rs = -0.081, P = 0.421).
Table 5. Time from recently RM date to recruitment and creatinine-adjusted urinary BPA concentration (μg/g Cr)*.
| Time from RM date to recruitment (days) | Mean±SD(n) | Median (P 25-P 75) | Min | Max |
|---|---|---|---|---|
| ≤5 | 11.93±51.29(26) | 0.45(0.32,1.66) | 0.10 | 262.46 |
| 6~18 | 10.42±36.80(25) | 1.15(0.51,3.68) | 0.26 | 185.26 |
| 19~114 | 1.50±1.76(26) | 0.78(0.32,1.88) | 0.02 | 6.79 |
| 115~352 | 3.09±3.48(25) | 1.69(0.72,4.22) | 0.05 | 10.93 |
* Wilcoxon χ2 = 6.48, P = 0.090
Discussion
In the present study, we found that the urinary BPA level of women who have experienced RM was significantly higher than the level measured in controls. The higher the urinary BPA levels of the women were, the stronger RM risk of 3~9 times was suggested. The time from recently RM date to recruitment does not influence the urinary BPA level, which has not been reported previously. The detection rates of urinary BPA reported in other countries range from 52% to 100% [38]. We found a reasonably similar detection rate in our study. The BPA level in our study was comparable to three previous studies [35, 39, 40] that reported median urinary BPA concentrations of 1.2μg/l, 1.3μg/l, and 1.2μg/l, respectively. After adjusting for creatinine, the BPA level was a little lower than two previous studies [31, 40] that reported median BPA levels of 1.7μg/g Cr and 2.2μg/g Cr, respectively. One study of a Chinese population without occupational exposure found a median urinary BPA concentration level below the LOD (0.31μg/l) [32]; the median urinary BPA of the controls in our study was 0.58ng/ml, slightly higher than the BPA level He reported. However, exposure to BPA is related to the changes in lifestyle in recent years. Thus, the exposure level to BPA may be different in different regions and over time [41–43].
To the best of our knowledge, this is the first epidemiologic study to investigate the association between urinary BPA level and RM risk in humans using a relatively large sample. The strengths of our methods also include the addition of an internal standard (d16-BPA), the relatively low limit of BPA detection and the adjustment of urinary BPA concentration by creatinine. In addition, we controlled the BMI variable in the logistic model, which was reported to be significantly associated with RM risk [44, 45].
Through two studies have been reported the association between the BPA exposure and miscarriage in women, the conclusion is inconsistent [22, 23]. To date, only Sugiura-Ogasawara and colleagues [11] have examined serum BPA levels using ELISA in 45 patients with a history of three or more (3–11) consecutive first-trimester miscarriages and 32 healthy women who had never given birth and had no history of infertility. The mean ± SD level of BPA in the patients was 2.59±5.23ng/ml, significantly higher than the level found in the controls (0.77±0.38ng/ml) (P = 0.024). This study suggested that high exposure to BPA may be associated with RM. Our results provided a different exposure biomarker in support of this conclusion.
Our findings were supported from animal and other human studies. It is clear that BPA has adverse effects on reproduction for animals in vitro and in vivo [17, 46, 47]. Lemos and colleagues showed that the success to achieve pregnancy decreased slightly with BPA concentration increasing in the terrestrial isopod Porcellio scaber, and there were 20% more miscarriages after exposure to 10 and 1000 mg BPA kg-1 dry soil [48]. In addition, an important study from China [19] found that zebrafish embryos exposed to BPA in early embryonic development exhibited higher levels of bioaccumulation and toxicity of BPA than in late embryonic development; they were also more likely to experience lethal or sublethal toxicity. For humans, BPA is a well- studied, well characterized, ubiquitous EDC. Previous studies demonstrated that serum BPA levels were associated with endometrial hyperplasia and ovarian dysfunction in females [27–29]. Balakrishnan and colleagues have demonstrated that BPA at low environmentally relevant levels can transfer across the human placenta [49]. What’s more, Nishikawa found that BPA‑GA is transferred into the fetus and deconjugated in the fetus [50]. There was a particularly worrying finding that human fetuses have also been contaminated with BPA [51, 52]. Serum BPA levels among women carrying fetuses with an abnormal karyotype were higher than the levels in women carrying fetuses with a normal karyotype [53]. In addition, a study found that there was a positive linear dose-response association between urinary BPA concentrations and implantation failure among women undergoing in vitro fertilization [54].
The major limitation of our study is that the BPA measurements were based on a spot urine sample. We could not acquire samples before or during the RM patients’ pregnancies, although this could have provided information about women’s daily BPA exposure during the critical period. Urinary BPA concentration has a moderate degree of intra-individual variability, making it difficult to accurately characterize exposure from a single measurement [31]. Braun et al. also mentioned that more than one sample may be necessary for BPA during pregnancy [55]. However, it has been reported that a single sample is predictive of BPA exposure over weeks or months, and a single sample can provide good sensitivity to classify a person’s exposure in epidemiologic studies [34, 56, 57]. Therefore, the urine samples we collected should be partly representative of the daily BPA exposure level of the subjects. An additional limitation is that the cases were not pregnant at the time of urine sample collection while the controls were. It cannot be excluded that the differences in BPA concentration between patients and controls could partly be due to one group being pregnant and the other not. There is recent evidence showing that serial urine concentrations of BPA were highly variable before and during pregnancy, these pregnancy induced changes may influence the absorption, distribution, metabolism, or excretion of BPA [55]. But, due to the limitation of the field conditions, we had difficulty to collect urine sample from the cases before or at the time of miscarriage. To resolve this problem, we consider to design a cohort study and to collect specimens according to the monitoring data in the future research. In this study, although we did not collect information about women chromosome abnormality and maternal endocrinologic disorders, because there is no evidence that the BPA level is associated with chromosome abnormalities and endocrine disorders, if the cases included a little number of such women, it would have underestimated the OR between the exposure and outcome. Calafat reported that BPA was associated with the use of medical devices [58]. Hence, our evidence need further studies in consideration of the medical devices, though according to our investigation of gynaecologists and obstetricians, most subjects who visited hospitals for RM were generally not treated with intravenous or intramuscular injections; instead, they were prescribed traditional Chinese medicine remedies for improving their endocrine secretion.
The biological mechanism of our findings and the above results of animals and human studies may be relevant to the chemical structure of BPA. It is similar to that of endogenous estrogens and can mimic the function of 17β-estradiol (E2) by combining with the estrogen receptors. So BPA can interfere with normal levels of hormones in the blood and functioning of estrogens, and further affect the reproductive development [55].
In conclusion, higher daily exposure to BPA was associated with an increased risk of RM, and that longitudinal studies are required to investigate the association between maternal BPA levels during pregnancy and RM.
Acknowledgments
We thank Jinfei Ding, Hao Peng, Jinzhong Xu, and Bo Yang for their kindly assistance in BPA measurement and paper writing.
Data Availability
All relevant data are within the paper.
Funding Statement
This project was supported by the National Natural Science Foundation of China (Grant No. 30872176) and the Soochow University Medical Development Foundation (EE126705). YP Shen received the funding and he had a role in study design, data collection and analysis.
References
- 1. Weiss A, Shalev E, Romano S. Hysteroscopy may be justified after two miscarriages. Human reproduction. 2005;20(9):2628–31. [DOI] [PubMed] [Google Scholar]
- 2. Daya S. Evaluation and management of recurrent spontaneous abortion. Current Opinion in Obstetrics and Gynecology. 1996;8(3):188–92. [PubMed] [Google Scholar]
- 3. Tulppala M, Palosuo T, Ramsay T, Miettinen A, Salonen R, Ylikorkala O. A prospective study of 63 couples with a history of recurrent spontaneous abortion: contributing factors and outcome of subsequent pregnancies. Human reproduction. 1993;8(5):764–70. [DOI] [PubMed] [Google Scholar]
- 4. Li T, Makris M, Tomsu M, Tuckerman E, Laird S. Recurrent miscarriage: aetiology, management and prognosis. Human reproduction update. 2002;8(5):463–81. [DOI] [PubMed] [Google Scholar]
- 5. Regan L, Owen EJ, Jacobs HS. Hypersecretion of luteinising hormone, infertility, and miscarriage. The Lancet. 1990;336(8724):1141–4. [DOI] [PubMed] [Google Scholar]
- 6. Stirrat G. Recurrent miscarriage I: definition and epidemiology. The Lancet. 1990;336(8716):673–5. [DOI] [PubMed] [Google Scholar]
- 7. Clifford K, Rai R, Watson H, Regan L. Pregnancy: An informative protocol for the investigation of recurrent miscarriage: preliminary experience of 500 consecutive cases. Human reproduction. 1994;9(7):1328–32. [DOI] [PubMed] [Google Scholar]
- 8. Daya S. Issues in the etiology of recurrent spontaneous abortion. Current Opinion in Obstetrics and Gynecology. 1994;6(2):153–9. [PubMed] [Google Scholar]
- 9. Greaves M, Cohen H, MacHin S, Mackie I. Guidelines on the investigation and management of the antiphospholipid syndrome. British journal of haematology. 2000;109(4):704–15. [DOI] [PubMed] [Google Scholar]
- 10. Homer HA, Li T-C, Cooke ID. The septate uterus: a review of management and reproductive outcome. Fertility and sterility. 2000;73(1):1–14. [DOI] [PubMed] [Google Scholar]
- 11. Sugiura-Ogasawara M, Ozaki Y, Sonta S-i, Makino T, Suzumori K. Exposure to bisphenol A is associated with recurrent miscarriage. Human reproduction. 2005;20(8):2325–9. [DOI] [PubMed] [Google Scholar]
- 12. Sugiura-Ogasawara M, Ozaki Y, Sonta S-i, Makino T, Suzumori K. PCBs, hexachlorobenzene and DDE are not associated with recurrent miscarriage. American Journal of Reproductive Immunology. 2003;50(6):485–9. [DOI] [PubMed] [Google Scholar]
- 13. Kang J-H, Kito K, Kondo F. Factors influencing the migration of bisphenol A from cans. Journal of Food Protection. 2003;66(8):1444–7. [DOI] [PubMed] [Google Scholar]
- 14. Le HH, Carlson EM, Chua JP, Belcher SM. Bisphenol A is released from polycarbonate drinking bottles and mimics the neurotoxic actions of estrogen in developing cerebellar neurons. Toxicology letters. 2008;176(2):149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kubwabo C, Kosarac I, Stewart B, Gauthier B, Lalonde K, Lalonde P. Migration of bisphenol A from plastic baby bottles, baby bottle liners and reusable polycarbonate drinking bottles. Food Additives and Contaminants. 2009;26(6):928–37. 10.1080/02652030802706725 [DOI] [PubMed] [Google Scholar]
- 16. Watts MM, Pascoe D, Carroll K. Chronic exposure to 17α-ethinylestradiol and bisphenol A-effects on development and reproduction in the freshwater invertebrate Chironomus riparius(Diptera: Chironomidae). Aquatic Toxicology. 2001;55(1):113–24. [DOI] [PubMed] [Google Scholar]
- 17. Honma S, Suzuki A, Buchanan DL, Katsu Y, Watanabe H, Iguchi T. Low dose effect of in utero exposure to bisphenol A and diethylstilbestrol on female mouse reproduction. Reproductive toxicology (Elmsford, NY). 2002;16(2):117 [DOI] [PubMed] [Google Scholar]
- 18. Herath CB, Jin W, Watanabe G, Arai K, Suzuki AK, Kazuyoshi Taya D. Adverse effects of environmental toxicants, octylphenol and bisphenol A, on male reproductive functions in pubertal rats. Endocrine. 2004;25(2):163–72. [DOI] [PubMed] [Google Scholar]
- 19. Duan Z-H, Zhang B-T, Zhu L. The toxicity and bioaccumulation of bisphenol A on developmental stages of zebrafish embryo China Environmental Scienc. 2008;3:018. [Google Scholar]
- 20. Zhang H-Q, Zhang X-F, Zhang L-J, Chao H-H, Pan B, Feng Y-M, et al. Fetal exposure to bisphenol A affects the primordial follicle formation by inhibiting the meiotic progression of oocytes. Molecular biology reports. 2012;39(5):5651–7. 10.1007/s11033-011-1372-3 [DOI] [PubMed] [Google Scholar]
- 21. Chao H-H, Zhang X-F, Chen B, Pan B, Zhang L-J, Li L, et al. Bisphenol A exposure modifies methylation of imprinted genes in mouse oocytes via the estrogen receptor signaling pathway. Histochemistry and cell biology. 2012;137(2):249–59. 10.1007/s00418-011-0894-z [DOI] [PubMed] [Google Scholar]
- 22. Lathi RB, Liebert CA, Brookfield KF, Taylor JA, vom Saal FS, Fujimoto VY, et al. Conjugated bisphenol A in maternal serum in relation to miscarriage risk. Fertility and sterility. 2014;102(1):123–8. 10.1016/j.fertnstert.2014.03.024 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen X, Chen M, Xu B, Tang R, Han X, Qin Y, et al. Parental phenols exposure and spontaneous abortion in Chinese population residing in the middle and lower reaches of the Yangtze River. Chemosphere. 2013;93(2):217–22. 10.1016/j.chemosphere.2013.04.067 [DOI] [PubMed] [Google Scholar]
- 24. Meeker JD, Ehrlich S, Toth TL, Wright DL, Calafat AM, Trisini AT, et al. Semen quality and sperm DNA damage in relation to urinary bisphenol A among men from an infertility clinic. Reproductive toxicology. 2010;30(4):532–9. 10.1016/j.reprotox.2010.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li D, Zhou Z, Qing D, He Y, Wu T, Miao M, et al. Occupational exposure to bisphenol-A (BPA) and the risk of self-reported male sexual dysfunction. Human reproduction. 2010;25(2):519–27. 10.1093/humrep/dep381 [DOI] [PubMed] [Google Scholar]
- 26. Bloom MS, vom Saal FS, Kim D, Taylor JA, Lamb JD, Fujimoto VY. Serum unconjugated bisphenol A concentrations in men may influence embryo quality indicators during in vitro fertilization. Environmental toxicology and pharmacology. 2011;32(2):319–23. 10.1016/j.etap.2011.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hiroi H, Tsutsumi O, Takeuchi T, Momoeda M, Ikezuki Y, Okamura A, et al. Differences in serum bisphenol a concentrations in premenopausal normal women and women with endometrial hyperplasia. Endocrine journal. 2004;51(6):595 [DOI] [PubMed] [Google Scholar]
- 28. Takeuchi T, Tsutsumi O, Ikezuki Y, Takai Y, Taketani Y. Positive relationship between androgen and the endocrine disruptor, bisphenol A, in normal women and women with ovarian dysfunction. Endocrine journal. 2004;51(2):165 [DOI] [PubMed] [Google Scholar]
- 29. Politch JA. Bisphenol A and risk assessment. Environmental Health Perspectives. 2006;114(1):A16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Völkel W, Colnot T, Csanády GA, Filser JG, Dekant W. Metabolism and kinetics of bisphenol A in humans at low doses following oral administration. Chemical research in toxicology. 2002;15(10):1281–7. [DOI] [PubMed] [Google Scholar]
- 31. Braun JM, Yolton K, Dietrich KN, Hornung R, Ye X, Calafat AM, et al. Prenatal bisphenol A exposure and early childhood behavior. Environmental Health Perspectives. 2009;117(12):1945 10.1289/ehp.0900979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. He Y, Miao M, Herrinton LJ, Wu C, Yuan W, Zhou Z, et al. Bisphenol A levels in blood and urine in a Chinese population and the personal factors affecting the levels. Environmental research. 2009;109(5):629–33. 10.1016/j.envres.2009.04.003 [DOI] [PubMed] [Google Scholar]
- 33. Matsumoto A, Kunugita N, Kitagawa K, Isse T, Oyama T, Foureman GL, et al. Bisphenol A levels in human urine. Environmental Health Perspectives. 2003;111(1):101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. García-Prieto A, Lunar ML, Rubio S, Pérez-Bendito D. Determination of urinary bisphenol A by coacervative microextraction and liquid chromatography-fluorescence detection. Analytica chimica acta. 2008;630(1):19–27. 10.1016/j.aca.2008.09.060 [DOI] [PubMed] [Google Scholar]
- 35. Ye X, Pierik FH, Hauser R, Duty S, Angerer J, Park MM, et al. Urinary metabolite concentrations of organophosphorous pesticides, bisphenol A, and phthalates among pregnant women in Rotterdam, the Netherlands: the Generation R study. Environmental research. 2008;108(2):260 10.1016/j.envres.2008.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cantonwine D, Meeker JD, Hu H, Sánchez BN, Lamadrid-Figueroa H, Mercado-García A, et al. Bisphenol a exposure in Mexico City and risk of prematurity: a pilot nested case control study. Environmental Health. 2010;9:62 10.1186/1476-069X-9-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hornung RW, Reed LD. Estimation of average concentration in the presence of nondetectable values. Applied occupational and environmental hygiene. 1990;5(1):46–51. [Google Scholar]
- 38. Vandenberg LN, Hauser R, Marcus M, Olea N, Welshons WV. Human exposure to bisphenol A (BPA). Reproductive Toxicology. 2007;24(2):139–77. [DOI] [PubMed] [Google Scholar]
- 39. Ouchi K, Watanabe S. Measurement of bisphenol A in human urine using liquid chromatography with multi-channel coulometric electrochemical detection. Journal of Chromatography B. 2002;780(2):365–70. [DOI] [PubMed] [Google Scholar]
- 40. Wolff MS, Engel SM, Berkowitz GS, Ye X, Silva MJ, Zhu C, et al. Prenatal phenol and phthalate exposures and birth outcomes. Environmental health perspectives. 2008;116(8):1092 10.1289/ehp.11007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yang M, Kim S-Y, Lee S-M, Chang S-S, Kawamoto T, Jang J-Y, et al. Biological monitoring of bisphenol A in a Korean population. Archives of environmental contamination and toxicology. 2003;44(4):0546–51. [DOI] [PubMed] [Google Scholar]
- 42. Lang IA, Galloway TS, Scarlett A, Henley WE, Depledge M, Wallace RB, et al. Association of urinary bisphenol A concentration with medical disorders and laboratory abnormalities in adults. JAMA: the journal of the American Medical Association. 2008;300(11):1303–10. 10.1001/jama.300.11.1303 [DOI] [PubMed] [Google Scholar]
- 43. LaKind JS, Levesque J, Dumas P, Bryan S, Clarke J, Naiman DQ. Comparing United States and Canadian population exposures from National Biomonitoring Surveys: Bisphenol A intake as a case study. Journal of Exposure Science and Environmental Epidemiology. 2012;22(3):219–26. 10.1038/jes.2012.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wang JX, Davies MJ, Norman RJ. Obesity increases the risk of spontaneous abortion during infertility treatment. Obesity research. 2002;10(6):551–4. [DOI] [PubMed] [Google Scholar]
- 45. Bellver J, Rossal LP, Bosch E, Zúñiga A, Corona JT, Meléndez F, et al. Obesity and the risk of spontaneous abortion after oocyte donation. Fertility and sterility. 2003;79(5):1136–40. [DOI] [PubMed] [Google Scholar]
- 46. Richter CA, Birnbaum LS, Farabollini F, Newbold RR, Rubin BS, Talsness CE, et al. In vivo effects of bisphenol A in laboratory rodent studies. Reproductive toxicology (Elmsford, NY). 2007;24(2):199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wetherill YB, Akingbemi BT, Kanno J, McLachlan JA, Nadal A, Sonnenschein C, et al. In vitro molecular mechanisms of bisphenol A action. Reproductive Toxicology. 2007;24(2):178–98. [DOI] [PubMed] [Google Scholar]
- 48. Lemos M, Van Gestel C, Soares A. Reproductive toxicity of the endocrine disrupters vinclozolin and bisphenol A in the terrestrial isopod Porcellio scaber(Latreille, 1804). Chemosphere. 2010;78(7):907–13. 10.1016/j.chemosphere.2009.10.063 [DOI] [PubMed] [Google Scholar]
- 49. Balakrishnan B, Henare K, Thorstensen EB, Ponnampalam AP, Mitchell MD. Transfer of bisphenol A across the human placenta. American journal of obstetrics and gynecology. 2010;202(4):393 e1-. e7. 10.1016/j.ajog.2010.01.025 [DOI] [PubMed] [Google Scholar]
- 50. Nishikawa M, Iwano H, Yanagisawa R, Koike N, Inoue H, Yokota H. Placental transfer of conjugated bisphenol A and subsequent reactivation in the rat fetus. Environmental health perspectives. 2010;118(9):1196 10.1289/ehp.0901575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sakurai K, Mori C. Fetal exposure to endocrine disruptors]. Nihon rinsho Japanese journal of clinical medicine. 2000;58(12):2508 [PubMed] [Google Scholar]
- 52. Brock JW, Yoshimura Y, Barr JR, Maggio VL, Graiser SR, Nakazawa H, et al. Measurement of bisphenol A levels in human urine. Journal of exposure analysis and environmental epidemiology. 2001;11(4):323 [DOI] [PubMed] [Google Scholar]
- 53. Yamada H, Furuta I, Kato EH, Kataoka S, Usuki Y, Kobashi G, et al. Maternal serum and amniotic fluid bisphenol A concentrations in the early second trimester. Reproductive Toxicology. 2002;16(6):735–9. [DOI] [PubMed] [Google Scholar]
- 54. Ehrlich S, Williams PL, Missmer SA, Flaws JA, Berry KF, Calafat AM, et al. Urinary bisphenol A concentrations and implantation failure among women undergoing in vitro fertilization. Environmental health perspectives. 2012;120(7):978 10.1289/ehp.1104307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Braun JM, Smith KW, Williams PL, Calafat AM, Berry K, Ehrlich S, et al. Variability of urinary phthalate metabolite and bisphenol A concentrations before and during pregnancy. Environmental health perspectives. 2012;120(5):739 10.1289/ehp.1104139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mahalingaiah S, Meeker JD, Pearson KR, Calafat AM, Ye X, Petrozza J, et al. Temporal variability and predictors of urinary bisphenol A concentrations in men and women. Environmental Health Perspectives. 2008;116(2):173 10.1289/ehp.10605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Teitelbaum S, Britton J, Calafat A, Ye X, Silva M, Reidy J, et al. Temporal variability in urinary concentrations of phthalate metabolites, phytoestrogens and phenols among minority children in the United States. Environmental research. 2008;106(2):257–69. [DOI] [PubMed] [Google Scholar]
- 58. Calafat AM, Weuve J, Ye X, Jia LT, Hu H, Ringer S, et al. Exposure to bisphenol A and other phenols in neonatal intensive care unit premature infants. Environmental Health Perspectives. 2009;117(4):639 10.1289/ehp.0800265 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.


