Abstract
B lymphopoiesis in bone marrow (BM) is critical for maintaining a diverse peripheral B cell pool to fight infection and establish lifelong immunity. The generation of immature B cells is reduced in Flt3-ligand (FL-/-) mice leading to deficiencies in splenic B cells. Here, we sought to understand the cellular basis of the spleen B cell deficiency in FL-/- mice. Significant reductions in transitional (TS) and follicular (FO) B cells were found in FL-/- mice, and increased frequencies, but not absolute numbers, of marginal zone (MZ) B cells. BAFF-R expression on splenic B cells and serum levels of B cell activating factor (BAFF) was comparable to wildtype (WT) mice. Mixed BM chimeras revealed that the reductions in TS and FO B cells were cell extrinsic. FL administration into FL-/- mice restored the deficiency in TS B cells and normalized the MZ compartment. Ki67 analysis revealed a significant decrease in the proliferative capacity of TS B cells in FL-/- mice. A Bcl2 transgene did not rescue TS cells in FL-/- mice, uncoupling FL-deficiency to Bcl2-dependent survival pathways. Upregulation of CD1d expression and adoptive transfer experiments suggested MZ skewing in FL-/- mice. These findings support an integral role for Flt3 signaling in peripheral B cell maturation.
Keywords: B lymphopoiesis, B cell maturation, BAFF, Flt3 signaling, follicular B cells, marginal zone B cells, proliferation, transitional B cells
Introduction
In adult mice, B-lymphocytes are produced in the bone marrow (BM) as one of the lineage progeny of pluripotent hematopoietic stem cells. Upon successful rearrangement of the immunoglobulin heavy and light chain genes, B cell precursors express surface IgM and then are tested for self-reactivity. Non-self-reactive, immature B cells either initiate B cell maturation in the BM or exit the marrow, enter the blood, and travel to the spleen to complete maturation 1. Three major subsets of B cells can be distinguished in the spleen. Transitional B cells are newly produced and short-lived 2,3. They survive only 2–4 days unless selected into one of the other two major splenic B cell subsets, follicular (FO), or marginal zone (MZ) B cells 2,4. FO B cells comprise the major pool of recirculating B cells, while MZ B cells reside in the marginal sinus and in mice, do not recirculate.
Various cellular pathways for B cell maturation in the periphery have been characterized 2–7. TS B cells are IgMhi, express the B-cell precursor antigen CD93/AA4, and varying levels of CD21/35. TS B cells are fractionated into two additional subsets, transitional 1 (T1) and transitional 2 (T2). T1 cells are CD21/35−, CD23−, and IgD−/lo, while T2 cells are CD21/35int, CD23+, and IgD+/hi. T1 cells give rise to T2 cells that are the major precursor for FO and MZ B cells. FO B cells are IgM+lo, CD21/35int, IgD+, CD23+, and CD24+lo, and MZ B cells are IgMhi, CD21/35hi, IgDlo, CD23−, and CD1dhi.
B cell maturation is dependent on multiple factors including responsiveness to multivalent antigen, BAFF-R signaling, and BCR signaling in order to select non-self-reactive B cells that comprise the long-lived mature B cell pool. T1 B cells are the least mature splenic B cell subset, are in the G0/G1 phase of the cell cycle, and are nonresponsive to multivalent antigen and B cell activating factor (BAFF). On the other hand, T2 B cells are in cycle and have acquired the ability to robustly respond to multivalent antigen and BAFF 1,4,8. BCR signaling is critical for the transition from T1 to T2 as mice lacking the cytoplasmic tail of Igα have no detectable T2, MZ, or mature B cells 3. In particular, BCR signaling has been hypothesized to induce a proliferative response at this stage necessary for the development of T2 B cells 3. The specific events that drive peripheral B cell maturation downstream of the BCR are unclear, but have been linked to the splenic microenvironment.
The selection of TS B cells into the FO or MZ B cell pools is a critical, yet incompletely understood step in peripheral B cell maturation. Currently, two models have been proposed to mechanistically understand the FO versus MZ cell fate decision: the BCR signal strength model and the production bottleneck model 9,10. The signal strength model posits that if a T2-follicular precursor receives a strong signal via its BCR, then it is induced to differentiate into a FO B cell in a Btk-dependent manner. Alternatively, if the same precursor reacts weakly to antigen, it survives through signals provided by BAFF and weak BCR signaling and becomes competent to inductive signals that promote the MZ B cell fate. The production bottleneck model posits that when B lymphopoiesis in BM is reduced, there is enhanced MZ B cell production. Although the mechanism driving this model is yet to be understood, expansion of the MZ compartment is hypothesized to be necessary to maintain a diverse repertoire of natural IgM.
Microenvironmental signals play a critical role in peripheral B cell maturation. BAFF is a type II membrane protein and a member of the TNF family expressed in either cell bound or soluble forms by radio-resistant stromal cells, monocytes, macrophages, dendritic cells, and T cells, albeit at low levels 11,12. BAFF production is influenced by cytokines including interferon-α, interferon-γ, granulocyte-colony stimulating factor, CD40-ligand, lipopolysaccharide, and peptidoglycans 11. T1 cells express low levels of the receptor for BAFF, BAFF-R, and are largely nonresponsive to BAFF-R signaling 11. In contrast, T2, FO, and MZ B cells are responsive to and dependent on BAFF-R signaling 11. The critical role of BAFF-R signaling in peripheral B cell survival and homeostasis has been determined through combined analysis of mutant and transgenic mice and exogenous administration experiments in vivo and in vitro (reviewed in 13).
Flt3-ligand (FL) is a soluble and membrane bound cytokine produced by stromal cells, hematopoietic progenitors, and activated T cells 14. FL binding to its cognate receptor, Flt3, is critical to establish normal levels of Flt3+ multipotential progenitors in BM, maintain the lymphoid progenitor pool, and regulate the number of B cells generated from lymphoid progenitors 15–18. Mice deficient in FL have reduced splenic and lymph node cellularity, reductions in B cells, NK cells, and DCs, and early thymic progenitors 19,20. Flt3 expression is silenced at an early stage in B cell development in BM (after the Pro-B cell stage) and is not re-expressed on splenic B cells (The Immunological Genome Project, http://www.immgen.org/)21. Thus, reductions in splenic B lymphocytes in FL-/- mice are likely cell extrinsic.
Herein, we document select deficiencies in T1, T2, and FO B cells in FL-/- mice. Serum levels of BAFF and cell surface expression of BAFF-R on splenic B cells in FL-/- mice were comparable to WT mice, suggesting BAFF-independent regulation. Radiation chimeras confirmed that the deficiencies in TS and FO B cell subsets were cell extrinsic. FL replacement therapy in FL-/- mice rescued the TS and FO B cell deficiencies and normalized frequencies of MZ B cells. We show that FL deficiency impairs the proliferation, but not survival of TS B cells. Finally, we provide two pieces of evidence that suggest that FL deficiency skews TS B cell maturation into the MZ B cell fate. First, FL-/- mice display an upregulation of CD1d, a hallmark of MZ B cells, starting in T1 cells. Second, WT T1 cells generated an increased frequency of MZ cells when adoptively transferred into FL-/- mice in comparison to WT mice. These new data suggest an integral indirect role for Flt3 signaling in regulation of B cell maturation in the spleen.
Results
Mice deficient for Flt3-ligand have reductions in TS and FO B cells in the spleen
Flt3 signaling sets the threshold for B lymphopoiesis in BM 15. Consistent with the reduction in B cell precursors in FL-/- mice, numbers of immature B cells that have completed the B lineage differentiation program are reduced (Supporting Information Fig. S1). Immature B cells in BM are identified as IgM+CD24hi and recirculating B cells as IgM+CD24lo 5,6. Enumeration of IgM+CD24lo recirculating B cells in the marrow revealed a statistically significant decrease (Supporting Information Fig. S1). This observation prompted further evaluation of peripheral B cell development in FL-/- mice.
Spleen cellularity is reduced in FL-/- mice and our results confirmed this finding (1.24 × 108 ± 8.85 × 106 vs. 6.74 × 107 ± 8.42 × 106, WT vs. FL-/-, respectively; P < 0.0001) 19. Percentages, and to a greater extent, numbers of total CD19+ splenic B lymphocytes are reduced in FL-/- mice (Fig. 1A–C). TS, FO, and MZ B subsets can be distinguished by differential expression of IgM and CD21/35. Total TS cells include recent emigrants from the BM and are reduced (Fig. 1A, 9.15 ± 0.72% vs. 2.84 ± 0.19% of CD19+ cells, WT vs. FL-/-, respectively; P < 0.0001). T1 cells are discriminated from T2 by differential expression of CD23. Both T1 and T2 subsets are reduced in FL-/- mice (Fig. 1A–C). Percentages of FO cells were not affected by FL deficiency, although absolute numbers were significantly reduced, consistent with the reduction in splenic cellularity (Fig. 1A–C). MZ B cells are not reduced by FL-deficiency 22. Indeed, percentages of MZ B cells are significantly increased in FL-/- mice (Fig. 1A and B). However, as a consequence of reduced spleen cellularity, absolute numbers of MZ B cells are comparable to WT mice (Fig. 1C). This result is identical for MZ precursors (MZP) (IgMhiCD21/CD35hiCD23+, data not shown) 7. Taken together, these data show selective reductions in TS and FO B splenic subsets in FL-deficient mice.
Figure 1.
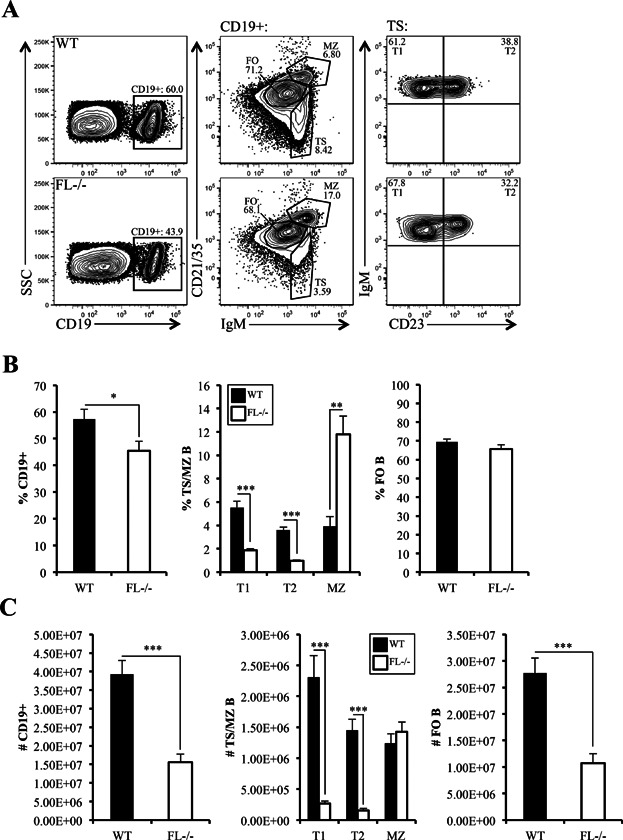
Impaired peripheral B cell maturation in FL-/- mice. (A) Flow cytometric analysis of splenic CD19+ B cells from a representative wild-type (WT) and FL-/- mouse further stained by CD21/35, IgM, and CD23 to examine transitional (TS), marginal zone (MZ), and follicular (FO) B cell subsets. TS B cells are further stained using CD23 to characterize T1 and T2 B cells (right panels). (B and C) Bar graphs illustrating the frequency (B) and absolute numbers (C) of total CD19+ and T1, T2, MZ, and FO B cell subsets in the spleens of WT and FL-/- mice. The bars represent WT (black) or FL-/- (white). (B) Frequencies reflect the proportion these cells represent within the CD19+ fraction of spleen. (A–C) Data are representative of 15–16 mice/genotype and six independent experiments. Error bars represent mean ± SEM. *, **, and *** represent statistically significant differences measured using the Student's t-test at P ≤ 0.05, P ≤ 0.005, and P < 0.0001, respectively.
Reduced BM B cell output does not explain defective peripheral B cell maturation in FL-/- mice
Hoxa9-/- and FL-/- mice exhibit similar reductions in B lymphopoiesis in BM 23. Therefore, we sought to determine if Hoxa9-/- mice had a similar defect in peripheral B cell maturation as FL-/- mice. As shown in Table1, TS B cells are reduced in Hoxa9-/- mice, but not to the same magnitude as in FL-/- mice. TS B cells in Hoxa9-/- mice are reduced 40%, while TS B cells in FL-/- mice are decreased 70% compared to WT. Furthermore, while slightly elevated, frequencies of MZ B cells in Hoxa9-/- mice are not significantly different from WT (Table1). Similar to FL-/- mice, HoxA9-/- mice displayed no alterations in the frequency of FO B cells (Table1). Thus, reductions in BM B cell output alone cannot explain the peripheral B cell maturation defect in FL-/- mice.
Frequencies of TS, MZ, and FO B cells in mice deficient for FL or HoxA9
| Mice | TS | MZ | FO |
|---|---|---|---|
| Wild-type | 9.3 ± 0.9 | 5.4 ± 0.9 | 63.1 ± 0.8 |
| HoxA9-/- | 5.6 ± 0.8* | 10.7 ± 2.7 | 63.3 ± 2.2 |
| FL-/- | 2.9 ± 0.3* | 16.3 ± 1.8* | 60.3 ± 2.3 |
Data represents mean ± SEM of peripheral B cell subsets (% of CD19+).
n = 6 mice/genotype.
Significance at P ≤ 0.01 between wild-type and FL-/- or HoxA9-/-.
Reductions in TS and FO B cell subsets in FL-/- mice are not due to defects in BAFF or BAFF-R
Flt3 expression is silenced at the Pro-B cell stage of B cell differentiation in BM and is not re-expressed on splenic B cells (The Immunological Genome Project, http://www.immgen.org) 21. Thus, the reduction in TS and FO B cells is not due to a cell intrinsic defect in peripheral B cell maturation directly regulated by Flt3 signaling. One growth factor important for peripheral B cell homeostasis and selection is BAFF 8,24. Serum levels of BAFF are not altered by FL deficiency (Fig. 2A). BAFF-R expression is upregulated at the T1 to T2 transition and no alteration in surface expression of BAFF-R was observed in T1 or T2 cells in FL-/- mice (Fig. 2B and C) 4. Likewise, BAFF-R expression on FO and MZ B cells was not impaired in FL-/- mice (Fig. 2B). These data suggest that the reductions in TS and FO B cell subsets in FL-/- mice are BAFF-independent.
Figure 2.
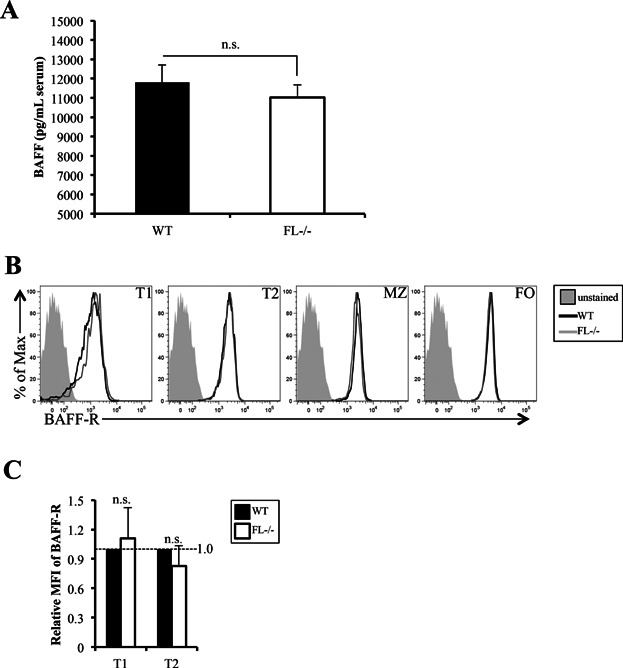
Reductions in TS and FO B cell subsets in FL-/- mice are not due to defects in BAFF or BAFF-R. (A) Concentration of BAFF (pg/mL) in the serum of wild-type (WT) and FL-/- mice as determined by ELISA. Data is representative of seven mice/genotype. (B) Flow cytometric analysis of BAFF-R in T1, T2, MZ, and FO B cell subsets. The filled histogram represents the unstained control; the black line indicates BAFF-R staining in T1, T2, MZ, or FO subsets in WT mice, and the gray line for FL-/- mice. (C) Bar graph depicting the relative mean fluorescence intensity (MFI) of BAFF-R in WT (normalized to 1, further described in Materials and Methods) and FL-/- T1 and T2 B cells. The bars represent WT (black) or FL-/- (white). (B and C) Data are representative of nine mice/genotype and three independent experiments. (A and C) Error bars represent mean ± SEM. n.s. signifies non-statistical differences measured using the Student's t-test (P > 0.05) between the means of different genotypes.
Reductions in TS and FO B cell subsets in FL-/- mice are cell extrinsic
Next, we set out to experimentally determine if the reduction in TS and FO B cells in FL-/- mice were cell extrinsic. To make this determination, mixed BM radiation chimeras were generated and examined after 10 weeks. CD45.2+ WT or FL-/- B cells were first gated on CD19+, and TS, FO, and MZ B cell subsets were then evaluated within this population (Fig. 3A). As shown in Figure 3A–C, transplantation of FL-/- BM cells into WT recipients corrected the deficiencies in TS and FO B cell subsets, and normalized MZ frequencies. Percentages and numbers of total CD19+ cells, TS, FO, and MZ B cell subsets in the chimeras were comparable to that generated from CD45.2+ WT mice (Fig. 3A–C). We conclude from this experimental data that the defective peripheral B cell maturation observed in the FL-/- mice is cell extrinsic.
Figure 3.
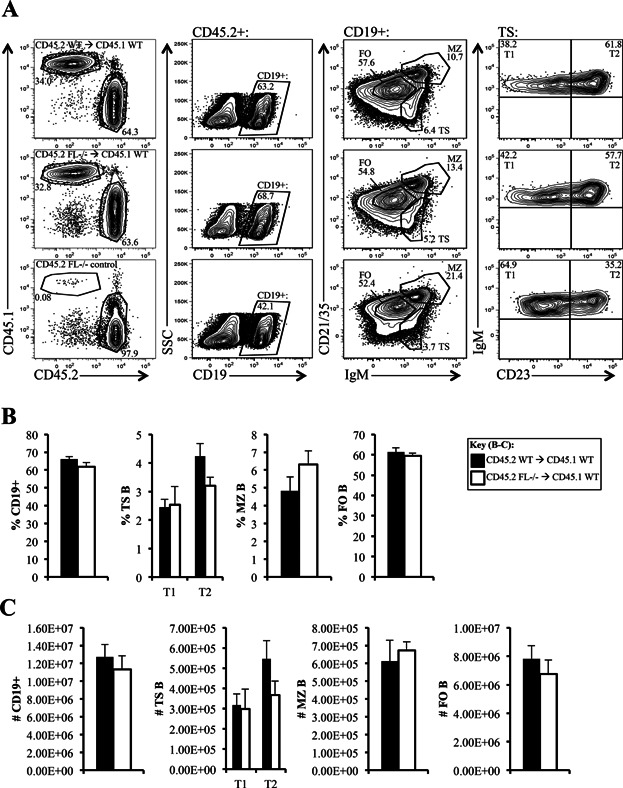
Cell extrinsic defect in peripheral B cell maturation in FL-/- mice. (A) Flow cytometric analysis of splenic B cells from a representative host CD45.1 wild-type (WT) mouse that received 2 million CD45.2 WT BM cells (top panels) or CD45.2 FL-/- BM cells (middle panels). CD45.2 FL-/- mice served as uninjected controls (bottom panels). At 10 weeks, spleens from these mice were harvested and initially analyzed for CD45.1 versus CD45.2 to identify transplanted cells as CD45.2+. CD45.2+ were further fractionated using CD19, CD21/35, IgM, and CD23 to examine total CD19+, TS, MZ, and FO B cell subsets. TS B cells are further stained using CD23 to characterize CD23− T1 and CD23+ T2 B cells (far right panels). (B and C) Bar graphs illustrating the frequency (B) and numbers (C) of total CD19+ and T1, T2, MZ, and FO B cell subsets in the spleens of CD45.1 WT hosts that received CD45.2 WT (black) or CD45.2 FL-/- bone marrow (white). Error bars represent mean +/− SEM. (B) Frequencies reflect the proportion these cells represent within the CD19+ fraction of spleen. (A–C) Data is representative of five chimeras per group.
Reductions in TS B cell subsets in FL-/- mice are corrected by exogenous FL administration
We showed reductions in frequencies and numbers of TS B cells in FL-/- mice. Next, we evaluated if exogenous administration of FL into FL-/- mice was sufficient to restore TS B cells. Ten micrograms of recombinant mouse FL was administered into FL-/- mice every other day for 8 days. PBS injected into WT or FL-/- mice served as positive and negative controls for FL restoration of TS B cells. On day 2 (Fig. 4A) or day 5 (Fig. 4B) post-FL or PBS injection, splenic B cells were evaluated by flow cytometry. On day 2 following cessation of FL administration, frequencies of TS B cells were unchanged compared to PBS injected FL-/- mice (Fig. 4A and C). In contrast, on day 5 following cessation of FL administration, frequencies of TS B cell numbers were substantially increased in FL-/- mice (Fig. 4B and D). These data suggest that the TS B cell pool is temporally sensitive to FL-induced alterations to the splenic microenvironment.
Figure 4.
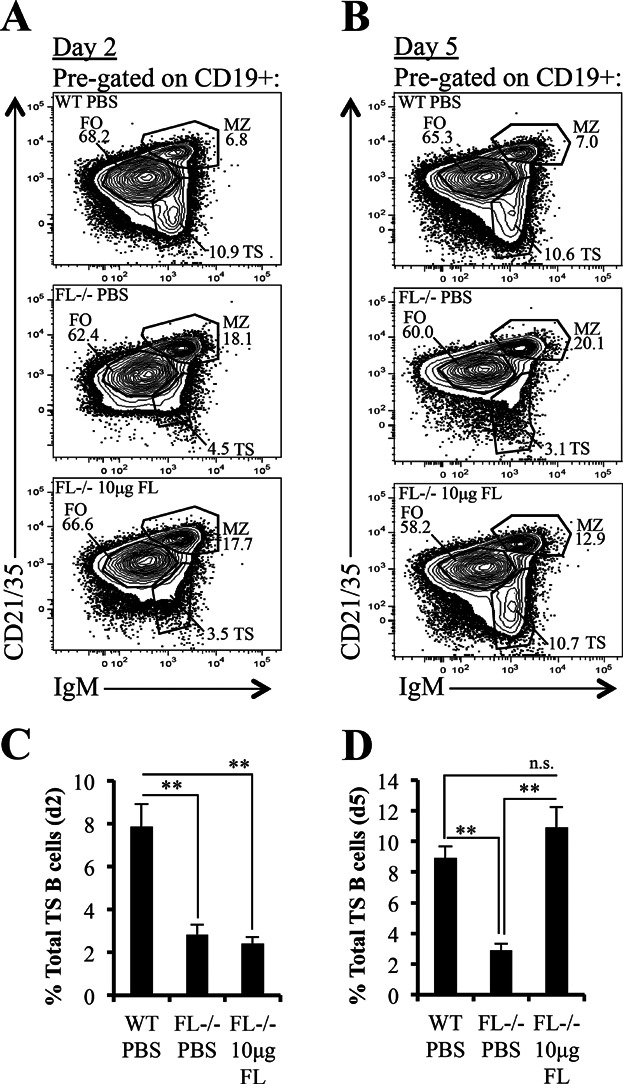
B cell deficiency in FL-/- mice is corrected by exogenous FL administration. (A and B) Flow cytometric analysis of spleen taken 2 days (A) or 5 days (B) after either the last injection of PBS (control mice) or FL. Spleen (pre-gated on CD19+) is stained with antibodies against CD21/35 and IgM to visualize TS, MZ, and FO B cell subsets in PBS-injected wild-type mice (WT PBS) (top panels), PBS-injected FL-/- mice (FL-/- PBS) (middle panels), and FL-injected FL-/- mice (FL-/- 10 µg FL) (bottom panels). (C and D) Bar graphs illustrating the cell frequencies of TS B cells harvested from spleens 2 days (C) or 5 days (D) after either the last injection of PBS (control mice) or FL. (A–D) Data are representative of 4–6 mice/genotype and 2–3 independent experiments. Error bars represent mean ± SEM. ** represent statistically significant differences measured using the Student's t-test at P ≤ 0.005 between the means of different conditions. n.s. signifies non-statistical differences measured using the Student's t-test (P > 0.05) between the means of different conditions.
Reductions in percentages of proliferating TS B cells in FL-/- mice
T1 cells are largely comprised of recent emigrants from the BM. Since the generation of immature B cells is reduced in BM of FL-/- mice, it was not surprising that the T1 population was reduced. Reductions in non-cycling T1 cells could be presumed to reduce the T2 subset as well. A previous study established that T2 B cells are cycling in vivo 4. We showed above that reduction in TS and FO B cells in FL-/- mice was cell extrinsic. To determine if the cell extrinsic defect altered the proliferative capacity of TS and FO B cells in vivo, we compared Ki67 expression in WT and FL-/- mice. Ki67 is a nuclear proliferation antigen expressed in G1, S, and G2 phases of the cell cycle 25. It is not expressed in quiescent or resting cells in G0. Importantly, Ki67 expression has been shown to be particularly informative regarding the potential for cellular division, not just the actual cycling state of a given cell 26. Consistent with that interpretation, the vast majority of T1 and T2 cells in WT mice express Ki67 protein, while substantially fewer MZP, MZ, and FO B cells are Ki67+ (Fig. 5A and B). Importantly, we document a statistically significant reduction in percentages of T1, T2, and FO B cells in FL-/- mice that express Ki67 protein (Fig. 5B). These data indicate that the cell extrinsic defect in FL-/- spleen negatively impacts the proliferative capacity of TS, and to a lesser, but statistically significant extent, FO B cells.
Figure 5.
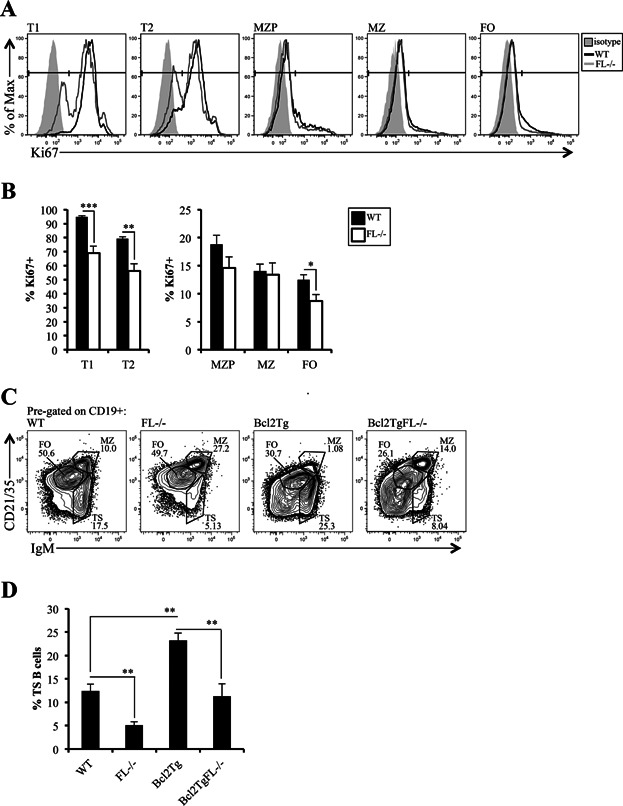
Proliferative capacity, but not survival is impaired in TS B cells in FL-/- mice. (A) Intracellular flow cytometric analysis of Ki67 in T1, T2, MZP, MZ, and FO B cell subsets. The filled histogram represents the wild-type (WT) isotype control; the black line indicates Ki67 staining in T1, T2, MZ, MZP, or FO subsets in WT, and the gray line for FL-/- mice. FL-/- isotype and WT and FL-/- unstained controls looked identical to WT isotype control staining (data not shown). T1, T2, MZ, and FO B cell subsets are immunophenotypically defined in Figures 1 and 3. MZP are CD19+ CD21/CD35hi IgM+ CD23+. (B) Bar graphs depicting the frequency of Ki67+ cells in WT and FL-/- T1, T2, MZP, MZ, and FO B cells. The bars represent WT (black) or FL-/- (white). (A and B) Data are representative of 11 mice/genotype and four independent experiments. (C) Flow cytometric analysis (pre-gated on CD19+) to examine TS, MZ, and FO B cell subsets in spleens of WT, FL-/-, Eu-Bcl2Tg (Bcl2Tg), and Eu-Bcl2Tg FL-/- (Bcl2TgFL-/-) mice. (D) Bar graph illustrating percentages of TS B cells across the four genotypes displayed in (C). (C and D) Data are representative of 5–8 mice/genotype and four independent experiments. (B and D) Error bars represent mean ± SEM. *, **, and *** represent statistically significant differences measured using the Student's t-test at P ≤ 0.05, P ≤ 0.005, and P < 0.0001, respectively, between the means of different genotypes.
Forced expression of a survival gene does not rescue TS B cells in FL-/- mice
Survival signals are critical for peripheral B cell maturation. To determine if the deficiency in TS B cells in FL-/- mice is due to impaired survival, we bred FL-/- to Eu-Bcl2Tg mice 27. Expression of the Bcl2 transgene did not rescue the decrease in splenic cellularity due to FL-deficiency (4.07 × 108 ± 4.67 × 107 vs. 2.53 × 108 ± 6.64 × 107, Eu-Bcl2Tg vs. Eu-Bcl2Tg FL-/-, respectively; P = 0.074). Although it did not reach statistical significance, it was similar (38%) to the decline in splenic cellularity we observed between WT and FL-/- (46%). Percentages of TS cells were increased in Eu-Bcl2Tg mice compared to WT (Fig. 5C and D). Next, we compared frequencies of TS cells between Eu-Bcl2Tg and Eu-Bcl2Tg FL-/- mice. We observed a similar reduction in percentages of TS cells between Eu-Bcl2Tg and Eu-Bcl2Tg FL-/- mice (51%) that we observed between WT and FL-/- mice (59%; Fig. 5C and D). Likewise, reductions in absolute numbers of TS cells between Eu-Bcl2Tg (2.19 × 107 ± 5.51 × 106) and Eu-Bcl2Tg FL-/- (5.21 × 106 ± 3.39 × 106) mice (76%, P = 0.036) were comparable to that displayed between WT (1.51 × 106 ± 3.51 × 105) and FL-/- (3.20 × 105 ± 4.92 × 104) mice (79%, P = 0.0049). These experimental findings suggest that reductions in TS cells in FL-/- mice are not due to impaired Bcl2-regulated cell survival pathways.
Evidence of marginal zone skewing within the T1 and T2 subsets in FL-/- mice
It has been observed that reduced B cell genesis in BM leads to enhanced MZ B cell production and a corresponding reduction in FO B cells (reviewed in 10). FL-/- mice that have reduced production of B cells in the BM faithfully recapitulate this phenomenon (Supporting Information Fig. S1 and Fig. 1). It has been postulated that under conditions of immunodeficiency, humoral mediators involved in homeostatic proliferation and differentiation might increase the MZ cell niche and drive MZ B cell expansion 9. We, therefore, theorized that loss of an appropriate environmental signal due to FL-deficiency might selectively promote MZ B cell development from TS cells. MZP are enriched in the IgMhi CD21/35hi CD23hi fraction of CD19+ splenic B cells that express high levels of the glycoprotein CD1d 9,28,29. As shown in Figure 6A, elevated CD1d expression is first detectable in CD19+ B cells expressing intermediate levels of CD21/35 then greatly upregulated on MZ B cells that express high levels of CD21/35. FL-/- mice displayed an overall increase in frequency of CD1dhi cells, suggesting MZ skewing (Fig. 6A). Next, we sought to determine if skewing of the MZ fate decision occurred in TS cells. TS cells were initially discriminated by expression of AA4.1 and CD19 and further fractionated into T1 and T2 cells by differential expression of CD21/35 and CD23 (Fig. 6B). Within AA4.1+ CD19+ TS cells, T1 cells were defined as CD21/35−lo CD23− and T2 cells were CD21/35lo/int CD23+. In accordance with Figure 1, TS cells were severely reduced in FL-/- mice (Fig. 6B). High expression of CD1d has been observed in T2 cells, and CD1dhi T2 cells are presumed MZP 9,29. Therefore, we examined the frequency of CD1dhi cells within T1 and T2 cells to determine whether FL-deficiency skewed TS cells towards the MZ cell fate. As seen in Figure 6B and C, significant increases in the frequency of CD1dhi cells could be detected within the T1 and T2 subsets in FL-/- mice. Finally, we conducted adoptive transfer experiments to provide additional evidence that TS cells are skewed towards MZ cell fate in FL-/- mice. T1 B cells were sorted from WT CD45.1 mice and adoptively transferred into CD45.2 WT or CD45.2 FL-/- mice. Four days later, mice were analyzed to examine the frequency of TS, MZ, and FO cells generated from the transferred WT CD45.1+ T1 cells. TS cells survive 2–4 days unless they are selected to become FO or MZ cells 2,4. Therefore, this time point allowed us to determine to what degree the transferred WT CD45.1 T1 cells were maintained as TS cells or became FO or MZ cells. Splenic cells were initially gated using CD45.1 and CD45.2 to identify CD45.1+ donor cells. Around twice as many CD45.1+ cells were recovered in FL-/- than in WT host mice, although this finding was not statistically significant (0.010 ± 0.003% vs. 0.005 ± 0.001% CD45.1+ cells, FL-/- vs. WT, respectively; P = 0.13, data not shown). CD45.1+ background staining in CD45.2 FL-/- PBS-injected mice was almost non-existent (12 ± 11 cells; 0.0003 ± 0.0003%, data not shown). CD45.1+ cells were further gated for CD19, and CD19+ cells were fractionated using IgM and CD21/35 to examine TS, MZ, and FO cells. In agreement with our findings that the peripheral B cell maturation defect in FL-/- mice is cell extrinsic (Fig. 3), WT T1 cells transferred into FL-/- mice were unable to maintain TS cells in comparison to those transferred into WT mice (Fig. 6D). Interestingly, WT T1 cells generated a statistically significant increase in the percentage of MZ cells in FL-/- mice than in their WT counterparts (Fig. 6D, 9.71 ± 0.88% vs. 6.03 ± 0.77%, FL-/- vs. WT, respectively; P = 0.0072), suggesting that TS cells are skewed toward the MZ cell fate in FL-/- mice. We found no difference in the ability of WT T1 cells to generate FO cells in WT or FL-/- mice (Fig. 6D). Taken together, these data suggest that FL-deficiency preferentially supports the differentiation of MZ B cells once TS cells enter the spleen.
Figure 6.
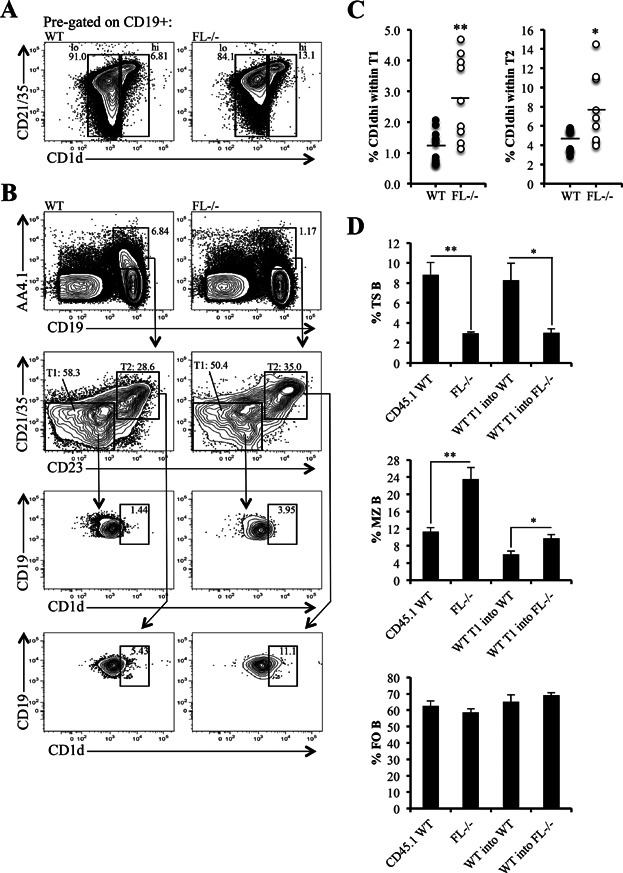
Evidence of marginal zone skewing within T1 and T2 subsets in FL-/- mice. (A) Flow cytometric analysis of splenic CD19+ B cells (pre-gated) from a representative wild-type (WT) and FL-/- mouse further stained by CD21/35 and CD1d to examine CD1dhi and CD1dlo peripheral B cell subsets. (B) Splenic cells were stained using CD19 and AA4.1 to initially resolve CD19+ AA4.1+ TS B cells. To examine T1 and T2 B cells, CD19+ AA4.1+ cells were further characterized using CD21/35 and CD23. T1 are defined as CD21/35-/lo CD23− and T2 are CD21/35lo/int CD23+ within CD19+ AA4.1+ TS B cells. T1 and T2 were subsequently analyzed with CD1d to determine the percentage of CD1dhi cells within these TS subsets. CD1dhi gates were set based on expression of CD1d in the MZ as in (A). (A and B) Data are representative of 9–11 mice/genotype and four independent experiments. (C) Scatter plots depict the frequency of CD1dhi cells within T1 and T2 B cells in each WT and FL-/- mouse in the analysis (n = 11 WT and 9 FL-/- mice). (D) Bar graphs illustrating percentages of TS, MZ, and FO B cells generated from WT CD45.1+ T1 B cells adoptively transferred into CD45.2 WT (WT T1 into WT) or CD45.2 FL-/- (WT T1 into FL-/-) mice. The bars labeled CD45.1 WT represent TS, MZ, and FO B cells from uninjected CD45.1 WT mice analyzed to ensure correct CD45.1+ and peripheral B cell subset gating. Bars labeled FL-/- PBS represent endogenous CD45.2+ TS, MZ, and FO B cells from PBS-injected CD45.2 FL-/- mice. CD45.1 background staining (controlled by PBS-injected CD45.2 FL-/- mice) was very minimal, averaging 0.0003 ± 0.0003% or 12 ± 11 CD45.1+ cells (data not shown). Data are representative of 5–9 mice/condition and five independent experiments. (C and D) * and ** represent statistically significant differences measured using the Student's t-test at P ≤ 0.05 and P ≤ 0.005, respectively.
Discussion
In this study, we show an integral indirect role for Flt3 signaling in regulation of peripheral B cell maturation in the spleen. B lymphopoiesis in BM provides a pool of immature cells that seed the peripheral lymphoid tissues. FL-deficiency reduces production of immature B cells. Numbers of T1, T2 and FO, but not MZ B cells are significantly reduced in spleens of FL-/- mice. Peripheral B cell maturation is contingent on BCR signaling, as well as survival and differentiation cues provided by the splenic microenvironment. One cytokine essential for T2, FO, and MZ B cells is BAFF. Serum levels of BAFF in FL-/- mice were comparable to WT mice and we found no alteration in surface expression of BAFF-R on splenic B cell subsets. Mixed radiation chimeras together with FL administration experiments confirmed that the reduction in TS and FO B cells was cell extrinsic. Ki67 analysis revealed that FL deficiency altered the proliferative potential of TS, and to a lesser extent FO B cells. Forced expression of Bcl2 did not rescue TS B cells in FL-/- mice, suggesting that impaired survival is not the basis of the TS cell deficiency in FL-/- mice. Finally, upregulation of CD1d in TS cells, accompanied by an iøncreased frequency of MZ cells generated from WT T1 cells adoptively transferred into FL-/- mice suggest that the FL-deficient splenic microenvironment skews TS cells towards the MZ cell fate.
The reductions in TS and FO, but not MZ B cells in FL-/- mice are similar to previous observations made in lymphopenic mice 7,10,30. An elegant study by Srivastava et al. showed that transplantation of AA4+ T2 or AA4+ T1 cells from WT mice into RAG2-/- hosts gave rise to MZ-like B cells while the same cells transferred into WT mice generated FO B cells 7. These results were interpreted that in a lymphopenic environment, factors that promote MZ B cell development are more readily available. FL-/- mice have deficiencies in TS and FO B cells and thus are lymphopenic compared to WT mice. Similar results were obtained in analysis of HoxA9-/- mice that also have reductions in B cell output from BM and reductions in splenic B cells (Table1) 31. Furthermore, we provide evidence that the FL-deficient splenic microenvironment preferentially supports the differentiation of MZ cells once TS cells enter the spleen. One factor essential for MZ B cell differentiation is Notch2 and its ligand, Delta-Like-1 (DL1) 29,32. Presumably, DL1 activation of Notch2 leads to upregulation of Deltex1 in MZ B cells. Notch2 transcripts are expressed at high levels in T2, FO, and MZ B cells 29. We hypothesize that under conditions of FL-deficiency, the microenvironmental signal that limits DL1 activation of Notch2 is reduced. At present, the nature of this signal is unknown. Regardless, TS cells must be highly sensitive to the MZ developmental signal as mild lymphopenia, as we document in FL-/- and HoxA9-/- mice, is sufficient to promote the MZ B cell fate.
Mixed radiation chimeras established with FL-/- BM cells showed normal distributions of TS, FO, and MZ B cell subsets. Frequencies of NK and DCs were normalized as well (data not shown). Moreover, FL administration experiments provided additional evidence that a FL-sensitive component exists in the splenic microenvironment that is necessary for peripheral B cell maturation. Given their well-known requirement for Flt3 signaling, DCs, the only splenic cell type that expresses Flt3, are of particular interest. DCs play a critical role in humoral immunity through production of soluble and cell-bound factors that directly regulate the survival, proliferation, and differentiation of naïve B cells, antigen-activated B cells, and terminally differentiated plasma cells 33–37. However, there are several lines of evidence that suggest that reductions in DCs due to FL deficiency are not the basis of the alterations in splenic B cell subsets we report. First, pDC deficiency as a consequence of targeted inactivation of the transcription factor E2-2 does not impair numbers of splenic B cells 38. Second, CD11c-DTR-EGFP mice allow inducible selective conditional deletion of splenic cDCs upon injection of diphtheria toxin 39. We obtained these mice and effectively depleted splenic cDCs as previously reported and found no alteration in numbers of splenic B cells or alterations in TS or MZ B cell subsets (Dolence et al., unpublished observations). Together, these experimental findings along with our previous report that FL replacement restored the bone marrow B cell deficiency in FL-/- mice strongly suggest that the alterations in TS and MZ B cells are the consequence of reduced output of B cells from the bone marrow 18. We note that conditional deletion of FL in mature B cells would strengthen this conclusion, but at present a FLfl/fl mouse has not been generated.
Due to the importance of BAFF for peripheral B cell homeostasis and selection, it was necessary to examine whether BAFF or BAFF-R was impacted by FL-deficiency 8,24. No differences in serum BAFF or BAFF-R expression were found in FL-/- mice. Although DCs, macrophages, and neutrophils are capable of producing BAFF in vitro, BAFF production by radiation-resistant stromal cells is sufficient for B cell survival and maturation 12,40. The selective deficiencies in TS and FO, but not MZ B cells in FL-/- mice are consistent with a BAFF-independent role as MZ B cells have a stringent dependence on BAFF 24. Further support for this interpretation is that overproduction of BAFF favors MZ B cell differentiation from T2 cells 41. Finally, since DCs are the only BAFF-producing cell type impacted by FL-deficiency, the finding that indicates defective peripheral B cell maturation in FL-/- mice is BAFF-independent is not surprising. We note that we cannot rule out the possibility that local reductions in BAFF, due to diminished numbers of DCs, might contribute to the deficiencies in TS and FO B cells in FL-/- mice. Additional experiments, employing transient BAFF replacement, are required to unambiguously rule out BAFF deficiency as a contributing factor in the TS and FO B cell reductions in FL-/- mice.
To our knowledge, there has only been a single report of a mouse model that phenocopies the select B cell deficiencies in FL-/- mice. Homeodomain-interacting protein Kinase-1 (HIPK1) is a ubiquitously expressed member of the HIPK family comprised of serine/threonine kinases that act as co-repressors for various homeodomain-containing transcription factors 42. HIPK1-/- mice exhibit a preferential loss of TS and FO but intact MZ B cells 43. HIPK1 has been implicated in regulation of apoptosis through a p53-dependent pathway 44. Whether deficiencies in HIPK1, in particular the loss of HIPK1-mediated survival signals, contribute to the reductions in TS and FO B cells in FL-/- is unknown. However, analysis of Eu-Bcl2Tg FL-/- mice indicates that if reductions in TS B cells are due to defective survival, then these signals are Bcl2-independent.
B cells are known to undergo homeostatic proliferation under conditions of lymphopenia. Microenvironmental cues have been suggested to promote homeostatic proliferation, but the exact nature of the signal is unknown. T1 and T2 displayed less proliferative capacity as measured by intracellular Ki67 expression in FL-/- mice. This is an intriguing finding and whether the impaired proliferative capacity influences the differentiation capacity of TS cells will be interesting to investigate. Much remains to be learned concerning the microenvironmental signals that dictate the FO versus MZ B cell fate decision. The FL-/- mouse provides a unique model to identify the nature of these signals.
Materials and Methods
Mice
Wild-type (C57Bl/6) mice were obtained from The Jackson Laboratory (Bar Harbor, Maine). FL-/- mice were obtained from Taconic Farms (Germantown, New York), then bred and maintained in our colony. PCR sequences and conditions for genotyping FL-/- mice have been described 15. HoxA9-/- mice have been described 31. B6-Ly5.2 congenic mice (CD45.1 WT) obtained from National Cancer Institute (NCI) (Frederick, Maryland) were used as recipients to establish mixed bone marrow radiation chimeras. CD45.1 WT mice (obtained from NCI) were also used as donors for adoptive transfer experiments, as T1 B cells were sorted and transferred into CD45.2+ WT or FL-/- mice. Eu-Bcl2Tg mice were kindly provided by Paul W. Kincade (Oklahoma Medical Research Foundation, Oklahoma City, Oklahoma) 45. Eu-Bcl2Tg FL-/- mice have been described 18. All mice in this study have been maintained on a C57Bl/6 genetic background for greater than 10 generations. Age-matched or littermate controls were used for individual experiments, and all mice analyzed in this study ranged from 8–12 weeks of age. All animals were bred and maintained at the Mayo Clinic animal facility and experiments were carried out with the approval of the Mayo Clinic Institutional Animal Care and Use Committee.
Flow cytometry
Methods for flow cytometry have been described 15,46–48. BM was harvested and stained with the following combination of antibodies: B220 APC, IgM APC-Cy7, and CD24 PE. Spleen was harvested, lyzed with ACK to remove red blood cells, and stained with combination of the following antibodies: CD19 (FITC, APC, and PE-Cy7), IgM (FITC, APC-Cy7), CD21/35 (PE, PerCP-Cy5.5), AA4.1 PE, CD23 (PE-Cy7, bio), BAFF-R APC, and CD1d FITC. Staining with CD45.1 PE-Cy7 and CD45.2 FITC antibodies were used to analyze the degree of chimerism of mixed bone marrow chimeras. Staining with CD45.1 PE and CD45.2 FITC antibodies were used to identify the CD45.1+ transferred cells in CD45.2+ hosts in adoptive transfer experiments. Incubation with streptavidin-APC or streptavidin-APC-eFluor 780 was used to visualize biotin-labeled CD23. All antibodies were obtained from eBioscience (San Diego, California), BD Biosciences (San Jose, California), or BioLegend (San Diego, California). For intracellular Ki67 staining, cells were initially stained for surface antigens (described above), followed by fixation and permeabilization using the Foxp3 staining buffer set (eBioscience) according to manufacturer's instructions. Ki67 or isotype control staining was performed using the FITC mouse anti-human Ki67 set per kit instructions (BD Biosciences). Flow cytometric analysis was performed on the LSRII or Canto cytometers (BD Biosciences). Data were analyzed with FlowJo software (Tree Star, Ashland, Oregon).
ELISA
The serum concentration of BAFF was calculated by ELISA using the mouse BAFF Quantikine ELISA kit (R&D Systems, Minneapolis, Minnesota). Serum was diluted 10-fold to fall in range of kit detection. The sensitivity for BAFF was 7.8 pg/mL.
Mixed bone marrow radiation chimeras
Chimeras were established by retro-orbitally transplanting either 2 million C57Bl/6 WT (CD45.2 WT) or FL-/- (CD45.2 FL-/-) BM cells into irradiated male B6-Ly5.2 congenic mice (CD45.1 WT). Mice were analyzed at 10 weeks after transplantation.
Flt3 ligand replacement therapy
Methods for Flt3 ligand replacement therapy have been previously described 18. Briefly, two cohorts of FL-/- mice were administered 10 µg Flt3 ligand (in 200 µL PBS) every other day for a total of five injections over 8 days. Control WT and FL-/- mice were injected with equivalent volumes of PBS. Two days following the last injection, one cohort of mice were euthanized, spleen harvested, and peripheral B cell subsets were analyzed by flow cytometry. The second cohort of mice was euthanized and spleen harvested 5 days following the last injection for flow cytometric analysis.
Adoptive transfer of T1 B cells
WT T1 B cells (CD19+ AA4.1+ CD21/35−lo CD23−) were sorted from male B6-Ly5.2 congenic mice (CD45.1 WT) and retro-orbitally adoptively transferred into C57Bl/6 WT (CD45.2 WT) or FL-/- (CD45.2 FL-/-). An average of 5.0-6.0 × 105 WT T1 B cells were adoptively transferred in 200 µL PBS into each of the CD45.2+ hosts per experiment. Mice were analyzed 4 days following adoptive transfer. Uninjected CD45.1 WT mice were analyzed along side mice that received T1 B cells to ensure correct CD45.1+ and peripheral B cell subset gating. CD45.2 FL-/- mice injected retro-orbitally with 200 µL PBS served to control for CD45.1 background staining.
Cell frequency, absolute number, and mean fluorescence intensity calculations
Cell subset frequencies were calculated by multiplying percentages of sequential gated populations. Absolute numbers were calculated by multiplying mononuclear cell counts obtained after BM or splenic harvest by cell subset frequencies. The frequencies and number calculations reflect mononuclear and doublet exclusion gates. A mean MFI was calculated for WT and FL-/- BAFF-R by averaging the MFI of BAFF-R from T1 and T2 B cell subsets from nine WT and nine FL-/- mice. The relative MFI of WT BAFF-R was normalized to 1 by dividing the WT mean MFI against itself, and relative MFI of FL-/- BAFF-R was calculated by dividing the mean FL-/- MFI by the mean WT MFI.
Statistical analysis
Statistics were performed using the unpaired Student's t-test. P values ≤ 0.05 were significant. All numerical data are presented as mean ± SEM.
Acknowledgments
The authors thank Dr. Rick Bram for helpful comments on the manuscript, and the Mayo Clinic Flow Cytometry Core Facility for technical expertise. This work is supported by R01 grants (R01HL096108 to KLM and R01AI083279 to VSS) from the National Institutes of Health. JJD is supported by National Institutes of Health training grant T32 AI07047-31. KLM and VSS conceived and designed experiments. KLM and JJD performed data analysis and made figures. JJD conceived and designed experiments, performed data analysis, made figures, and wrote the manuscript with KLM. All authors have read and approved the final manuscript.
Conflict of Interest
None declared.
Supporting Information
Additional supporting information may be found in the online version of this article at the publisher's web-site.
Figure S1: Immature and recirculating B cells are severely reduced in the BM of FL-/- mice.
References
- Lindsley RC, Thomas M, Srivastava B. Allman D. Generation of peripheral B cells occurs via two spatially and temporally distinct pathways. Blood. 2007;109:2521–2528. doi: 10.1182/blood-2006-04-018085. [DOI] [PubMed] [Google Scholar]
- Allman D, Lindsley RC, DeMuth W, Rudd K, Shinton SA. Hardy RR. Resolution of three nonproliferative immature splenic B cell subsets reveals multiple selection points during peripheral B cell maturation. J. Immunol. 2001;167:6834–6840. doi: 10.4049/jimmunol.167.12.6834. [DOI] [PubMed] [Google Scholar]
- Loder F, Mutschler B, Ray RJ, Paige CJ, Sideras P, Torres R, Lamers MC. Carsetti R. B cell development in the spleen takes place in discrete steps and is determined by the quality of B cell receptor-derived signals. J. Exp. Med. 1999;190:75–89. doi: 10.1084/jem.190.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Bahlburg A, Andrews SF, Yu KO, Porcelli SA. Rawlings DJ. Characterization of a late transitional B cell population highly sensitive to BAFF-mediated homeostatic proliferation. J. Exp. Med. 2008;205:155–168. doi: 10.1084/jem.20071088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allman DM, Ferguson SE. Cancro MP. Peripheral B cell maturation. I. Immature peripheral B cells in adults are heat-stable antigenhi and exhibit unique signaling characteristics. J. Immunol. 1992;149:2533–2540. [PubMed] [Google Scholar]
- Allman DM, Ferguson SE, Lentz VM. Cancro MP. Peripheral B cell maturation. II. Heat-stable antigen(hi) splenic B cells are an immature developmental intermediate in the production of long-lived marrow-derived B cells. J. Immunol. 1993;151:4431–4444. [PubMed] [Google Scholar]
- Srivastava B, Quinn WJ, 3rd, Hazard K, Erikson J. Allman D. Characterization of marginal zone B cell precursors. J. Exp. Med. 2005;202:1225–1234. doi: 10.1084/jem.20051038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu BL, Harless SM, Lindsley RC, Hilbert DM. Cancro MP. Cutting edge: BLyS enables survival of transitional and mature B cells through distinct mediators. J. Immunol. 2002;168:5993–5996. doi: 10.4049/jimmunol.168.12.5993. [DOI] [PubMed] [Google Scholar]
- Pillai S, Cariappa A. Moran ST. Marginal zone B cells. Annu. Rev. Immunol. 2005;23:161–196. doi: 10.1146/annurev.immunol.23.021704.115728. [DOI] [PubMed] [Google Scholar]
- Martin F. Kearney JF. Marginal-zone B cells. Nat. Rev. Immunol. 2002;2:323–335. doi: 10.1038/nri799. [DOI] [PubMed] [Google Scholar]
- Mackay F, Silveira PA. Brink R. B cells and the BAFF/APRIL axis: fast-forward on autoimmunity and signaling. Curr. Opin. Immunol. 2007;19:327–336. doi: 10.1016/j.coi.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Gorelik L, Gilbride K, Dobles M, Kalled SL, Zandman D. Scott ML. Normal B cell homeostasis requires B cell activation factor production by radiation-resistant cells. J. Exp. Med. 2003;198:937–945. doi: 10.1084/jem.20030789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancro MP. Peripheral B-cell maturation: the intersection of selection and homeostasis. Immunol. Rev. 2004;197:89–101. doi: 10.1111/j.0105-2896.2004.0099.x. [DOI] [PubMed] [Google Scholar]
- Wodnar-Filipowicz A. Flt3 ligand: role in control of hematopoietic and immune functions of the bone marrow. News Physiol. Sci. 2003;18:247–251. doi: 10.1152/nips.01452.2003. [DOI] [PubMed] [Google Scholar]
- Dolence JJ, Gwin K, Frank E. Medina KL. Threshold levels of Flt3-ligand are required for the generation and survival of lymphoid progenitors and B-cell precursors. Eur. J. Immunol. 2011;41:324–334. doi: 10.1002/eji.201040710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitnicka E, Bryder D, Theilgaard-Monch K, Buza-Vidas N, Adolfsson J. Jacobsen SE. Key role of flt3 ligand in regulation of the common lymphoid progenitor but not in maintenance of the hematopoietic stem cell pool. Immunity. 2002;17:463–472. doi: 10.1016/s1074-7613(02)00419-3. [DOI] [PubMed] [Google Scholar]
- Sitnicka E, Buza-Vidas N, Ahlenius H, Cilio CM, Gekas C, Nygren JM, Mansson R, Cheng M, Jensen CT, Svensson M, et al. Critical role of FLT3 ligand in IL-7 receptor independent T lymphopoiesis and regulation of lymphoid-primed multipotent progenitors. Blood. 2007;110:2955–2964. doi: 10.1182/blood-2006-10-054726. [DOI] [PubMed] [Google Scholar]
- Dolence JJ, Gwin KA, Shapiro MB. Medina KL. Flt3 signaling regulates the proliferation, survival, and maintenance of multipotent hematopoietic progenitors that generate B cell precursors. Exp. Hematol. 2014;42:380–393. doi: 10.1016/j.exphem.2014.01.001. , and . e383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna HJ, Stocking KL, Miller RE, Brasel K, De Smedt T, Maraskovsky E, Maliszewski CR, Lynch DH, Smith J, Pulendran B, et al. Mice lacking flt3 ligand have deficient hematopoiesis affecting hematopoietic progenitor cells, dendritic cells, and natural killer cells. Blood. 2000;95:3489–3497. [PubMed] [Google Scholar]
- Schwarz BA, Sambandam A, Maillard I, Harman BC, Love PE. Bhandoola A. Selective thymus settling regulated by cytokine and chemokine receptors. J. Immunol. 2007;178:2008–2017. doi: 10.4049/jimmunol.178.4.2008. [DOI] [PubMed] [Google Scholar]
- Holmes ML, Carotta S, Corcoran LM. Nutt SL. Repression of Flt3 by Pax5 is crucial for B-cell lineage commitment. Genes Dev. 2006;20:933–938. doi: 10.1101/gad.1396206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buza-Vidas N, Cheng M, Duarte S, Nozad H, Jacobsen SE. Sitnicka E. Crucial role of FLT3 ligand in immune reconstitution after bone marrow transplantation and high-dose chemotherapy. Blood. 2007;110:424–432. doi: 10.1182/blood-2006-09-047480. [DOI] [PubMed] [Google Scholar]
- Gwin KA, Shapiro MB, Dolence JJ, Huang ZL. Medina KL. Hoxa9 and Flt3 signaling synergistically regulate an early checkpoint in lymphopoiesis. J. Immunol. 2013;191:745–754. doi: 10.4049/jimmunol.1203294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiemann B, Gommerman JL, Vora K, Cachero TG, Shulga-Morskaya S, Dobles M, Frew E. Scott ML. An essential role for BAFF in the normal development of B cells through a BCMA-independent pathway. Science. 2001;293:2111–2114. doi: 10.1126/science.1061964. [DOI] [PubMed] [Google Scholar]
- Scholzen T. Gerdes J. The Ki-67 protein: from the known and the unknown. J. Cell. Physiol. 2000;182:311–322. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- van Oijen MG, Medema RH, Slootweg PJ. Rijksen G. Positivity of the proliferation marker Ki-67 in noncycling cells. Am. J. Clin. Pathol. 1998;110:24–31. doi: 10.1093/ajcp/110.1.24. [DOI] [PubMed] [Google Scholar]
- Strasser A, Whittingham S, Vaux DL, Bath ML, Adams JM, Cory S. Harris AW. Enforced BCL2 expression in B-lymphoid cells prolongs antibody responses and elicits autoimmune disease. Proc. Natl. Acad. Sci. U S A. 1991;88:8661–8665. doi: 10.1073/pnas.88.19.8661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cariappa A, Tang M, Parng C, Nebelitskiy E, Carroll M, Georgopoulos K. Pillai S. The follicular versus marginal zone B lymphocyte cell fate decision is regulated by Aiolos, Btk, and CD21. Immunity. 2001;14:603–615. doi: 10.1016/s1074-7613(01)00135-2. [DOI] [PubMed] [Google Scholar]
- Saito T, Chiba S, Ichikawa M, Kunisato A, Asai T, Shimizu K, Yamaguchi T, Yamamoto G, Seo S, Kumano K, et al. Notch2 is preferentially expressed in mature B cells and indispensable for marginal zone B lineage development. Immunity. 2003;18:675–685. doi: 10.1016/s1074-7613(03)00111-0. [DOI] [PubMed] [Google Scholar]
- Carvalho TL, Mota-Santos T, Cumano A, Demengeot J. Vieira P. Arrested B lymphopoiesis and persistence of activated B cells in adult interleukin 7(-/)- mice. J. Exp. Med. 2001;194:1141–1150. doi: 10.1084/jem.194.8.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence HJ, Helgason CD, Sauvageau G, Fong S, Izon DJ, Humphries RK. Largman C. Mice bearing a targeted interruption of the homeobox gene HOXA9 have defects in myeloid, erythroid, and lymphoid hematopoiesis. Blood. 1997;89:1922–1930. [PubMed] [Google Scholar]
- Hozumi K, Negishi N, Suzuki D, Abe N, Sotomaru Y, Tamaoki N, Mailhos C, Ish-Horowicz D, Habu S. Owen MJ. Delta-like 1 is necessary for the generation of marginal zone B cells but not T cells in vivo. Nat. Immunol. 2004;5:638–644. doi: 10.1038/ni1075. [DOI] [PubMed] [Google Scholar]
- Dubois B, Massacrier C, Vanbervliet B, Fayette J, Briere F, Banchereau J. Caux C. Critical role of IL-12 in dendritic cell-induced differentiation of naive B lymphocytes. J. Immunol. 1998;161:2223–2231. [PubMed] [Google Scholar]
- Jego G, Palucka AK, Blanck JP, Chalouni C, Pascual V. Banchereau J. Plasmacytoid dendritic cells induce plasma cell differentiation through type I interferon and interleukin 6. Immunity. 2003;19:225–234. doi: 10.1016/s1074-7613(03)00208-5. [DOI] [PubMed] [Google Scholar]
- MacLennan I. Vinuesa C. Dendritic cells, BAFF, and APRIL: innate players in adaptive antibody responses. Immunity. 2002;17:235–238. doi: 10.1016/s1074-7613(02)00398-9. [DOI] [PubMed] [Google Scholar]
- Poeck H, Wagner M, Battiany J, Rothenfusser S, Wellisch D, Hornung V, Jahrsdorfer B, Giese T, Endres S. Hartmann G. Plasmacytoid dendritic cells, antigen, and CpG-C license human B cells for plasma cell differentiation and immunoglobulin production in the absence of T-cell help. Blood. 2004;103:3058–3064. doi: 10.1182/blood-2003-08-2972. [DOI] [PubMed] [Google Scholar]
- Kingston D, Schmid MA, Onai N, Obata-Onai A, Baumjohann D. Manz MG. The concerted action of GM-CSF and Flt3-ligand on in vivo dendritic cell homeostasis. Blood. 2009;114:835–843. doi: 10.1182/blood-2009-02-206318. [DOI] [PubMed] [Google Scholar]
- Cisse B, Caton ML, Lehner M, Maeda T, Scheu S, Locksley R, Holmberg D, Zweier C, den Hollander NS, Kant SG, et al. Transcription factor E2-2 is an essential and specific regulator of plasmacytoid dendritic cell development. Cell. 2008;135:37–48. doi: 10.1016/j.cell.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung S, Unutmaz D, Wong P, Sano G, De los Santos K, Sparwasser T, Wu S, Vuthoori S, Ko K, Zavala F, et al. In vivo depletion of CD11c+ dendritic cells abrogates priming of CD8+ T cells by exogenous cell-associated antigens. Immunity. 2002;17:211–220. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litinskiy MB, Nardelli B, Hilbert DM, He B, Schaffer A, Casali P. Cerutti A. DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nat. Immunol. 2002;3:822–829. doi: 10.1038/ni829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thien M, Phan TG, Gardam S, Amesbury M, Basten A, Mackay F. Brink R. Excess BAFF rescues self-reactive B cells from peripheral deletion and allows them to enter forbidden follicular and marginal zone niches. Immunity. 2004;20:785–798. doi: 10.1016/j.immuni.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Ecsedy JA, Michaelson JS. Leder P. Homeodomain-interacting protein kinase 1 modulates Daxx localization, phosphorylation, and transcriptional activity. Mol. Cell. Biol. 2003;23:950–960. doi: 10.1128/MCB.23.3.950-960.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra FM, Gommerman JL, Corfe SA, Paige CJ. Rottapel R. Homeodomain-interacting protein kinase (HIPK)-1 is required for splenic B cell homeostasis and optimal T-independent type 2 humoral response. PloS ONE. 2012;7:e35533. doi: 10.1371/journal.pone.0035533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo S, Lu Y, Debbas M, Lin AW, Sarosi I, Itie A, Wakeham A, Tuan J, Saris C, Elliott G, et al. Characterization of cells and gene-targeted mice deficient for the p53-binding kinase homeodomain-interacting protein kinase 1 (HIPK1) Proc. Natl. Acad. Sci. U S A. 2003;100:5431–5436. doi: 10.1073/pnas.0530308100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser A, Harris AW. Cory S. Bcl-2 transgene inhibits T cell death and perturbs thymic self-censorship. Cell. 1991;67:889–899. doi: 10.1016/0092-8674(91)90362-3. [DOI] [PubMed] [Google Scholar]
- Gwin K, Frank E, Bossou A. Medina KL. Hoxa9 regulates Flt3 in lymphohematopoietic progenitors. J. Immunol. 2010;185:6572–6583. doi: 10.4049/jimmunol.0904203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, Pongubala JM, Reddy KL, Lancki DW, Dekoter R, Kieslinger M, Grosschedl R. Singh H. Assembling a gene regulatory network for specification of the B cell fate. Dev. Cell. 2004;7:607–617. doi: 10.1016/j.devcel.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Medina KL, Garrett KP, Thompson LF, Rossi MI, Payne KJ. Kincade PW. Identification of very early lymphoid precursors in bone marrow and their regulation by estrogen. Nat. Immunol. 2001;2:718–724. doi: 10.1038/90659. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Immature and recirculating B cells are severely reduced in the BM of FL-/- mice.


