Abstract
Background
Impaired admission glucose (AG) is considered to significantly increase risk on both early and late death of the patients with ST-segment elevation myocardial infarction (STEMI), especially for non-diabetic patients; however, some reports contradict the relationship. We therefore conducted a meta-analysis to clarify this issue.
Material/Methods
PubMed, EMBASE, Web of Science, and Cochrane Library databases were systematically searched to identify all related prospective cohort studies. The relative risks (RR) with their 95% confidence interval (CI) were pooled quantitatively.
Results
The pooled RR of early outcome events indicated patients with glucose concentrations ≥6.1–11.1 mmol/L had a 4.38-fold (95% CI, 3.23–5.94) higher early mortality. The pooled RR of late outcome events indicated that the patients with glucose concentrations ≥7.8–11.1 mmol/L had a 1.65-fold (95% CI, 1.33–2.04) higher late mortality based on in-hospital or 30-day survivors.
Conclusions
High AG may be a helpful prognostic marker of significantly increased risk on early death in non-diabetic patients with STEMI, and has an explicit but prognostic adverse impact on long-term mortality but not early mortality in these patients.
MeSH Keywords: Blood Glucose, Meta-Analysis, Myocardial Infarction
Background
Increased plasma glucose is a common feature in the acute phase of myocardial infarction, ranging from 3% to 71% in patients without diabetes [1,2]. Moreover, when serum markers of necrosis may still be normal, elevated plasma glucose levels can be detected within minutes of presentation, and then help to make appropriate decisions on treatment. It seems that the categorical variable elevated admission plasma glucose serves as a more powerful predictor than fasting glucose and the other elements of risk prediction markers such as elevated serum markers of myocardial infraction [3]. The patients with high admission glucose are more likely to develop restenosis and require repeat revascularization procedures than those with normal admission glucose. They also have increased risk for repeated myocardial infarction (MI) [4], stent thrombosis [5], and death [6], especially in non-diabetic patients [2], although some studies show inconsistent effects on the risk of late mortality [7–10]. Notably, most of these studies were conducted in the trials of fibrinolytic therapy as initial reperfusion strategy. Currently, limited evidence is available to propose admission glucose levels as an adverse prognostic factor in ST-segment elevation myocardial infarction (STEMI) patients treated with primary percutaneous coronary intervention (PCI) [11].
We therefore performed a meta-analysis of prospective studies published through August 2014 to evaluate the prognostic utility of admission glucose on early and late mortality in STEMI patients undergoing PCI without previous diagnosis of diabetes mellitus (DM).
Material and Methods
Selection of studies
Pertinent articles were searched in the electronic databases PubMed, EMBASE, Web of Science, the Cochrane Library from January 2000 to August 2014 using the terms “glycemic level” OR “glucose level” OR “blood glucose” OR “hyperglycemia” in conjunction with each of the following words: “percutaneous coronary intervention” OR “stent” OR “revascularization” OR “angioplasty” OR “PCI” OR “stenting” OR “reperfusion” OR “catheterization” OR “myocardial infarction”. In addition, conference proceedings/abstracts from major cardiology meetings were also searched in our analysis. For studies that did not report outcomes of interest, we contacted the authors for more information. The search was restricted to English- or Chinese-language articles.
Inclusion criteria
Only studies fulfilling the following criteria were included in the meta-analysis: (1) prospective clinical trials or cohort studies in which all outcomes data had been collected prospectively; (2) the outcome was clearly defined as mortality after STEMI, including early (<30 days after admission) or late (>6 months after discharge) mortality; (3) admission glucose or hyperglycemia was quantified; (4) sufficient data on mortality or relative risks (RR) or odds risks (OR) and their 95% confidence interval (CI) were reported; (5) receiving PCI in adult non-diabetic patients with STEMI in each study group. In the case of a series of articles based on the same study, only the last published report was selected for analysis and the previous could be reviewed as supplementaries to missing data where applicable.
With a standardized manner, article search and review were performed independently by two investigators (Z-X.H and R.L). A third investigator (C-J.Z) was involved to adjudicate wherever discrepancies between the investigators occurred.
Data abstraction
The following data on pre-specified forms were abstracted: authors, year of publication, location of the study group, baseline features, death, myocardial infarction, characteristics of the study population (sample size, source of population and distribution of age, sex), follow-up duration, the RRs or ORs overall and in each subgroup and the corresponding CIs or standard errors, and the confounding factors matched or adjusted in the studies. The end-point of interest for the present analysis was the predictive value of admission glucose level for mortality in the first 30 days and late mortality in 30-day survivors. Two reviewers (Z-X.H and R.L) independently extracted the data in duplicate using a standardized protocol. Any disagreements were adjudicated by a third investigator (C-J.Z).
Study quality assessment
For assessment of trial quality, key indicators of study quality were extracted and methodological quality of each study was assessed by non-blinded independent reviewers according to the Newcastle-Ottawa Scale [12]. We assigned a categories of good (fulfilling 5 or more of the criteria), fair (meeting 4 of this criteria), and poor (fewer than 4 of this criteria) quality to all 4 criteria for quality standards. Discrepancies were also decided by discussion and consensus was made.
Statistical analyses
Relative risks for mortality were calculated separately for patients with high and low AG in each study. Unadjusted RRs were pooled using both fixed-effects or DerSimonian and Laird random-effects models, weighting by the inverse of the variance (1/SE2) for each separate trial. Forest plots were generated to assess the RR estimates and corresponding 95% CIs across studies for graphical presentations. Statistical heterogeneity was assessed by conducting Q tests. P<0.1 was considered representative of significant statistical heterogeneity. I2 values of more than to 50% represent high heterogeneity, respectively. When effects were high heterogeneous, the randomized-effects model was used, otherwise, the fixed-effects model was used. In addition, the sources of heterogeneity were explored and meta-regression was performed. Variables included in the subgroup analyses were proportion of men, sample of participants, country of origin, and mean age of participants. We performed both the Egger test and Begg test to assess potential publication bias graphically using a funnel plot, in which log RR were plotted against their corresponding standard errors. Statistical analysis was performed by using Stata version 8.2 (Stata Corporation, College Station, TX, USA).
Results
Literature search
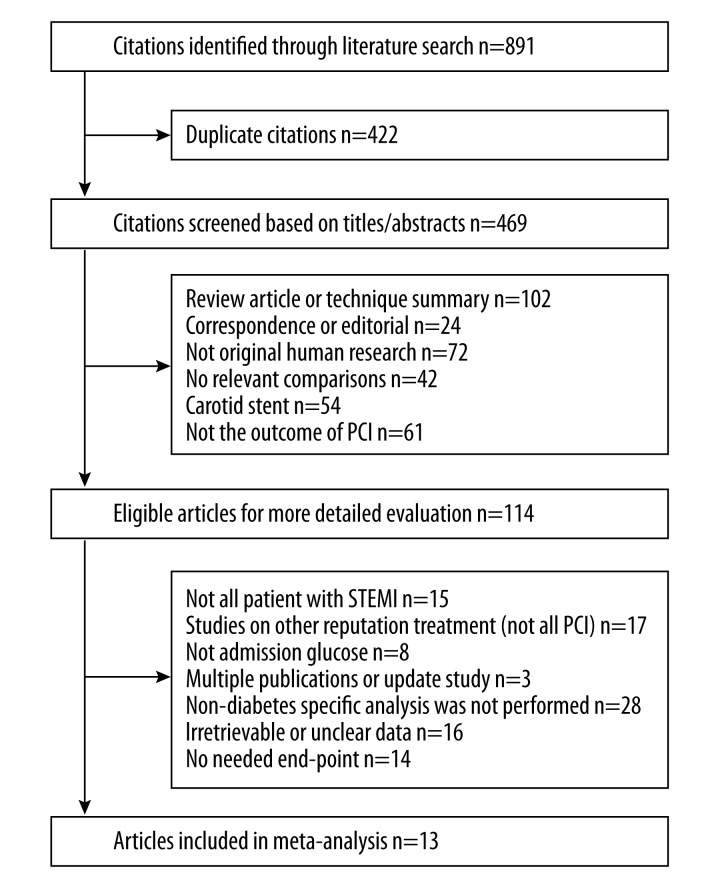
Totally, 1287 potentially relevant citations were found after an initial search. After excluding duplicates and screening the titles/abstracts, full publications of the remaining 119 articles were retrieved for further evaluation. Ultimately, of these 119 articles, 13 articles met the predetermined inclusion criteria and provided data adequate for meta-analysis (Figure 1).
Figure 1.
Study flow diagram of study selection process.
Study characteristics
The 13 trials included in the meta-analysis were summarized in Table 1. Seven of the selected cohort studies [8,13–18] reported both the early and late outcome events, whereas 5 studies [19–23] only reported the early outcome events and one study [24] only report the late outcome event. Within the 13 trials, the mean age for non-diabetic participants ranged from 55 to 65 years, and the proportion of men in majority of the studies ranged from 68% to 88%, while 1 study reported mortalities stratified by sex [24].
Table 1.
Characteristics of cohort studies evaluating prognostic utility of admission glucose on early and late mortality.
| Author and Year | Participants | Mortality outcome | Direct stent (%) | Multiple vessel diseased (%) | Prior MI | Time to PCI (hour) | Final TIMI 3 (%) | Cutoff levels | Study quality | |
|---|---|---|---|---|---|---|---|---|---|---|
| Early | Late | |||||||||
| Ishihara et al. (2005) [8] | 590 W and M (0.80) with mean age 63.2 years in Japan | 30-day | 3-year | 75 | 35 | 12 | 4.7 | 88 | 11 mmol/L | Good |
| Kosuge et al. (2005) [21] | 591 W and M (0.76) with mean age 65.9 years in Japan | Hospitalization | NR | 80 | 10 | 10 | 3.51 | 90 | 11 mmol/L | Good |
| Vis et al. (2007) [17] | 208 W and M(0.683) with mean age (NR) years in Netherlands | 30-day | 1-year | NR | 49 | 20 | NR | 72 | 11.1 mmol/L | Good |
| Gasior et al. (2008) [16] | 958 W and M (0.78) with mean age 57.0 years in Poland | Hospitalization | 1-year | 73 | 51 | 16 | 4.58 | 92 | 7.8 mmol/L | Good |
| Monte et al. (2008) [19] | 126 W and M (NR) with mean age 63.7 years in Italy | 30-day | NR | NR | NR | NR | NR | NR | 6.1 mmol/L | Fair |
| Ergelen et al. (2010) [14] | 1870 W and M(0.86) with mean age 55.7 years in Turkey | Hospitalization | More than 21 months | 84 | 54 | 9.6 | 3.16 | 89 | 11.1 mmol/L | Good |
| Li et al. (2010) [20] | 115 W and M (0.73) with mean age 65.8 years in china | Hospitalization | NR | NR | NR | NR | 6.70 | NR | 7.8 mmol/L | Good |
| Timmer et al. (2011) [13] | 4176 W and M(0.74) with mean age 62.2 years in Netherlands | 30-day | 1-year | 87 | 49 | 8.9 | NR | 92 | 8.1 mmol/L | Good |
| Planer et al. (2012) [15] | 2839 W and M(0.77) with mean age 59.5 years in USA and Europe | 30-day | 3-year | NR | NR | 9.7 | 1.75 | NR | 8.1 mmol/L | Good |
| Hoebers et al. (2012) [18] | 1437 W and M(0.72) with mean age 61.0 years in Netherlands | 30-day | 3-year | 83 | 33 | 12 | 3.06 | 91 | 7.8 mmol/L | Good |
| Otten et al. (2013) [24] | 2872 M (1.0) with mean age 61.8 years in Netherlands | NR | 1-year | NR | NR | 10 | NR | NR | NR | Good |
| Otten et al. (2013) [24] | 115 W with mean age 66.5 years in Netherlands | NR | 1-year | NR | NR | 5.6 | NR | NR | NR | Fair |
| Zhang et al. (2013) [22] | 853 W and M (0.70) with mean age 62.1 years in china | Hospitalization | NR | 90 | 53 | NR | NR | 84 | 10 mmol/L | Good |
| Ekmekci et al. (2013) [23] | 503 W and M (0.88) with mean age 55.2 years in Turkey | Hospitalization | NR | NR | NR | NR | 3.56 | 92 | 8.1 mmol/L | Good |
CABG – coronary artery bypass graft; LVEF – left ventricular ejection fraction; M – men; NR – no report; PCI – percutaneous coronary intervention; RR – relative risk; TIMI – thrombolysis in myocardial infarction; W – women.
Admission glucose and early mortality
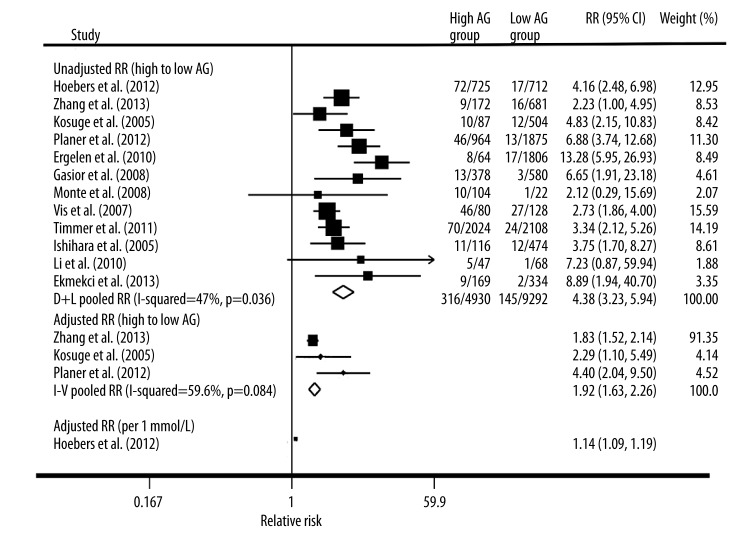
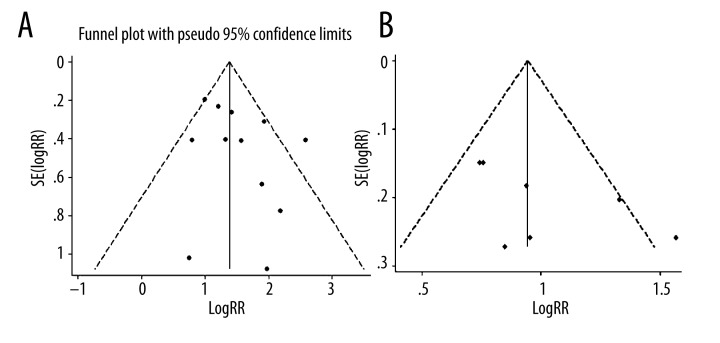
At short-term follow-up, the point estimates of the unadjusted RR were consistently more than 1 in all studies, whereas 2 studies did not show statistically significant associations. As depicted in Figure 2, the pooled unadjusted relative risk of early mortality after STEMI in patients who had high AG was 4.38 (95% CI, 3.23–5.94) compared with patients with low AG. Statistical heterogeneity was significant for the analysis (I2=47.0%; P for heterogeneity 0.036) and stratified analyses showed that age and proportion of men were significantly related with the results (Table 2). Adjusted relative risks of early mortality after STEMI in patients with high AG were reported in 3 of the 12 studies [15,21,22], with a pooled relative risk of 1.92 (95% CI, 1.63–2.26; Figure 2). One trial [18] showed that AG also had significant effect on early mortality (adjusted RR [per 1 mmol/L AG increased], 1.14; 95% CI, 1.09–1.19; Figure 2). Visual inspection of the funnel plot for the studies revealed symmetry. The funnel plot for the visual assessment of publication bias suggested no significant asymmetry (Figure 3A), and the Egger test (P=0.193) and Begg test (P=0.193) both indicated the absence of substantial publication bias.
Figure 2.
Forest plot of relative risk (RR) and 95% CI for high vs. low category of admission glucose and early death risk.
Table 2.
Subgroups and metareg analysis of the association of admission glucose on early mortality.
| Subgroups | Number of studies | Pooled RR (95% CI) | Heterogeneity | Meta-regression (P value**) | |
|---|---|---|---|---|---|
| P value* | I2 | ||||
| Follow-up time | |||||
| Hospitalization | 6 | 5.890 (3.127, 11.095) | 0.065 | 51.8% | 0.226 |
| 30-day | 6 | 3.708 (2.784, 4.938) | 0.207 | 30.4% | |
| Ethnicity | |||||
| Yellows | 4 | 3.540 (2.255, 5.558) | 0.509 | 0.0% | 0.467 |
| Whites | 8 | 4.834 (3.226, 7.244) | 0.011 | 61.3% | |
| Mean age | |||||
| >60 years | 8 | 3.278 (2.628, 4.088) | 0.736 | 0.0% | 0.003 |
| ≤60 years | 4 | 8.482 (5.495, 13.092) | 0.561 | 0.0% | |
| Men proportion | |||||
| >75% | 6 | 6.566 (4.520, 9.537) | 0.340 | 11.8% | 0.008 |
| ≤75% | 6 | 3.129 (2.462, 3.978) | 0.652 | 0.0% | |
| Cutoff level | |||||
| >8.1 mmol/L | 5 | 4.157 (2.320, 7.451) | 0.006 | 72.2% | 0.626 |
| ≤8.1 mmol/L | 7 | 4.453 (3.367, 5.889) | 0.484 | 0.0% | |
| Sample size | |||||
| ≤1000 | 8 | 3.254 (2.466, 4.294) | 0.481 | 0.0% | 0.224 |
| >1000 | 4 | 5.583 (3.236, 9.631) | 0.014 | 71.8% | |
CI – confidence interval; RR – relative risk;
P<0.1 was considered significant;
P<0.05 was considered significant.
Figure 3.
Funnel plots with 95% CI for (A) early death risk and late death risk based on in-hospital or 30-day survivors (B). RR, relative risk; SE, standard error.
Admission glucose and late mortality based on in-hospital or 30-day survivors
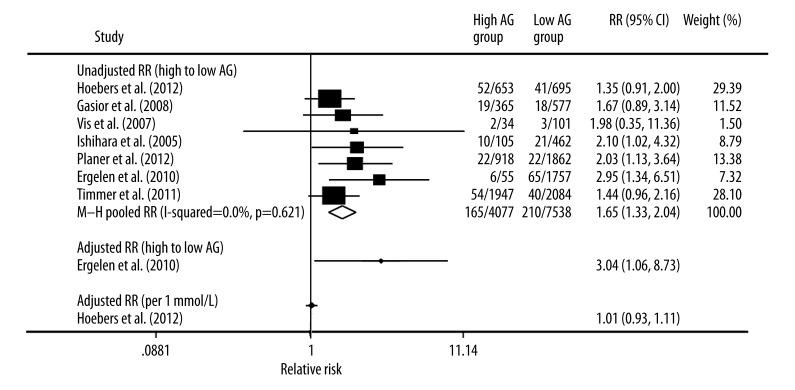
Seven trials showed that high AG was associated with a significantly higher risk of later mortality compared with lower AG group (pooled unadjusted RR, 1.65; 95% CI, 1.33–2.04; Figure 4). There was no statistically significant heterogeneity among the studies (I2 =0.0%; P for heterogeneity 0.621). In the stratified analysis by follow-up time, ethnicity, mean age, proportion of men, cutoff level, and sample size, inconsistencies in these factors were not significantly related with the results. Moreover, 1 trial [14] reported the adjusted RR of late mortality after STEMI in patients who had high AG compared with patients with low AG on admission. In this trial, the RR of late mortality was significantly higher in the patients with high AG than that in the other patients (RR, 3.04; 95% CI, 1.06–8.73; Figure 4). One trial [18] showed that AG had no significant effect on later mortality (adjusted RR of per 1 mmol/L AG increased, 1.01; 95% CI, 0.93–1.11; Figure 4). As shown in Figure 3B, we did not find a significant publication bias for Egger’s test (P=0.081) and Begg’s test (P=0.133).
Figure 4.
Forest plot of relative risk (RR) and 95% CI for high vs. low category of admission glucose and late death risk based on in-hospital or 30-day survivors.
Discussion
The main finding from the 6 cohort studies indicated that the elevated AG was significantly associated with an increased risk of early death in the non-diabetes STEMI patients following PCI. Stratified analyses demonstrated that age and proportion of men may be the source of heterogeneity for early mortality but not late mortality based on in-hospital or 30-day survivors.
The mechanisms underlying the adverse effect of high AG in the STEMI patients with PCI are likely multifactorial, such as augmenting platelet-dependent thrombus formation [25], loss of the endothelial glycocalyx layer [26], inflammatory changes with adhesion molecule production [27], and direct glycation of coagulation factors to impair their function [28]. Recent animal studies have shown that increased myocardial uptake and metabolism of glucose during ischemia was associated with preservation of myocardial function [30], and elevated free fatty acid levels reduced myocardial contractility and increased myocardial oxygen demand [31]. Hyperglycemia may precipitate an osmotic diuresis and deplete stroke volumes through interfering with the Frank-Starling mechanism [32]. Hyperglycemia also attenuated ischemic preconditioning by decreasing the activity of K-ATP channels [33].
The present meta-analysis showed that admission glucose was significantly associated with an increased risk of death for non-diabetic patients with STEMI following PCI. In term of the late mortality, the mortality based on in-hospital or 30-day survivors have their own strengths, with the former applied to evaluate the long-term risk of death before treatment and the latter applied to predict the long-term risk of death for patients still alive after 30 days of onset. Consistently, the meta-analysis also revealed a statistically significant increase of risk in patients who underwent PCI that was not consistently identified in the individual studies, whereas the prognostic effect was worse compared with early mortality. This indicates that the AG level was primarily an important marker of early risk, reflecting, at least in part, the response to more severe stress due to larger infarctions and/or more severe hemodynamic compromise. On the other hand, the discrepancies between prognostic effect of early mortality and late mortality could result from the long-term benefits of early aggressive treatment.
These results suggest that physicians need to be aware that it is indispensable for the rapid delivery of appropriate treatment. At present, insulin-only and insulin-glucose with or without K infusions, which are used for strict control of glycemia following STEMI, seem to be the most acceptable management strategy [34]. The Hi-5 study demonstrated that early intensive treatment with insulin significantly decreased mortality in patients with AG>144 mg/dL [35], although detrimental effects, such as excessive volume overload, hyperglycemia, and hypoglycemia, were clinically observed [36]. Strict glycemic control with insulin treatment after STEMI was downgraded from a class Ib to a class IIa recommendation in the recent update of the American Heart Association guidelines [18]. Recently, a new therapeutic approach, glucagon like peptide-1(GLP-1) infusion [36], was proposed, which might improve cardiac function and reduce infarct size; it heralds a promising alternative approach for glycometabolic control in patients with STEMI.
This study did have several limitations that merit consideration when interpreting the results, which include study selection bias, between-study heterogeneity, and inability to adjust for baseline differences because individual level data were not available. In the meta-analysis, the Egger’s regression test and visual inspection of a funnel plot for publication bias did not show a substantially bias. Nevertheless, it is still very likely that negative studies are under-published, even though the results of tests for publication bias were not significant. Moreover, the present study was based on observational studies; hence, patients in observational studies are subject to a large treatment bias and other confounding effects because of the lack of random allocation.
Conclusions
Taken together, the present meta-analysis revealed that impaired AG may be an effective prognostic marker of significantly increased risk on early death in non-diabetic patients with STEMI. To further confirm this conclusion, more high-quality and larger samplings of studies will be required in the future.
Footnotes
Conflict of interest statement
None declared.
Source of support: Departmental sources
References
- 1.Del OM, Merino-Torres JF, Argente M, et al. Detection of glucose abnormalities in patients with acute coronary heart disease: study of reliable tools in clinical practice. J Endocrinol Invest. 2012;35:71–76. doi: 10.3275/7769. [DOI] [PubMed] [Google Scholar]
- 2.Capes SE, Hunt D, Malmberg K, Gerstein HC. Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview. Lancet. 2000;355:773–78. doi: 10.1016/S0140-6736(99)08415-9. [DOI] [PubMed] [Google Scholar]
- 3.Morrow DA, Antman EM, Charlesworth A, et al. TIMI risk score for ST-elevation myocardial infarction: A convenient, bedside, clinical score for risk assessment at presentation: An intravenous nPA for treatment of infarcting myocardium early II trial substudy. Circulatio. 2000;102:2031–37. doi: 10.1161/01.cir.102.17.2031. [DOI] [PubMed] [Google Scholar]
- 4.Norhammar AM, Ryden L, Malmberg K. Admission plasma glucose. Independent risk factor for long-term prognosis after myocardial infarction even in nondiabetic patients. Diabetes Care. 1999;22:1827–31. doi: 10.2337/diacare.22.11.1827. [DOI] [PubMed] [Google Scholar]
- 5.Ishihara M, Kojima S, Sakamoto T, et al. Acute hyperglycemia is associated with adverse outcome after acute myocardial infarction in the coronary intervention era. Am Heart J. 2005;150:814–20. doi: 10.1016/j.ahj.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 6.Wahab NN, Cowden EA, Pearce NJ, et al. Is blood glucose an independent predictor of mortality in acute myocardial infarction in the thrombolytic era? J Am Coll Cardiol. 2002;40:1748–54. doi: 10.1016/s0735-1097(02)02483-x. [DOI] [PubMed] [Google Scholar]
- 7.Hoebers LP, Damman P, Claessen BE, et al. Predictive value of plasma glucose level on admission for short and long term mortality in patients with ST-elevation myocardial infarction treated with primary percutaneous coronary intervention. Am J Cardiol. 2012;109:53–59. doi: 10.1016/j.amjcard.2011.07.067. [DOI] [PubMed] [Google Scholar]
- 8.Ishihara M, Kagawa E, Inoue I, et al. Impact of admission hyperglycemia and diabetes mellitus on short- and long-term mortality after acute myocardial infarction in the coronary intervention era. Am J Cardiol. 2007;99:1674–79. doi: 10.1016/j.amjcard.2007.01.044. [DOI] [PubMed] [Google Scholar]
- 9.Kosiborod M, Rathore SS, Inzucchi SE, et al. Admission glucose and mortality in elderly patients hospitalized with acute myocardial infarction: implications for patients with and without recognized diabetes. Circulation. 2005;111:3078–86. doi: 10.1161/CIRCULATIONAHA.104.517839. [DOI] [PubMed] [Google Scholar]
- 10.Hsu CW, Chen HH, Sheu WH, et al. Initial serum glucose level as a prognostic factor in the first acute myocardial infarction. Ann Emerg Med. 2007;49:618–26. doi: 10.1016/j.annemergmed.2006.10.023. [DOI] [PubMed] [Google Scholar]
- 11.Keeley EC, Boura JA, Grines C. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet. 2003;361:13–20. doi: 10.1016/S0140-6736(03)12113-7. [DOI] [PubMed] [Google Scholar]
- 12.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–5. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 13.Timmer JR, Hoekstra M, Nijsten MWN, et al. Prognostic value of admission glycosylated hemoglobin and glucose in nondiabetic patients with ST-segment-elevation myocardial infarction treated with percutaneous coronary intervention. Circulation. 2011;124:704–11. doi: 10.1161/CIRCULATIONAHA.110.985911. [DOI] [PubMed] [Google Scholar]
- 14.Ergelen M, Uyarel H, Cicek G, et al. Which is worst in patients undergoing primary angioplasty for acute myocardial infarction? Hyperglycaemia? Diabetes mellitus? Or both? Acta Cardiol. 2010;65:415–23. doi: 10.2143/AC.65.4.2053900. [DOI] [PubMed] [Google Scholar]
- 15.Planer D, Witzenbichler B, Guagliumi G, et al. Impact of hyperglycemia in patients with ST-segment elevation myocardial infarction undergoing percutaneous coronary intervention: The HORIZONS-AMI trial. Int J Cardiol. 2013;167:2572–79. doi: 10.1016/j.ijcard.2012.06.054. [DOI] [PubMed] [Google Scholar]
- 16.Gasior M, Pres D, Stasik-Pres G, et al. Effect of blood glucose levels on prognosis in acute myocardial infarction in patients with and without diabetes, undergoing percutaneous coronary intervention. Cardiol J. 2008;15:422–30. [PubMed] [Google Scholar]
- 17.Vis MM, Sjauw KD, van der Schaaf RJ, et al. In patients with ST-segment elevation myocardial infarction with cardiogenic shock treated with percutaneous coronary intervention, admission glucose level is a strong independent predictor for 1-year mortality in patients without a prior diagnosis of diabetes. Am Heart J. 2007;154:1184–90. doi: 10.1016/j.ahj.2007.07.028. [DOI] [PubMed] [Google Scholar]
- 18.Hoebers LP, Damman P, Claessen BE, et al. Predictive value of plasma glucose level at admission for short and long term mortality in patients with ST elevation myocardial infarction treated with primary percutaneous coronary intervention. Am J Cardiol. 2012;109:53–59. doi: 10.1016/j.amjcard.2011.07.067. [DOI] [PubMed] [Google Scholar]
- 19.De Monte A, Perkan A, Vitrella G, et al. Impact of hyperglycemia on clinical outcome in patients undergoing percutaneous coronary intervention (PCI) for ST-segment elevation myocardial infarction (STEMI). European Journal of Internal Medicine 7th Congress of the European Federation of Internal Medicine Compendium of Oral Communications; 2008. pp. 45–46. [Google Scholar]
- 20.Li Y, Qiu H, Wang W, et al. Alteration of stress hormones and glucose levels after percutaneous coronary intervention in patients with acute myocardial infarction and its influence on prognosis. Chinese Journal of Practical Internal Medicine. 2010;30:61–63. [Google Scholar]
- 21.Kosuge M, Kimura K, Kojima S, et al. Effects of glucose abnormalities on in-hospital outcome after coronary intervention for acute myocardial infarction. Circ J. 2005;69:375–79. doi: 10.1253/circj.69.375. [DOI] [PubMed] [Google Scholar]
- 22.Zhang JW, Zhou YJ, Cao SJ, et al. Impact of stress hyperglycemia on in-hospital stent thrombosis and prognosis in nondiabetic patients with ST-segment elevation myocardial infarction undergoing a primary percutaneous coronary intervention. Coron Artery Dis. 2013;24:352–56. doi: 10.1097/MCA.0b013e328361a942. [DOI] [PubMed] [Google Scholar]
- 23.Ekmekci A, Cicek G, Uluganyan M, et al. Admission hyperglycemia predicts inhospital mortality and major adverse cardiac events after primary percutaneous coronary intervention in patients without diabetes mellitus. Angiology. 2014;65:154–59. doi: 10.1177/0003319713488930. [DOI] [PubMed] [Google Scholar]
- 24.Otten AM, Ottervanger JP, Timmer JR, et al. Age-dependent differences in diabetes and acute hyperglycemia between men and women with ST-elevation myocardial infarction: a cohort study. Diabetol Metab Syndr. 2013;5:34. doi: 10.1186/1758-5996-5-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shechter M, Merz CN, Paul-Labrador MJ, Kaul S. Blood glucose and platelet-dependent thrombosis in patients with coronary artery disease. J Am Coll Cardiol. 2000;35:300–7. doi: 10.1016/s0735-1097(99)00545-8. [DOI] [PubMed] [Google Scholar]
- 26.Williams SB, Goldfine AB, Timimi FK, et al. Acute hyperglycemia attenuates endothelium-dependent vasodilation in humans in vivo. Circulation. 1998;97:1695–701. doi: 10.1161/01.cir.97.17.1695. [DOI] [PubMed] [Google Scholar]
- 27.Marfella R, Siniscalchi M, Esposito K, et al. Effects of stress hyperglycemia on acute myocardial infarction: role of inflammatory immune process in functional cardiac outcome. Diabetes Care. 2003;26:3129–35. doi: 10.2337/diacare.26.11.3129. [DOI] [PubMed] [Google Scholar]
- 28.Esposito K, Nappo F, Marfella R, et al. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: role of oxidative stress. Circulation. 2002;106:2067–72. doi: 10.1161/01.cir.0000034509.14906.ae. [DOI] [PubMed] [Google Scholar]
- 29.Allison SP, Tomlin PJ, Chamberlain MJ. Some effects of anaesthesia and surgery on carbohydrate and fat metabolism. 1969. Br J Anaesth. 1998;81:273–77. doi: 10.1093/bja/81.2.273. [DOI] [PubMed] [Google Scholar]
- 30.Eberli FR, Weinberg EO, Grice WN, et al. Protective effect of increased glycolytic substrate against systolic and diastolic dysfunction and increased coronary resistance from prolonged global underperfusion and reperfusion in isolated rabbit hearts perfused with erythrocyte suspensions. Circ Res. 1991;68:466–81. doi: 10.1161/01.res.68.2.466. [DOI] [PubMed] [Google Scholar]
- 31.Shah B, Amoroso NS, Sedlis SP. Hyperglycemia in nondiabetic patients presenting with acute myocardial infarction. Am J Med Sci. 2012;343:321–26. doi: 10.1097/MAJ.0b013e31822fb423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ishihara M, Inoue I, Kawagoe T, et al. Impact of acute hyperglycemia on left ventricular function after reperfusion therapy in patients with a first anterior wall acute myocardial infarction. Am Heart J. 2003;146:674–78. doi: 10.1016/S0002-8703(03)00167-4. [DOI] [PubMed] [Google Scholar]
- 33.Ishihara M, Inoue I, Kawagoe T, et al. Effect of acute hyperglycemia on the ischemic preconditioning effect of prodromal angina pectoris in patients with a first anterior wall acute myocardial infarction. Am J Cardiol. 2003;92:288–91. doi: 10.1016/s0002-9149(03)00627-1. [DOI] [PubMed] [Google Scholar]
- 34.Mehta SR, Yusuf S, Diaz R, et al. Effect of glucose-insulin-potassium infusion on mortality in patients with acute ST-segment elevation myocardial infarction: the CREATE-ECLA randomized controlled trial. JAMA. 2005;293:437–46. doi: 10.1001/jama.293.4.437. [DOI] [PubMed] [Google Scholar]
- 35.Bucciarelli-Ducci C, Bianchi M, De Luca L, et al. Effects of glucose-insulin-potassium infusion on myocardial perfusion and left ventricular remodeling in patients treated with primary angioplasty for ST-elevation acute myocardial infarction. Am J Cardiol. 2006;98:1349–53. doi: 10.1016/j.amjcard.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 36.Nikolaidis LA, Mankad S, Sokos GG, et al. Effects of glucagon-like peptide-1 in patients with acute myocardial infarction and left ventricular dysfunction after successful reperfusion. Circulation. 2004;109:962–65. doi: 10.1161/01.CIR.0000120505.91348.58. [DOI] [PubMed] [Google Scholar]