Summary
Background
The objective of this study was a comparative evaluation of radiography and MRI in the diagnostics of sacroiliitis in patients with a clinical diagnosis of spondyloartropathy, according to the current ASAS criteria.
Material/Methods
Sacroiliac joints radiograms and MRI were conducted in 101 consecutive patients, aged 19–71 yrs (mean age: 40.6 yrs). The patients were referred by a senior rheumatologist, with symptoms of the chronic back pain. The sacroiliac joints were assessed on AP radiograms of the pelvis according to the modified New York criteria for ankylosing spondylitis. MRI was performed to look for active and chronic inflammatory lesions.
Results
Of 14 patients with radiographic sacroiliitis according to modified New York criteria, only 50% had sacroiliitis on MRI. The sensitivity and specificity of conventional radiography were 22% and 94% and of MRI were 71% and 90%. Cohen’s kappa coefficient was κ=0.0187, agreement of radiograms and MRI was 58%. Among 86 patients displaying no sacroiliitis on radiograms, MRI showed sacroiliitis in 34 patients (39.5%). Positive predictive value was 0.429, negative predictive value was 0.605.
Conclusions
MRI allowed to diagnose sacroiliitis in 39,5 % of patients in preradiographic stage. MRI ruled out the presence of active inflammatory lesions in 60.4% of patients with sacroiliitis on radiograms according to modified New York criteria.
MeSH Keywords: Magnetic Resonance Imaging, Radiography, Sacroiliitis, Spondylarthropathies
Background
Inflammation of peripheral joints with accompanying inflammation of the spine, or spondyloarthropathies (spondyloarthritis, SpA), are a group of chronic inflammatory rheumatoid diseases involving peripheral joints (arthritis), entheses (enthesitis), digits (dactylitis), and the axial spine, typically, primarily in the form of sacroiliitis [1,2]. This group comprises 5 conditions: ankylosing spondylitis (AS), reactive arthritis (ReA), psoriatic arthritis (PsA), spondylitis associated with non-specific inflammatory bowel diseases (IBD) such as Crohn’s disease and ulcerative colitis, and undifferentiated spondyloarthropathy (uSpA) [1]. Depending on the dominant symptoms, SpA is differentiated into axial SpA (axSpA) – where the clinical picture involves mainly sacroiliitis, and peripheral SpA – where arthritis, enthesitis or dactylitis are the most pronounced symptoms [1,3].
The clinical presentation of spondyloarthropathies differs from rheumatoid arthritis (RA) mainly by the presence of the so-called chronic back pain (CBP) and the symptoms of enthesitis, dactylitis, uveitis, or history of IBD.
The hallmarks of SpA in laboratory findings include HLA-B27 (human leukocyte antigen)receptor presence, particularly common in patients with axSpA, as well as the absence of rheumatoid factor in SpA (hence the historical term for this disease group – seronegative spondyloarthropathies) which is, in turn, typical for RA [1,2,4].
The leading, and, most typically, the initial characteristic axSpA manifestation is sacroiliitis, diagnosed in a radiological examination on the basis of the so called New York criteria dating back to 1966, and, in the case of AS, the modified New York criteria of 1984 [1,5]. Sacroiliitis on radiographs is among the diagnostic criteria for sacroiliitis according to European Spondyloarthropathy Study Group Criteria (ESSG) and Amor criteria [5], which, in combination with clinical data, have been used by rheumatologists in everyday practice for diagnosing SpA. The above criteria are not, however, optimal, i.e. due to a delay of up to 9 years in diagnosing sacroiliitis on radiographs [6,7].
In 2009, ASAS introduced new axSpA classification criteria, whereby MRI of the sacroiliac joints was incorporated alongside radiological examination [8].
Thereby, for the first time in the field of rheumatology, MRI was introduced into the diagnostics of inflammatory lesions in joints, and imaging (MRI and radiographs) gained the basic criterion status for the purpose of diagnosing sacroiliitis.
According to the new ASAS criteria, the initial imaging modality for the diagnostics of sacroiliitis remains the conventional radiographic examination, based on the modNY criteria for AS [1,3]. MRI of sacroiliac joints (SIJs) is a second-line method of choice, conducted when the radiograph does not meet modNY criteria, i.e. when Grade 0, Grade 1 sacroiliitis, or unilateral Grade 2 sacroiliitis is found, yet clinical symptoms and laboratory findings indicate SpA [1,3].
Studies in the literature of the subject have for many years now demonstrated MRI to be more sensitive and specific in detecting sacroiliitis than radiographs [2,9–11]. Bone marrow edema (BME) is MRI-evident several years prior to the development of structural changes visible on radiograph, thus facilitating early treatment, more effective than treatment started in patients with established disease [2]. Hence, MRI is being postulated as the first-line imaging modality in the diagnostics of sacroiliitis, particularly in the early stages of the inflammatory condition, in the so called pre-radiographic stage.
The study was aimed at a comparative evaluation of radiography and MRI in the diagnostics of sacroiliitis in patients with a clinical diagnosis of SpA according to the current ASAS criteria.
Material and Methods
Radiographs and MRI were conducted in 101 consecutive patients, including 40 males and 61 females, aged 19–71 yrs (mean age: 40.6 yrs). The patients were referred by a senior rheumatologist, with symptoms of the so-called chronic back pain (CBP) and other clinical symptoms possibly indicative of SpA, such as psoriatic-like skin and nail lesions, positive family history of SpA, or non-specific inflammatory bowel disease. CBP is defined as back pain lasting ≥3 months with an onset preceding 45 yrs of age.
All patients underwent radiographs and MRI of the SIJs, within an interval of no longer than 2 weeks. The radiographs and MR scans were evaluated by senior radiologists, independently of one another, i.e. without the knowledge of the outcome of the other imaging modality or the clinical data. Each joint was evaluated separately. A total of 202 joints were evaluated.
The study was approved by the institutional Ethical Committee and all patients gave their written informed consent.
AP pelvis with L-spine radiograph
Radiographs were conducted with Quantum device. Each patient underwent AP pelvis and L-spine radiograph. The 1984 modified New York criteria for AS (modNY) for diagnosing sacroiliitis, based on the 1966 New York (NY) criteria (Table 1), were applied [1,3,8]. According to the modNY criteria, sacroiliitis is diagnosed where bilateral Grade 2 inflammatory lesions, or minimum unilateral Grade 3 lesions in sacroiliac joints are evident [1,2]. According to the NY criteria, Grade 1 sacroiliitis is already diagnostic.
Table 1.
New York sacroiliitis radiological grading criteria.
| Grade 0 | No abnormalities (sacroiliac joints normal) |
| Grade 1 | Suspicious for abnormalities (blurring of the joint margins) |
| Grade 2 | Minimal abnormalities (solitary erosions and juxta-articular sclerosis in small sacral or iliac areas) |
| Grade 3 | Advanced abnormalities (manifested juxta-articular sclerosis, numerous erosions with widening of joint space, possible partial ankylosis) |
| Grade 4 | Complete ankylosis |
Magnetic resonance imaging
All patients presented for the MRI with their GFR or creatinine level laboratory findings. Prior to the examination they filled out a contraindications (and other information) questionnaire. The examination was conducted with Siemens Avanto 1.5T device, with Body MATRIX Tim Coil. The MRI SI protocol comprised T2- weighted image in sagittal plane, T1, T2, T1FS, DIFF and STIR sequences in oblique coronal plane, which is parallel to the posterior cortex of the S2 vertebral body seen on a sagittal image, and PD FS oblique axial plane (Table 2).
Table 2.
MRI protocol for SI joints in SpA.
| Seq. | FOV | TR | TE | Matrix | Layer thickness | Flip |
|---|---|---|---|---|---|---|
| Sagittal T2 TSE | 300 | 5800 | 77 | 320 | 5 mm | 150 |
| Axial PD TSE FAT SAT | 270 | 3000 | 35 | 320 | 3 mm | 180 |
| Coronal TIRM TSE | 260 | 160 | 38 | 256 | 3 mm | 148 |
| Coronal T1 TSE FAT SAT | 260 | 590 | min 9.9 | 384 | 3 mm | 180 |
| Coronal T1 TSE | 260 | 600 | min 9.9 | 384 | 3 mm | 180 |
| Coronal T2 TSE | 260 | 4900 | 87 | 256 | 3 mm | 180 |
Contrast agent, Gadovist, was administered in amount of 0.1 ml/kg body weight in cases where BME or effusion in the joint cavities were visualized. MR images were assessed for the presence of active and chronic inflammatory lesions (Table 3). According to ASAS criteria, detection of active inflammatory lesions in the form of BME was the grounds for sacroiliitis diagnosis [1,2].
Table 3.
Active and chronic sacroiliitis lesions in MRI according to ASAS [2].
| Active inflammatory lesions | Chronic inflammatory lesions |
|---|---|
| Bone marrow edema | Subchondral sclerosis |
| Capsulitis | Erosions |
| Synovitis | Fatty transformation of bone marrow |
| Enthesitis | Syndesmophytes, ankylosis |
The lesion required to diagnose sacroiliitis in MRI was BME in a typical juxta articular location, subchondrally, in the iliac or sacral bone, visible either in one layer (if a minimum of two areas with BME are present in various quadrants of SI joints), or in at least two parallel layers (where at least a single area of BME is found) [1,8,12].
Synovitis, capsulitis or enthesitis in SIJs, where not accompanied by BME, could not be taken as diagnostic parameters for sacroiliitis [1,2].
Statistical analysis was conducted with Excel and Statistica software. MRI and the clinical symptom of chronic back pain were taken as reference for statistical calculations.
The basic purposes of the analysis were:
the evaluation of the specificity of CBP with regard to radiographs and MRI findings;
a comparative evaluation of radiography against MRI in terms of the number of diagnosed sacroiliitis cases and disease severity by radiographs according to NY and the modified NY criteria.
Results
Of 101 analyzed subsequent patients with a clinical suspicion of axial SpA (i.e. with CBP), radiographs of SIJs were bilaterally non-diagnostic in 1 patient, and unilaterally non-diagnostic in further 4 (joints obscured by bowel content). These joints were excluded from the further comparative analysis of conventional radiographs against MRI.
Comparison of the classification criterion – CBP against radiographs and MRI results
Of 101 patients referred with CBP symptoms, inflammatory lesions in SIJs were disclosed:
in radiographic examination: in 14 out of 100 examined patients (14%) (F: M – 8: 6) according to modNY criteria, and in 41 out of 100 according to NY criteria (41%) (F: M – 22: 19).
in MRI: active sacroiliitis lesions were found in 41 out of 101 patients (41%) (F: M – 25: 16).
The number of diagnosed sacroiliitis cases by radiographs and by MRI
196 joints were evaluated in radiographic examination out of total 202 qualified for the evaluation. The picture of 6 joints was non-diagnostic, in 1 patient bilaterally.
Radiographic inflammatory lesions were found, broken down into grades as follows (Table 4):
Table 4.
Number of joints with MR features of sacroiliitis with regard to consecutive grades of sacroiliitis on radiographs.
| Grade of sacroiliitis | X-ray | MRI | ||||
|---|---|---|---|---|---|---|
| Total | (%) | No changes | (%) | Sacroiliitis | (%) | |
| 0 | 134 | 66% | 105 | 78% | 29 | 22% |
| 1 | 21 | 11% | 12 | 57% | 9 | 43% |
| 2 | 33 | 16% | 21 | 64% | 12 | 36% |
| 3 | 8 | 4% | 3 | 38% | 5 | 62% |
| 4 | – | – | – | – | – | – |
| N | 6 | 3% | 3 | 50% | 3 | 50% |
| Total | 202 | 100% | 144 | 71% | 58 | 29% |
N – non diagnostic joints.
Grade 0 – in 134 joints (66% of the total number of SIJs evaluated in radiographic examination).
Grade 1 – in 21 joints (10% of the total number of SIJs evaluated in radiographic examination).
Grade 2 – in 33 joints (16% of the total number of SIJs evaluated in radiographic examination).
Grade 3 – in 8 joints (4% of the total number of SIJs evaluated in radiographic examination).
No cases of complete SIJs ankylosis (grade 4) were found.
On radiographs, inflammatory lesions of SIJs were found in 62 out of the evaluated 196 joints (32%). In 20 patients (49% of the 41 patient in the group) inflammatory lesions were located unilaterally (R/L – 9/11 patients), and in 21 patients bilaterally (51%).
In MRI, BME fulfilling ASAS sacroiliitis criteria was found in 58 out of the evaluated 202 joints (29%). In 24 patients (58,5% of the 41 patient in the group) inflammatory lesions were located unilaterally (R/L – 8/16 patients), and in 17 patients bilaterally (41.5%).BME was accompanied by synovitis in 19 joints (9%), enthesitis in 4 joints (2%), capsulitis in 2 joints (1%), erosions in 32 joints (16%), sclerosis in 107 joints (53%) and fatty transformation in 129 joints (64%).
All BME areas were enhanced by contrast. It was solely the administration of contrast that enabled the additional diagnosis of synovitis and capsulitis.
Comparison of radiographs against MRI
Among 14 patients with sacroiliitis by radiographs according to modNY criteria, 7 were confirmed with inflammatory lesions in MRI (true positive results in radiographic examination) (50%, 7/14), further 7 were not confirmed with sacroiliitis features in MRI (no BME nor other active inflammatory lesions, false positive in radiographic examination) (50%). Kappa coefficient κ=0.06, the agreement of radiographs and MRI was 60%.
Of 41 diagnoses of sacroiliitis by radiographs according to NY criteria, 22 patients were confirmed with the inflammation in MRI, whereas 18 were not confirmed (44% vs. 50%). Kappa coefficient κ=0.23, the agreement of radiographs and MRI was 63%.
Among 86 patients without sacroiliitis features by radiographs according to modNY criteria (in i.e. preradiographic stage of the disease), MRI confirmed the lack of inflammatory lesions in 53 cases (62%), in further 33 inflammatory lesions in SIJs were found (38%) (Figure 1).
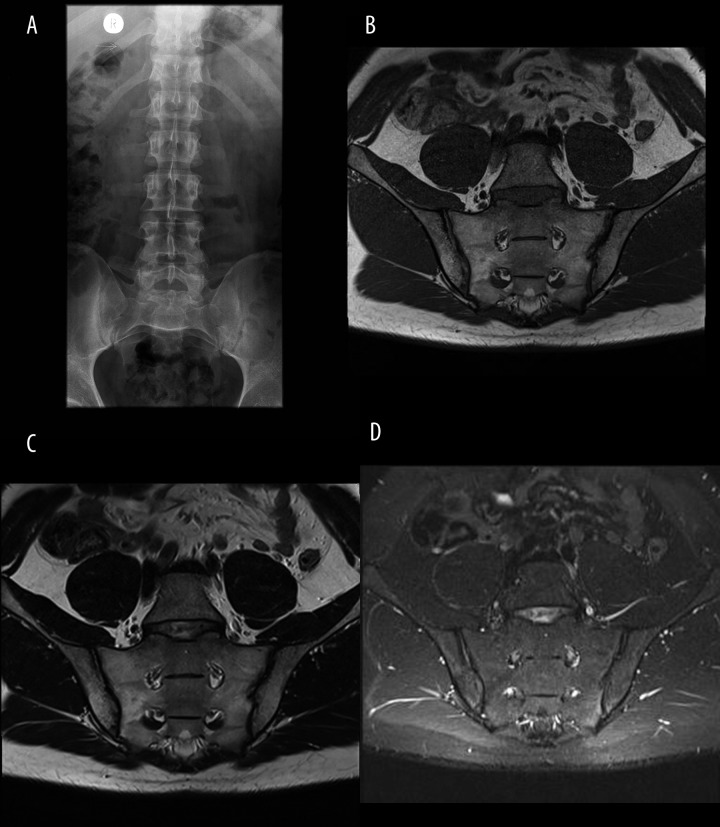
Figure 1.
Bilateral sacroiliitis Grade I on radiograph (A); no active inflammatory lesions on MRI on T1 (B), T2 (C), and TIRM (D) sequencies, except for fatty infiltration of bone marrow in the right ilium and sacrium.
Of 59 patients without sacroiliitis cases by radiographs according to NY criteria, MRI confirmed the lack of inflammatory lesions in 41 patients, and did not confirm the lack of inflammatory lesions 18 (31%) (Figures 2, 3).
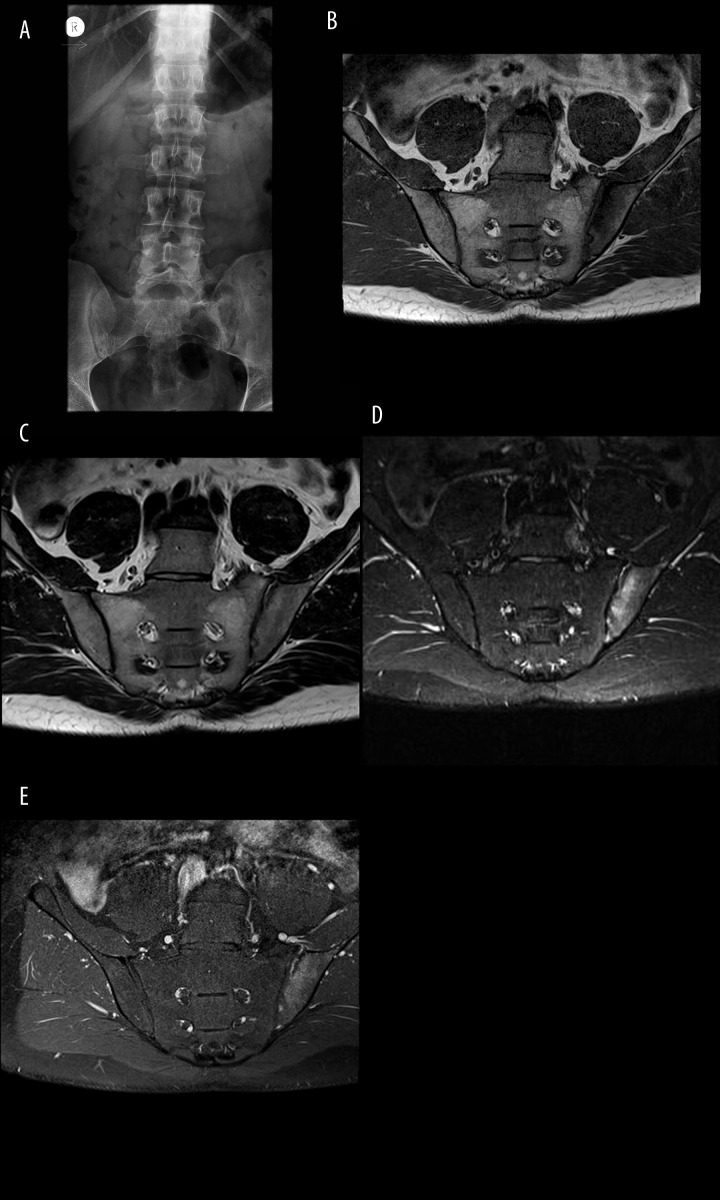
Figure 2.
Bilateral sacroiliitis Grade 0 on radiograph (A); active sacroiliitis on MRI of the left SIJ, characterized by the presence of BME, and synovitis and active bone erosion in the left iliac bone on T1 (B), T2 (C), TIRM (D) and T1FSCE sequencies.
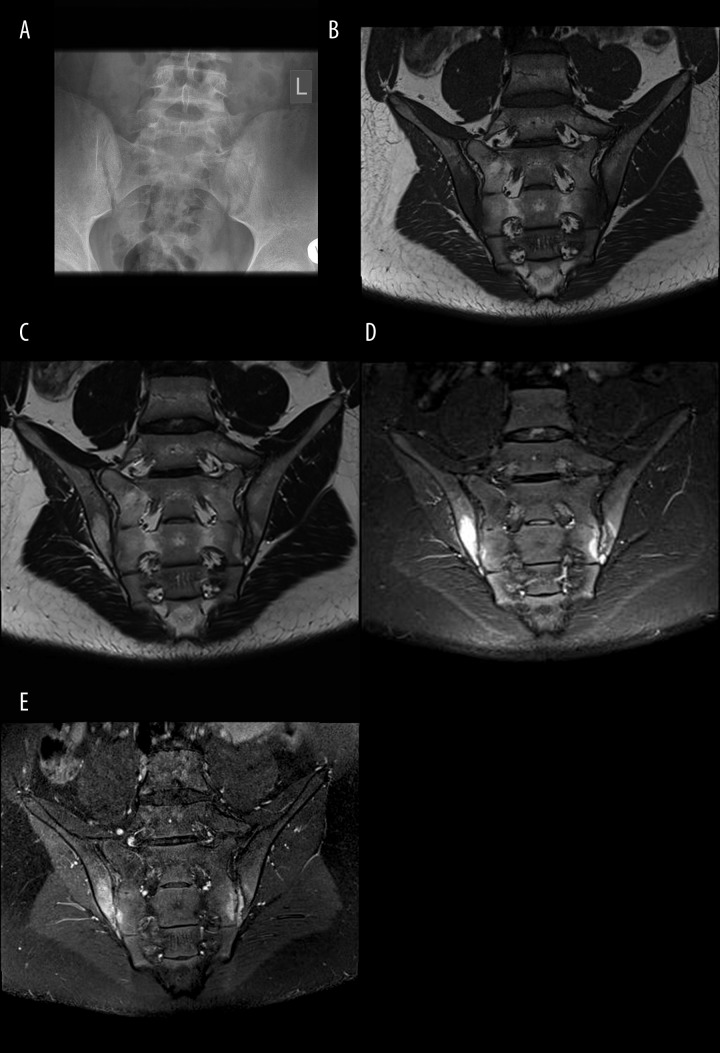
Figure 3.
Sacroiliitis Grade I of the left SIJ on radiograph (A); bilateral active sacroiliitis on MRI, characterized by the presence of BME, erosions, synovitis on T1 (B), T2 (C), and TIRM (D) and T1FSCE (E) sequencies.
The findings have been listed in Table 5.
Table 5.
Correlation of the number of sacroiliitis diagnoses by radiographs and MRI.
| MRI | ||||
|---|---|---|---|---|
| Radiographs acc. modNY | Nb of patients | No sacroiliitis | With sacroiliitis | Total |
| No sacroiliitis | 53 (53%) | 33 (33%) | 86 (86%) | |
| With scroiliitis | 7 (7%) | 7 (7%) | 14 (14%) | |
| Total | 60 (60%) | 40 (40%) | 100 (100%) | |
| Comparison | Agreement between radiographs and MRI – 60% kappa Cohen coeficient= 0.06; 95%; CI: 0–0.29 |
|||
| RM | ||||
| Radiographs acc. NY | Nb of patients | No sacroiliitis | With sacroiliitis | Total |
| No sacroiliitis | 41 (41%) | 18 (18%) | 59 (59%) | |
| With scroiliitis | 19 (19%) | 22 (22%) | 41 (41%) | |
| Total | 60 (60%) | 40 (40%) | 100 (100%) | |
| Comparison | Agreement between radiographs and MR – 63% kappa Cohen coeficient=0.23; 95%; CI: 0.04–0.43 |
|||
Comparison radiographs against MRI in terms of subsequent sacroiliitis severity grades according to NY and modNY criteria
In the total group of 196 joints evaluated in radiographic examination according to NY criteria (6 joints non-diagnostic, including one patient bilaterally non-diagnostic), grade 1 and higher sacroiliitis was disclosed in 62 joints (31.6% of 196).
In the same group of 196 patients, MRI-evident sacroiliitis was found in 58 joints (29%).
Sacroiliitis by radiographs broken down by grades:
Sacroiliitis Grade 0 on radiographs (no inflammatory lesions in SIJs) in 134 joints – confirmed in MRI in 105 (78%) joints, in other 29 (22%) abnormalities were found.
Sacroiliitis Grade 1 on radiographs in 21 joints – in 9 (42.8%) of these BME was disclosed in MRI, the remaining 12 (57.1%) joints were normal by MRI.
Sacroiliitis Grade 2 on radiographs in 33 joints – in 12 (36%) of these BME was disclosed, 21 (64%) joints were normal by MRI.
Sacroiliitis Grade 3 on radiographs in 8 joints – in 5 (62.5%) of these BME was disclosed, 3 (37.5%) joints were normal by MRI.
The agreement between radiographs and MRI on singular joints was 67.46%, kappa coefficient κ=0.04 (Table 6).
Table 6.
Number of sacroiliitis cases diagnosed on radiographs acc. to NY criteria, modNY critera, and MRI.
| MRI | ||||
|---|---|---|---|---|
| X-rays acc. to modNY | Numer of joints | No sacroiliitis | With sacroiliitis | Total |
| No sacroiliitis | 123 | 46 | 169 | |
| With sacroiliitis | 18 | 9 | 27 | |
| Total | 141 | 55 | 196 | |
| Comparison | Aagreement between x-rays and MRI – 67.4% kappa Cohen coeficient=0.04; 95% CI: 0–0.2351 |
|||
Sacroiliitis on radiographs and in MRI in correlation with the final diagnosis of SpA
Sensitivity and specificity of imaging methods with regards to final clinical diagnosis were the following (Table 7):
Table 7.
Sacroiliitis with regards to final clinical diagnosis of SpA.
| Patients | ||||
|---|---|---|---|---|
| SIJ | No SpA | With SpA | Total | |
| Radiographs acc. modNY | No sacroiliitis | 47 (47%) | 39 (39%) | 86 (86%) |
| Sacroiliitis | 3 (3%) | 11 (11%) | 14 (14%) | |
| Total | 50 (50%) | 50 (50%) | 100 (100%) | |
| Radiographs acc. NY | No sacroiliitis | 36 (36%) | 23 (23%) | 59 (59%) |
| Sacroiliitis | 14 (14%) | 27 (27%) | 41 (41%) | |
| Total | 50 (50%) | 50 (50%) | 100 (100%) | |
| MRI | No sacroiliitis | 45 (45%) | 15 (15%) | 60 (59%) |
| Sacroiliitis | 5 (5%) | 36 (35%) | 41 (41%) | |
| Total | 50 (50%) | 51 (50%) | 101 (100%) | |
For radiographs acc. NY criteria – 54% and 72%.
For radiographs acc. modny criteria – 22% and 94%.
For MRI – 71% and 90%.
Correlation of HLA-B27 presence with MRI result
HLA-B27 was found in 32 (31.6%) out of 101 patients referred for imaging diagnostics, including 16 female and 16 male patients. In this group, 17 out of 32 patients (53.1%) exhibited inflammatory lesions by MRI (7 female and 10 male), whereas no sacroiliitis features were disclosed in the remaining 15 (46.8%) patients. Of 69 patients with absent HLA-B27, sacroiliitis features by MRI were found in 24 (35%), whereas no features of inflammation by MRI were detected in 45 patients (65%) (Table 8).
Table 8.
HLA-B27 antigen and sacroiliitis in MRI.
| Sacroiliitis in MRI | HLA-B27 | ||
|---|---|---|---|
| Absent | Present | Total | |
| 0 | 45 | 15 | 60 |
| 1 | 24 | 17 | 41 |
| Total | 69 | 32 | 101 |
Discussion
According to American College of Rheumatology (ACR) classification of 1983, [13] spondyloarthropathies fall into Group II of rheumatic diseases. The incidence and the number of SpA diagnoses have been reported to grow yearly. In some countries, SpA diagnoses tend to be more common than diagnoses of RA and other rheumatic disease diagnoses [14]. On the one hand, this is due to the increasing SpA incidence, including nonspecific bowel inflammation diseases’ incidence, on the other, to the introduction of ASAS diagnostic criteria in 2009, which incorporated MRI of SIJs in the set of clinical diagnostic criteria for the disease alongside conventional radiography [8]. ASAS criteria decisively increased the detectability of SpA, as in Europe and the USA the prevalence of all SpA forms diagnosed according to AMOR and ESSG criteria was previously known to be 0.3–1.06% [14,15], whereas now has gone up to 1–1.4% of axSpA cases alone, diagnosed according to ASAS criteria [16].
Diagnostics of sacroiliitis, which is the primary feature and a hallmark of axSpA, is primarily based on clinical examination and laboratory testing. The clinical criterion used by rheumatologists when referring patients for accessory examinations (laboratory or radiological), is CBP. Our study has demonstrated that among all patients referred with CBP symptom, SIJs were normal on radiographs and MRI images in majority of cases. Inflammatory lesions on radiographs according to modNY criteria were found in as little as 14% of patients (according to NY criteria in 41%), and in MRI – in 41% (Table 5). Similar findings were obtained by Williamson et al. [17], in whose material 58% of patients suffering with CBP did not reveal MRI-evident inflammatory lesions. Also, the so-called inflammatory back pain has unsatisfactory sensitivity and specificity [18]. Hence, the reason behind the small number of sacroiliitis cases confirmed in radiological examinations may be suboptimal clinical selection. This is pointed out by Vossen MHE at al. [6], suggesting Diagnostic Berlin algorithm to improve patient selection for MRI.
The obtained results indicate higher sensitivity of MRI over radiographs for detecting inflammatory lesions. They could also be indicative of superiority of NY criteria as compared with the modified NY criteria, yet further analyses showed most Grade 0 and 1 sacroiliitis diagnoses by radiographs not to be confirmed in MRI (results IV, Table 4).
Antigen HLA-B27,which is specific for SpA, was found in only 32 out of 101 patients referred for imaging diagnostics (32%) (Table 8). The majority of scientific reports confirm the antigen to be present in 46–95% axSpA patients, including 42–75% patients in pre-radiographic stage [5,19–23]. There exist, however, studies demonstrating that e.g. in psoriatic arthritis (one of SpA forms) the antigens was detected in as little as 20% of patients [17]. In our own research, practically comparable proportions of HLA-B27 positive patients were found in MR examination to have and not to have sacroiliitis (54% vs. 45%) (Table 8). Likewise, in the group of HLA-B27 negative patients, BME was revealed in MRI in 35% of patients, whereas sacroiliitis features were not confirmed in 65% (Table 8). Our findings could confirm that BME meeting ASAS criteria is not a sufficiently specific SpA criterion, this being supported by the presence of slight bone marrow edema in 30% of well patients or patients with CBP [24]. The obtained own findings could also suggest that BME does in fact correspond to inflammation, as well as HLA-B27 tends to be often in axSpA. In our results half of the patients with SpA (25/51) were HLA-B27 positive and 78% (25/32) patients with antigen HLA-B27 have SpA (Table 9). The issue must be further investigated into. Chary-Valckenaere et al. [24] documented BME to be as significant a diagnostic criterion as HLA-B27 presence. Both parameters have diagnostic and prognostic value. Rudawaleit M et al. demonstrated the probability of SpA in HLA-B27 positive patients with sacroiliitis by MRI to equal 90% [25]. Onna et al. [26] found that in HLA-B27 positive patients with sacroiliitis by MRI, the probability of SIJs inflammation is 88% where sacroiliitis was disclosed in initial MRI, and 27% where the initial MRI was negative.
Table 9.
HLA-B27 antigen and final diagnosis of axSpA.
| Antigen HLA B27 | axSpA | ||
|---|---|---|---|
| Absent | Present | Total | |
| 0 | 43 | 26 | 69 |
| 1 | 7 | 25 | 32 |
| Total | 50 | 51 | 101 |
In Heuft-Dorenbosch et al. material of patients with inflammatory back pain (IBP) features <2 years, HLA-B27 was found in 73% patients with sacroiliitis by MRI, and in 33% with no sacroiliitis features on MRI. Among HLA-B27 positive patients, half of them were confirmed with sacroiliitis by MRI (similarly to our own research), whereas in HLA-B27 negative patients, inflammatory lesions of SIJs were visible in MRI in 16.6% of subjects (in our material in 35%) [5]. The issue of BME symptom‘s specificity remains open and is, as of today, difficult to interpret due to the limited number of reported histopathological examinations of SIJs in patients with SpA. As opposed to RA, mononuclear cell infiltrates found in SIJs correlate with MRI-evident BME only in some cases [27–30].
Both among axSpA patients and the so called imaging arm axSpA patients, in most publications men prevail [5,18]. In our material women were the majority, and we found sacroiliitis by MRI more commonly in female patients (F: M – 25: 16). HLA -B27 was present in an equal number of female and male patients (F: M – 16: 16). Sacroiliitis in MRI in HLA-B27 - positive patients was, however, more commonly disclosed in men (F: M – 7: 10). In a study by Heuft-Dorenbosch et al. [5] it was also women who prevailed (62%). The authors explain that possibly, contrary to an established AS of many years prevalent in men, early forms of axSpA are more common in women. The authors found HLA-B27 in 46% of SpA patients, more commonly in men (contrary to our own findings). In their study, women were more commonly HLA-B27 negative and did not reveal lesions in MRI. It is feasible, then, that they were not suffering from axSpA, which would point to a low specificity of the clinical criteria [5]. Similar conclusions are presented by Williamson et al. [17], based on MRI of SIJs in patients with PsA: sacroiliitis is frequently MRI – evident in this form of SpA (38%), yet it is difficult to diagnose in a clinical examination (35%).
The basic purpose of our study was a comparative analysis of radiographic examination and MRI in the diagnostics of early sacroiliitis according to ASAS criteria.
ASAS-OMERACT working group developing diagnostic standards for SpA demonstrated MRI of SI joints to be the best modality for diagnosing and evaluating the severity of sacroiliitis in patients with early inflammation and negative radiographs [24]. This was confirmed by our research. According to ASAS criteria, inflammatory lesions of SIJs on radiographs were diagnosed in 14% of patients, whereas in MRI in 41% (table 5). De Hooge et al. [23] found inflammatory lesions in SIJs considerably less frequently in MRI of patients with CBP (in 34% of patients in baseline examination and in 21% in the follow-up MRI in 3 months’ time). A study by Barkham et al. [30], in turn, which monitored SIJs for the evaluation of the efficacy of biological treatment of SpA, found active inflammatory lesions in SIJs in all patients in baseline MRI, including 70% bilaterally (in our material bilateral lesions by MRI were found in 41% of patients). In radiographic examination [30] sacroiliitis was visible in 53% of these patients, with lesions not meeting modNY criteria in 41% of them. In our material, we found radiographic lesions which did not fulfill modNY criteria in a vast majority of patients. Such patients, according to ASAS criteria, are candidates for MRI. In our 86 patients who did not meet the modified New York criteria, sacroiliitis by MRI was diagnosed in 33 patients (38%) (Table 5). In Blum et al. study [31] sacroiliitis by MRI was established in 75% of patients in pre-radiographic stage (i.e. patients not meeting modNY criteria).
Patients with a diagnosis of defined radiographic lesions in SIJs according to the modified NY criteria do not require further MR diagnostics. There were no agreement between radiographs and MRI examinations (kappa coefficient κ=0.06, 60%. According to ASAS criteria, radiographic picture is an indication for introducing treatment. Our preliminary findings showed that in half of the patients this may not be justified. 14% of patients who were diagnosed with sacroiliitis on radiographs (i.e. fulfilling modNY criteria), MRI ruled out sacroiliitis in 50% of them. Such disproportion of the number of sacroiliitis diagnoses between radiography and MRI was similar to NY criteria where Grade 1 already indicates sacroiliitis; the number of false positive results (overdiagnoses) by radiographs concerned 46% of the subjects. According to NY criteria the agreement of radiograph and MRI examinations was low (kappa coefficient κ=0.23, 63%).
Oostveen et al. [32] analyzed MR images in CBP patients HLA0B27 positive with sacroiliitis not meeting the modified New York criteria. BME by MRI was found in 11 out of 25 examined patients (44%). In our material, there were 11 out of 86 (28%) cases meeting identical sets of criteria (Table 8).
MRI is considered a leading diagnostic modality for sacroiliitis [17]. Our preliminary research confirmed the superior sensitivity and specificity of MRI over radiographic examination. Sensitivity and specificity of imaging exams with regards to final clinical diagnosis of SpA were: for radiographs acc. NY criteria – 54% and 72%, for radiographs acc. modNY – 22% and 94%, and for MRI – 71% and 90. In Blum et al. prospective study [31], in 44 patients with IBP symptoms, MRI sensitivity was 95%, specificity 100%, whereas radiographic examination sensitivity was 19%, and specificity 47%. In a study by Aydin at al. [12], the sensitivity and specificity of MRI against clinical examination were 66% and 94%, respectively.
The characteristic feature of the leading SpA form, namely AS, is the symmetric involvement of SIJs, whereas a unilateral, asymmetric location of BME indicates other SpA forms, most frequently PsA [33]. However, MRI indicates early stages of all axSpA forms to be unilateral, a fact which has been known since as early as 2005, and possibly beyond [32,34]. In our MRI, unilateral inflammatory lesions also prevailed (24: 17), which, nonetheless, could have resulted from the majority of female patients (F: M – 61: 40) and a slight majority of PsA cases over conclusively confirmed cases of AS.
In our own research we have rarely observed active inflammatory lesions other than BME, such as synovitis, capsulitis or enthesitis. On their own, not accompanied by BME, they are not diagnostically valid, yet their presence confirms sacroiliitis diagnosis [24]. De Hooge et al. [23] found synovitis in <7% of cases, including one synovitis case not accompanied by BME, which according to ASAS criteria is not axSpA-typical. In our material all synovitis cases coexisted with BME. The inflammation may also be located in the ligamentous, posterior portion of SI joints [2]. In our material we found startlingly few enthesitis cases (as few as 4), which is surprising in so far as etiopathogenesis of SpA is reported to involve tendinous elements of SIJs (where fibrocartilage prevails) as site of predilection [2].
Regarding chronic inflammatory lesions, we found: erosions in 16% of subjects, sclerosis in 53%, and fatty infiltration of bone marrow in 64% of subjects. These results are similar to reported in studies by other authors [35,36].
The limitation for our research was the lack of a control patient group with nonspecific back pain (the so called mechanical back pain, MBP) or well patients, to examine the specificity of BME. It has been documented that BME as an independent parameter, especially in HLA-B27 negative patients, has low specificity for axSpA [37,38]. The authors found it in 30% of well patients and patients with non-specific back pain. In a 2010 study [9], BME, erosions and fatty infiltration were found in 27% of patients from the control group with nonspecific back pain, and in 24% of well volunteers. Possibly, quantitative research into signal intensity, the evaluation of the extensity of marrow edema will improve the method’s specificity [24]. Administration of a contrast agent, basically (apart from the few synovitis and capsulitis cases) does not improve the diagnostic merit of T1 and STIR sequences [23,39]. There is research in progress aimed at evaluating the utility of diffusion weighted MRI (diffusion weighted imaging – DWI) [40] and whole-body MRI [41].
Conclusions
Our research confirmed MRI to visualize early inflammatory lesions [2,36]. Application of MRI in patients not disclosing lesions by radiographs according to the modified NY criteria allowed us to diagnose sacroiliitis in 38% of patients in a pre-radiographic stage and ruled out inflammation in 50% of patients with sacroiliitis by radiographic examination according to modNY criteria.
The agreement of radiographic and MR imaging was documented in only 60% of cases, including 53 patients in whom both modalities ruled out the presence of inflammatory lesions, and only 7 in whom they both confirmed sacroiliitis features.
The comparable number of true positive and negative results by radiographs in patients meeting the modNY criteria questions the validity of taking radiographs in SpA with the purpose of diagnosing inflammation. Radiographs, however, remain invaluable in terms of initial differential diagnostics of pelvic and back pain.
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Sudoł-Szopińska I, Urbanik A. Diagnostic imaging of sacroiliac joint and the spine in the course of spondyloarthropathies. Pol J Radiol. 2013;78(2):43–49. doi: 10.12659/PJR.889039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lacout A, Rousselin B, Pelage J-P. CT and MRI of spine and sacroiliac involvement in spondyloartropathy. Am J Roentgenol. 2008;191:1016–23. doi: 10.2214/AJR.07.3446. [DOI] [PubMed] [Google Scholar]
- 3.Sieper J, Rudwaleit M, Baraliakos X, et al. The assessment of Spondylo Artritis international Society (ASAS) handbook: a guide to assess spondyloarthritis. Ann Rheum Dis. 2009;68(Suppl 2):ii1–44. doi: 10.1136/ard.2008.104018. [DOI] [PubMed] [Google Scholar]
- 4.McMichael A, Bowness P. HLA-B27: natural function and pathogenic role in spondyloarthritis. Arthritis Res. 2002;4(Suppl,3):153–58. doi: 10.1186/ar571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heuft-Dorenbosch L, Landewe R, Weijers R, et al. Performance of various criteria sets in patients with inflammatory back pain of short duration; the Maastricht early spondyloarthritis clinic. Ann Rheum Dis. 2007;66:92–98. doi: 10.1136/ard.2006.053918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vossen MHE, den Broeder AA, Hendriks-Roelofs F, et al. Improvement in deployment of MRI in the sacroiliac joints in patients suspected for spondyloarthritis using a targeted intervention: a case study. Rheumatol. 2013;52:933–38. doi: 10.1093/rheumatology/kes405. [DOI] [PubMed] [Google Scholar]
- 7.Heuft-Dorenbosch L, Weijers R, Landewe R, et al. Magnetic resonance imaging changes of sacroiliac joints in patients with recent-onset inflammatory back pain: inter-reader reliability and prevalence of abnormalities. Arthritis Res Ther. 2006;8:R11. doi: 10.1186/ar1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rudwaleit M, vanderHeijde D, Landewé R, et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis. 2009;68:777–83. doi: 10.1136/ard.2009.108233. [DOI] [PubMed] [Google Scholar]
- 9.Weber U, Lambert RG, Ostergaard M, et al. The diagnostic utility of magnetic resonance imaging in spondyloarthritis. An international multricenter evaluation of one hundred eighty seven subjects. Arthritis Rheum. 2010;62:3048–58. doi: 10.1002/art.27571. [DOI] [PubMed] [Google Scholar]
- 10.Heuft-Dorenbosch L, Landewe R, Weijers R, et al. Combining information obtained from magnetic resonance imaging and conventional radiographs to detect sacroiliitis in patients with recent onset inflammatory back pain. Ann Rheum Dis. 2006;65:804–8. doi: 10.1136/ard.2005.044206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rudwaleit M, Baraliakos X, Listing J, et al. Magnetic resonance imaging of the spine and the sacroiliac joints in ankylosing spondylitis and undifferentiated spondyloarthritis during treatment with etanercept. Ann Rheum Dis. 2005;64:1305–10. doi: 10.1136/ard.2004.032441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aydin SZ, Maksymowych WP, Bannett AN, et al. Validation of the ASAS criteria and definition of a positive MRI of sacroiliac joint in an inception cohort of axial spondyloarthritis followed up for 8 years. Ann Rheum Dis. 2012;71:56–60. doi: 10.1136/ard.2011.153064. [DOI] [PubMed] [Google Scholar]
- 13.Decker JL. Glossary Subcommittee of The ARA Committee on Rheumatologic Practice, American Rheumatism Association nomenclature abd classification of arthritis and rheumatism. Arthritis Rheum. 1983;26:1029–32. doi: 10.1002/art.1780260813. [DOI] [PubMed] [Google Scholar]
- 14.Saraux A, Guillemin F, Guggenbuhl P, et al. Prevalence of spondyloarthropatjies in France: 2001. Ann Rheum Dis. 2005;64:1431–35. doi: 10.1136/ard.2004.029207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Angelis R, Salaffi F, Grassi W. Prevalence of spondyloarthropaties in Italian population sample: a regional community-based study. Rheumatology. 2007;36:14–21. doi: 10.1080/03009740600904243. [DOI] [PubMed] [Google Scholar]
- 16.Reveille JD, Weisman MH. The epidemiology of back pain, axial spondyloarthritis and HLA-B27 in the United States. Am J Med Sci. 2013;345:431–36. doi: 10.1097/maj.0b013e318294457f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williamson L, Dockerty JL, Dalbeth N, et al. Clinical assessment of sacroiliitis and HLA-B27 are poor predictors of sacroiliitis diagnosed with magnetic resonance imaging in psoriatic arthritis. Rheumatology. 2004;43:85–88. doi: 10.1093/rheumatology/keg475. [DOI] [PubMed] [Google Scholar]
- 18.van der Berg R, de Hooge M, van Gaalen F, et al. Percentage of patients with spondyloarthritis in patients referred because of chronic back pain and performance of classification criteria: experience from the Spondyloarthritis Caugh Early (SPACE) cohort. Rheumatol. 2013;52:1492–99. doi: 10.1093/rheumatology/ket164. [DOI] [PubMed] [Google Scholar]
- 19.Hanly JG, Mitchell MJ, Barnes DC, MacMillan L. Early recognition of sacroiliitis by magnetic resonance imaging and single photon emission computed tomography. J Rheumatol. 1994;21:2088–95. [PubMed] [Google Scholar]
- 20.Puhakka JB, Jurik AG, Schiottz-Christiansen B, et al. Magnetic resonance imaging of sacroiliitis in early seronegative spondyloathropathy. Abnormalities correlated to clinical and laboratory findings. Rheumatology (Oxford) 2004;43:234–37. doi: 10.1093/rheumatology/keh008. [DOI] [PubMed] [Google Scholar]
- 21.Bollow M, Braun J, Hamm B, et al. Early sacroiliitis in patients with spondyloartropathy: evaluation with dynamic gadolinium-enhanced MR imaging. Radiology. 1995;194:529–36. doi: 10.1148/radiology.194.2.7824736. [DOI] [PubMed] [Google Scholar]
- 22.Chung HY, Machado P, van der Hejide D, et al. Hla-B27 positive patients differ from HLA-B27 negative patients in clinical presentation and imaging: results from DESIR cohort of patients with recent onset axial spondyloarthritis. Ann Rheum Dis. 2011;70:1930–36. doi: 10.1136/ard.2011.152975. [DOI] [PubMed] [Google Scholar]
- 23.de Hooge M, van den Berg R, Navarro-Compan V, et al. Magnetic resonance imaging of the sacroiliac joints in the early detection of spondyloarthritis: no added value of gadolinium compared to short tau inversion recovery sequence. Rheumatol. 2013;52:1220–24. doi: 10.1093/rheumatology/ket012. [DOI] [PubMed] [Google Scholar]
- 24.Chary-Valckenaere I, d’Agostino M-A, Loeuille D. Role for imaging studies in ankylosing spondylitis. Joint Bone Spine. 2011;78:138–43. doi: 10.1016/j.jbspin.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 25.Rudwaleit M, van der Hoijde D, Khan MA, et al. How to diagnose axial spondyloartropathy earlier. Ann Rheum Dis. 2004;63:535–43. doi: 10.1136/ard.2003.011247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Onna M, Jurik AG, van der Heijde D, et al. HLA-B27 and gender independently determine the likelyhood of a positive MRI of the sacroiliac Joints in patients with early inflammatory back pain: a 2-year MRI follow-up study. Ann Rheum. 2011;70:1981–85. doi: 10.1136/annrheumdis-2011-200025. [DOI] [PubMed] [Google Scholar]
- 27.Weber U, Ostergaard M, Lambert RGW, Maksymowych WP. The impact of MRI on the clinical management of inflammatory arthrides. Skeletal Radiol. 2011;40(9):1153–73. doi: 10.1007/s00256-011-1204-5. [DOI] [PubMed] [Google Scholar]
- 28.McQueen FM, Gao A, Ostergaard M, et al. High grade MRI bone edema is common within the surgical field in rheumatoid arthritis patients undergoing joint replacement and is associated with osteitis in subchondral bone. Ann Rheum Dis. 2007;66:1581–87. doi: 10.1136/ard.2007.070326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jimenez-Boj E, Noebauer-Huhmann I, Hanslik-Schnabel B, et al. Bone erosions and bone marrow edema as defined by magnetic resonance imaging reflect true bone marrow infiltration in rheumatoid arthritis. Arthritis Rheum. 2007;56:1118–24. doi: 10.1002/art.22496. [DOI] [PubMed] [Google Scholar]
- 30.Barkham N, Keen HI, Coates LC, et al. Clinical and imaging efficacy of infliximab in HLA-B27-positive patients with magnetic resonance imaging determined early sacroiliitis. Arthritis Rheum. 2009;60:946–54. doi: 10.1002/art.24408. [DOI] [PubMed] [Google Scholar]
- 31.Blum U, Buitrago-Tellez C, Mundinger A, et al. Magnetic resonance imaging (MRI) for detection of active sacroiliitis – a prospective study compering conventional radiography, scintigraphy, and contrast-enhanced MRI. J Rheumatol. 1996;23:2107–15. [PubMed] [Google Scholar]
- 32.Oosteveen J, Prevo R, den Boer J, van de Laar M. Early detection of sacroiliitis on magnetic resonance imaging and subsequent development of sacroiliitis on plain radiography. A prospective, longitudinal study. J Reumatol. 1999;26:1953–58. [PubMed] [Google Scholar]
- 33.Canella C, Schau B, Ribeiro E, et al. MRI in seronegative spondyloarthritis: imaging features and differential diagnosis in the spine and sacroiliac joints. Am J Roentgenol. 2013;200:149–57. doi: 10.2214/AJR.12.8858. [DOI] [PubMed] [Google Scholar]
- 34.Bollow M, Hermann K-G, Biedermann T, et al. Very early spondyloarthritis: where the inflammation in the sacroiliac joints starts. Ann Rheum Dis. 2005;64:1644–46. doi: 10.1136/ard.2004.034967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bennett AN, McGonagle D, O’Connor P, et al. Severity of baseline magnetic resonance imaging-evident sacroiliitis and HLA-B27 status in early inflammatory back pain predict radiographically evident ankylosing spondylitis at eight years. Arthritis Rheum. 2008;58:3413–18. doi: 10.1002/art.24024. [DOI] [PubMed] [Google Scholar]
- 36.Landeve RBM, Tuberge A. Clinical Assessment of outcome research in spondyloarthritis. Curr Rheumatol Res. 2009;11:334–39. doi: 10.1007/s11926-009-0048-7. [DOI] [PubMed] [Google Scholar]
- 37.Weber U, Ostergaard M, Hodler J, et al. Frequent detection of sacroiliac joint abnormalities on MRI in healthy subjects and patients with non-specific back pain. Arthritis Rheum. 2009;60(Suppl 10):S757. [Google Scholar]
- 38.Bigot J, Loeuille D, Chary-Valckenaere, et al. Determination of the best diagnostic criteria of sacroiliitis with MRI. J Radiol. 1999;80:1649–57. [PubMed] [Google Scholar]
- 39.Madsen K, Egund N, Jurik AG. Grading of inflammatory disease activity in the sacroiliac joints with magnetic resonance imaging: comparison between short-tau inversion recovery and gadolinium contrast-enhanced sequences. J Rheumatol. 2010;37:393–400. doi: 10.3899/jrheum.090519. [DOI] [PubMed] [Google Scholar]
- 40.Bozgeyik Z, Ozgocmen S, Kocakoc E. Role of diffusion-weighted MRI in the detection of early active sacroiliitis. Am J Roentgenol. 2008;191:980–86. doi: 10.2214/AJR.07.3865. [DOI] [PubMed] [Google Scholar]
- 41.Althoff CE, Sieper J, Song I-H, et al. Active inflammation and structural change in early active axial spondyloarthritis as detected by whole-body MRI. Ann Rheum Dis. 2013;72:967–73. doi: 10.1136/annrheumdis-2012-201545. [DOI] [PubMed] [Google Scholar]