Abstract
Background
Although many studies have estimated the association between the butyrylcholinesterase (BCHE) K variant and Alzheimer’s disease (AD) risk, the results are still controversial. We thus conducted this meta-analysis.
Material/Methods
We searched NCBI, Medline, Web of Science, and Embase databases to find all eligible studies. Odds ratios (ORs) with 95% confidence intervals (CIs) were used to assess the strength of the association.
Results
We found a significant association between BCHE K variant and AD risk (OR=1.20; 95% CI 1.03–1.39; P=0.02). In the stratified analysis by ethnicity, we observed a significant association between BCHE K variant and AD risk in Asians (OR=1.32; 95% CI 1.02–1.72; P=0.04). However, no significant association between BCHE K variant and AD risk in Caucasians was found (OR=1.14; 95% CI 0.95–1.37; P=0.16). When stratified by the age of AD onset, we found that late-onset AD (LOAD) was significantly associated with BCHE K variant (OR=1.44; 95% CI 1.05–1.97; P=0.02). No significant association between BCHE K variant and early-onset AD (EOAD) risk was observed (OR=1.16; 95% CI 0.89–1.51; P=0.27). Compared with non-APOE ɛ4 and non-BCHE K carriers, no significant association between BCHE K variant and AD risk was found (OR=1.11; 95% CI 0.91–1.35; P=0.30). However, APOE ɛ4 carriers showed increased AD risk in both non-BCHE K carriers (OR=2.81; 95% CI 1.75–4.51; P=0.0001) and BCHE K carriers (OR=3.31; 95% CI 1.82–6.02; P=0.0001).
Conclusions
The results of this meta-analysis indicate that BCHE K variant might be associated with AD risk.
Keywords: Alzheimer Disease, Butyrylcholinesterase, Genetics
Background
Alzheimer’s disease (AD) is one of the most common forms of dementia. The clinical manifestations of AD include loss of memory, and behavioral and cognitive disorders [1]. The survival time for patients with AD is generally 4 to 6 years after diagnosis. Thus, AD is a major public health concern. However, the etiology and pathogenesis of AD remain unclear. Recently, accumulating evidence shows that genetic factors might be involved in the development of AD [2].
Butyrylcholinesterase (BCHE) is a hydrolytic enzyme that can catalyze the hydrolysis of excess acetylcholine neurotransmission in the synaptic space. Darreh-Shori et al. suggested that low cerebrospinal fluid (CSF) levels of BCHE might predict extensive incorporation in neuritic plaques, greater central neurodegeneration, and increased neurotoxicity [3]. They also found that BCHE levels correlated with cerebral glucose metabolism, cerebral β-amyloid load, and CSF P-tau [4]. Diamant et al. reported an association of the BChE-K variant with impaired interaction with the fibrillogenic beta-amyloid protein [5]. Shenhar-Tsarfaty also suggested that this variant could influence metabolic syndrome [6].
BCHE K variant is one of the most common polymorphism in the BCHE gene. This is an alanine-to-threonine substitution in the 539 amino acid position (Ala539Thr). This polymorphism is associated with a 30% reduction of serum BCHE activity [7]. Many studies have been conducted to evaluate the association between BCHE K variant and AD risk [8–32]. However, the results are controversial and inconsistent. Therefore, we performed a meta-analysis to assess the association between BCHE K variant and AD risk.
Material and Methods
Search for studies
We searched NCBI, Medline, Web of Science, and Embase databases to find all eligible studies. The last retrieval date was October 29, 2014. The following terms and keywords were used: (“Alzheimer’s disease” or “Alzheimer disease”) and (“Butyrylcholinesterase” or “BCHE”). All relevant studies were retrieved.
Inclusion and exclusion criteria
The inclusion criteria were as follows: (1) the study should be case-control or a cohort design; and (2) the study should focus on the association between BCHE K variant and AD risk. The exclusion criteria were as follows: (1) animal studies; (2) reviews or abstracts; and (3) duplications.
Data extraction
According to the inclusion criteria, 2 investigators extracted the data independently. Any discrepancy was adjudicated by the third investigator. The following data was extracted from each study: first author, year, ethnicity, age and sex of patients, sample size, and genotyping results from BCHE and APOE genes.
Statistical analysis
The odds ratio (OR) and its 95% confidence interval (95% CI) were used to estimate the strength of the association between BCHE K variant and AD risk. A recessive model (KK vs. WW+WK) was applied. We estimated the heterogeneity by using the chi-square-based Q-test, which was considered significant at P<0.10. A fixed-effects model was used in the absence of heterogeneity; otherwise, a random-effects model was used. Subgroup analyses were performed based on ethnicity, APOE ɛ4 status, and the age at AD onset. We conducted sensitivity analysis by excluding every study individually and recalculating the OR and 95% CI. Potential publication bias was estimated using Egger’s linear regression test and the funnel plot. All statistical analyses were conducted using STATA software version 11.0 (Stata Corporation, College Station, TX). All P values were 2-sided, with a significance level of 0.05.
Results
Study characteristics
A total of 25 eligible case-control studies (3850 cases and 3947 controls) met the inclusion criteria [8–32]. Among these 25 case-control studies, 5 studies focused on Asians and 20 focused on Caucasians. The main characteristics of the included studies investigating the association of BCHE K variant and AD risk are presented in Table 1.
Table 1.
Characteristics of the case-control studies included in meta-analysis.
| First author | Year | Ethnicity | Age | Sex | Case (n) | Control (n) | BCHE K allele frequency (%) | APOE ɛ4 allele frequency (%) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Case | Control | Case | Control | |||||||
| Lehmann | 1997 | Caucasian | >65 | Mixed | 74 | 104 | 13 | 9 | 41 | 16 |
| Brindle | 1998 | Caucasian | 75.4 | Mixed | 138 | 165 | 20.3 | 18.8 | 31.4 | 14 |
| Crawford | 1998 | Caucasian | 76.4 | Mixed | 391 | 201 | 17.2 | 14.4 | 75 | 67.4 |
| Hiltunen | 1998 | Caucasian | 73 | Mixed | 59 | 59 | 12 | 22 | 100 | 100 |
| Kehoe | 1998 | Caucasian | NA | Mixed | 181 | 262 | 21 | 22 | 56.5 | 28.1 |
| Singleton | 1998 | Caucasian | 78.3 | Mixed | 119 | 83 | 20 | 17 | 65.5 | 21.4 |
| Yamada | 1998 | Asian | 85.1 | Mixed | 48 | 107 | 31.7 | 31.2 | NA | NA |
| Grubber | 1999 | Caucasian | NA | Mixed | 169 | 193 | 18.8 | 23 | 18 | 25.4 |
| Ki | 1999 | Asian | 73 | Mixed | 78 | 74 | 23 | 16 | 28 | 7 |
| Tilley | 1999 | Caucasian | 81 | Mixed | 177 | 118 | 20 | 19 | 31 | 11 |
| Wiebusch | 1999 | Caucasian | 78 | Mixed | 135 | 70 | 25 | 16 | 43 | 18 |
| Yamamoto | 1999 | Asian | 68.2 | Mixed | 149 | 200 | 15.9 | 15.7 | NA | NA |
| Lee | 2000 | Asian | 69.1 | Mixed | 89 | 101 | 13.5 | 12.3 | 24.2 | 6.9 |
| Mattila | 2000 | Caucasian | >65 | Mixed | 80 | 67 | 21 | 15 | 13 | 11 |
| McIlroy | 2000 | Caucasian | 77.7 | Mixed | 175 | 187 | 26.8 | 14.4 | 34.5 | 31.6 |
| Kim | 2001 | Asian | 71.7 | Mixed | 164 | 293 | 11.7 | 10.1 | NA | NA |
| Prince | 2001 | Caucasian | NA | Mixed | 204 | 186 | 20.9 | 20.1 | NA | NA |
| Raygani | 2004 | Caucasian | 75 | Mixed | 105 | 129 | 24.2 | 12 | 25.9 | 6.2 |
| Combarros | 2005 | Caucasian | 75 | Mixed | 187 | 172 | 10 | 15 | 12 | 8 |
| Cook | 2005 | Caucasian | NA | Mixed | 212 | 316 | 27 | 20 | NA | NA |
| Deniz-Naranjo | 2007 | Caucasian | >60 | Mixed | 282 | 312 | 19.5 | 19.4 | 48.9 | 22.1 |
| Piccardi | 2007 | Caucasian | 76.8 | Mixed | 158 | 118 | 21 | 19 | 18.9 | 5.5 |
| Mateo | 2008 | Caucasian | 71.3 | Mixed | 231 | 221 | 12 | 10 | NA | NA |
| Bizzarro | 2010 | Caucasian | 73.3 | Mixed | 167 | 129 | 10.1 | 10.2 | NA | NA |
| Simão-Silva | 2013 | Caucasian | 74.5 | Mixed | 78 | 80 | 23 | 21 | NA | NA |
NA – not available.
Association between BCHE K variant and AD risk
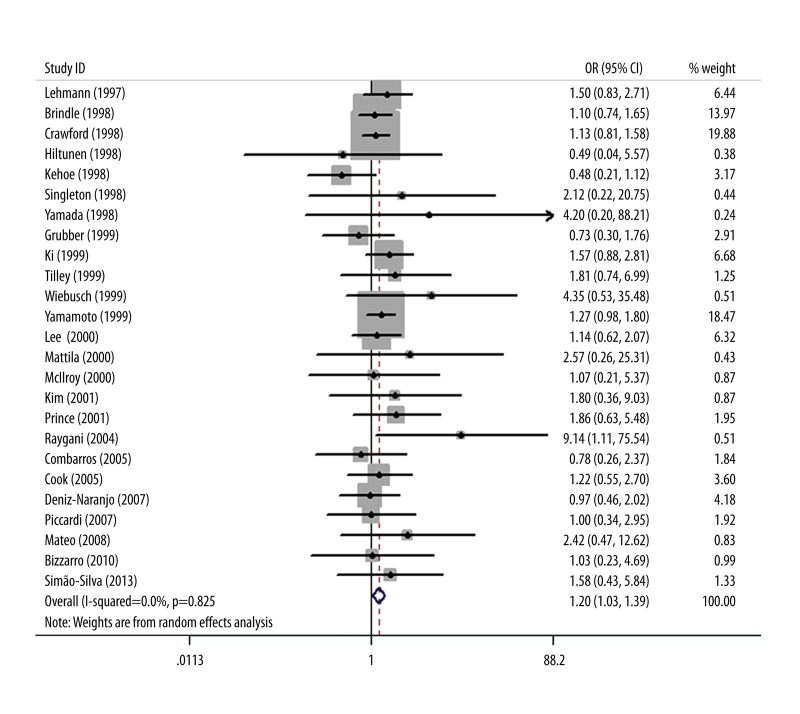
We found a significant association between BCHE K variant and AD risk (OR=1.20; 95% CI 1.03–1.39; P=0.02; Figure 1). In the stratified analysis by ethnicity (Table 2), we observed a significant association between BCHE K variant and AD risk in Asians (OR=1.32; 95% CI 1.02–1.72; P=0.04), but no significant association between BCHE K variant and AD risk in Caucasians was found (OR=1.14; 95% CI 0.95–1.37; P=0.16). When stratified by age at AD onset, we found that late-onset AD (LOAD) was significantly associated with BCHE K variant (OR=1.44; 95% CI 1.05–1.97; P=0.02). No significant association between BCHE K variant and early-onset AD (EOAD) risk was observed (OR=1.16; 95% CI 0.89–1.51; P=0.27).
Figure 1.
Meta-analysis of BCHE K variant and AD risk.
Table 2.
Results from this meta-analysis.
| OR (95% CI)* | P | I2 (%) | |
|---|---|---|---|
| Overall | 1.20 (1.03–1.39) | 0.02 | 0 |
| Asian | 1.32 (1.02–1.72) | 0.04 | 0 |
| Caucasian | 1.14 (0.95–1.37) | 0.16 | 0 |
| EOAD | 1.16 (0.89–1.51) | 0.27 | 33 |
| LOAD | 1.44 (1.05–1.97) | 0.02 | 15 |
EOAD – early-onset Alzheimer’s disease; LOAD – late-onset Alzheimer’s disease.
OR values refer to association between BCHE K variant and AD risk.
Compared with non-APOE ɛ4 and non-BCHE K carriers, no significant association between BCHE K variant and AD risk was found (OR=1.11; 95% CI 0.91–1.35; P=0.30). However, APOE ɛ4 carriers showed increased AD risk in both non-BCHE K carriers (OR=2.81; 95%CI 1.75–4.51; P=0.0001) and BCHE K carriers (OR=3.31; 95% CI 1.82–6.02; P=0.0001). Results are listed in Table 3.
Table 3.
APOE ɛ4 and BCHE K variant interaction.
| APOE ɛ4 | BCHE K | OR (95% CI) | P | I2 (%) |
|---|---|---|---|---|
| − | − | Reference | – | – |
| − | + | 1.11 (0.91–1.35) | 0.30 | 45 |
| + | − | 2.81 (1.75–4.51) | 0.0001 | 52 |
| + | + | 3.31 (1.82–6.02) | 0.0001 | 69 |
Sensitivity analysis and publication bias
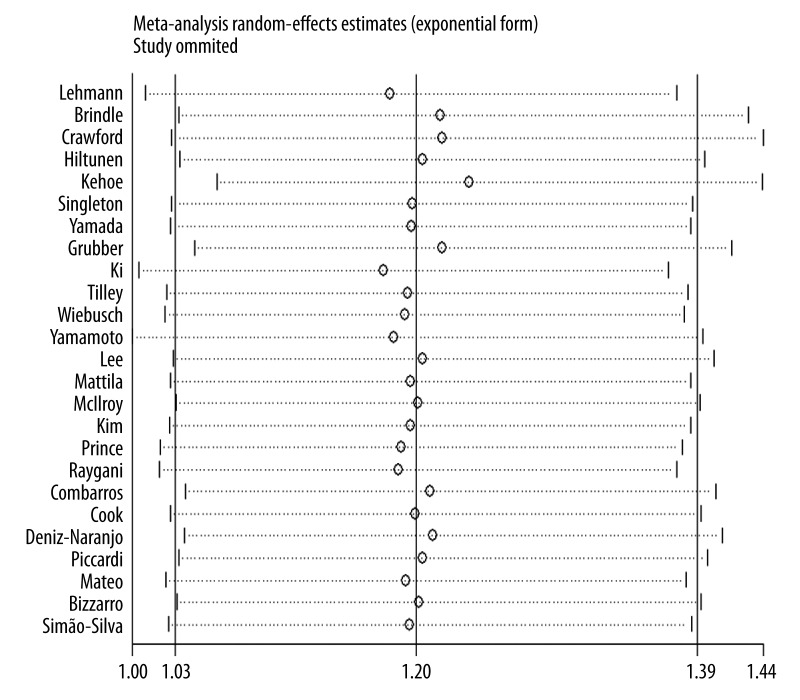
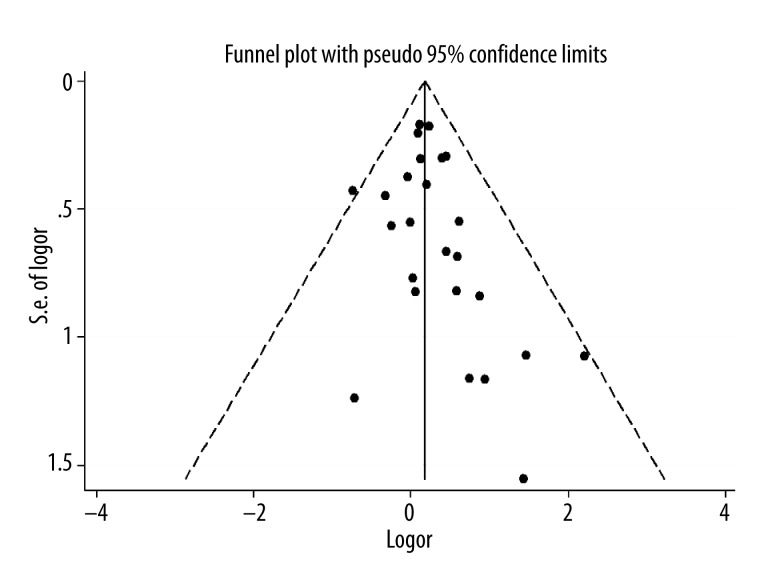
In the sensitivity analysis, the impact of each study on the pooled OR was checked by repeating the meta-analysis when omitting each study. This sensitivity analysis validated the stability of the results from this meta-analysis (Figure 2). The shape of funnel plots did not show any evidence of obvious asymmetry (Figure 3). Furthermore, the Egger’s test result suggested that there was no significant publication bias (P=0.67).
Figure 2.
Sensitivity analysis of BCHE K variant and AD risk.
Figure 3.
Funnel plot of association between BCHE K variant and AD risk.
Discussion
This meta-analysis with a total of 3850 cases and 3947 controls systematically evaluated the association between BCHE K variant and AD risk. Results from this meta-analysis suggested that BCHE K variant was significantly associated with AD risk. In the subgroup analysis by ethnicity, a significant association between BCHE K variant and AD risk in Asians was found, but this result was not found in Caucasians. This difference suggests that race might play a role in AD. Only 5 studies were included in our meta-analysis; thus, more studies with Asians are needed to confirm our results. In the stratified analysis by APOE ɛ4 status, APOE ɛ4 carriers, but not APOE ɛ4 non-carriers with BCHE K variant, showed an increased AD risk. This result suggests that gene-gene interaction also plays an important role in the development of AD. More studies should be conducted to assess the interaction between other genes and BCHE K variant. When stratified by age at AD onset, we found that the risk of LOAD, but not EOAD, was significantly associated with BCHE K variant. This information indicates that age also has a critical role in AD development.
Carson et al. found that BCHE activity was associated with the amyloid and the neuritic component in neuritic plaques [33]. Guillozet et al. also found that BCHE activity increased in the AD brain [34]. The activity of serum BCHE-K to hydrolyze butyrylthiocholine was found to be reduced by 30% relative to BCHE-U [7]. Thus, BCHE K variant might be associated with a decreased AD risk. However, Lopez et al. reported that BCHE K variant carriers were refractory to cholinesterase inhibitor therapy [35]. Additionally, Podoly et al. found that BCHE K variant had an elevated AD risk due to inefficient interference with amyloidogenic processes [36]. Furthermore, Ballard et al. found that BuCh E may play a role in the phosphorylation of tau, relevant to therapeutic inhibition of the enzyme [37]. Alkalay et al. found no association between BChE activity and amyloid loads in the AD brain [38]. Thus, the pathological role of BCHE K variant was still controversial. Our meta-analysis confirmed that this polymorphism might be associated with AD risk. More studies are needed to investigate the mechanism by which BCHE K variant could impact the risk of AD [39,40].
This meta-analysis had several limitations. First, results of this meta-analysis were based on unadjusted OR, because not all studies offered the adjusted ORs. Second, although the number of included studies was relatively large, the sample size and statistical power was still limited. Third, we only included the published studies; thus, publication bias and selection bias might exist. Forth, lack of sufficient eligible data on BCHE K variant and AD limited our further stratified analyses.
Conclusions
In conclusion, this study shows that BCHE K variant is associated with AD risk. More well-conducted studies with larger sample size are warranted to confirm our results.
Footnotes
Conflicts of interest
None.
Source of support: The work was supported by the opening project of the key laboratory of comprehensive utilization of advantage plants resources in Hunan South, Hunan University of Science and Engineering (No. XNZW14C10); the construct program of the key discipline in Hunan Province (2012); and the aid program for science and technology innovative research teams in higher educational institutions of Hunan Province (No. 2012-318)
References
- 1.Finder VH. Alzheimer’s disease: a general introduction and pathomechanism. J Alzheimers Dis. 2010;22(Suppl 3):5–19. doi: 10.3233/JAD-2010-100975. [DOI] [PubMed] [Google Scholar]
- 2.Kim DH, Yeo SH, Park JM, et al. Genetic markers for diagnosis and pathogenesis of Alzheimer’s disease. Gene. 2014;545(2):185–93. doi: 10.1016/j.gene.2014.05.031. [DOI] [PubMed] [Google Scholar]
- 3.Darreh-Shori T, Brimijoin S, Kadir A, et al. Differential CSF butyrylcholinesterase levels in Alzheimer’s disease patients with the ApoE epsilon4 allele, in relation to cognitive function and cerebral glucose metabolism. Neurobiol Dis. 2006;24(2):326–33. doi: 10.1016/j.nbd.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 4.Darreh-Shori T, Forsberg A, Modiri N, et al. Differential levels of apolipoprotein E and butyrylcholinesterase show strong association with pathological signs of Alzheimer’s disease in the brain in vivo. Neurobiol Aging. 2011;32(12):2320.e15–32. doi: 10.1016/j.neurobiolaging.2010.04.028. [DOI] [PubMed] [Google Scholar]
- 5.Diamant S, Podoly E, Friedler A, et al. Butyrylcholinesterase attenuates amyloid fibril formation in vitro. Proc Natl Acad Sci USA. 2006;103:8628–33. doi: 10.1073/pnas.0602922103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shenhar-Tsarfaty S, Berliner S, Bornstein NM, Soreq H. Cholinesterases as biomarkers for parasympathetic dysfunction and inflammation-related disease. J Mol Neurosci. 2014;53:298–305. doi: 10.1007/s12031-013-0176-4. [DOI] [PubMed] [Google Scholar]
- 7.Bartels CF, Jensen FS, Lockridge O, et al. DNA mutation associated with the human butyrylcholinesterase K-variant and its linkage to the atypical variant mutation and other polymorphic sites. Am J Hum Genet. 1992;50(5):1086–103. [PMC free article] [PubMed] [Google Scholar]
- 8.Lehmann DJ, Johnston C, Smith AD. Synergy between the genes for butyrylcholinesterase K variant and apolipoprotein E4 in late-onset confirmed Alzheimer’s disease. Hum Mol Genet. 1997;6(11):1933–36. doi: 10.1093/hmg/6.11.1933. [DOI] [PubMed] [Google Scholar]
- 9.Brindle N, Song Y, Rogaeva E, et al. Analysis of the butyrylcholinesterase gene and nearby chromosome 3 markers in Alzheimer disease. Hum Mol Genet. 1998;7(5):933–35. doi: 10.1093/hmg/7.5.933. [DOI] [PubMed] [Google Scholar]
- 10.Crawford F, Fallin D, Suo Z, et al. The butyrylcholinesterase gene is neither independently nor synergistically associated with late-onset AD in clinic- and community-based populations. Neurosci Lett. 1998;249(2–3):115–18. doi: 10.1016/s0304-3940(98)00423-6. [DOI] [PubMed] [Google Scholar]
- 11.Hiltunen M, Mannermaa A, Helisalmi S, et al. Butyrylcholinesterase K variant and apolipoprotein E4 genes do not act in synergy in Finnish late-onset Alzheimer’s disease patients. Neurosci Lett. 1998;250(1):69–71. doi: 10.1016/s0304-3940(98)00453-4. [DOI] [PubMed] [Google Scholar]
- 12.Kehoe PG, Williams H, Holmans P, et al. The butyrylcholinesterase K variant and susceptibility to Alzheimer’s disease. J Med Genet. 1998;35(12):1034–35. doi: 10.1136/jmg.35.12.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singleton AB, Smith G, Gibson AM, et al. No association between the K variant of the butyrylcholinesterase gene and pathologically confirmed Alzheimer’s disease. Hum Mol Genet. 1998;7(5):937–39. doi: 10.1093/hmg/7.5.937. [DOI] [PubMed] [Google Scholar]
- 14.Yamada M, Sodeyama N, Itoh Y, et al. Butyrylcholinesterase K variant and cerebral amyloid angiopathy. Stroke. 1998;29(12):2488–90. doi: 10.1161/01.str.29.12.2488. [DOI] [PubMed] [Google Scholar]
- 15.Grubber JM, Saunders AM, Crane-Gatherum AR, et al. Analysis of association between Alzheimer disease and the K variant of butyrylcholinesterase (BCHE-K) Neurosci Lett. 1999;269(2):115–19. doi: 10.1016/s0304-3940(99)00426-7. [DOI] [PubMed] [Google Scholar]
- 16.Ki CS, Na DL, Kim JW, et al. No association between the genes for butyrylcholinesterase K variant and apolipoprotein E4 in late-onset Alzheimer’s disease. Am J Med Genet. 1999;88(2):113–15. doi: 10.1002/(sici)1096-8628(19990416)88:2<113::aid-ajmg2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 17.Tilley L, Morgan K, Grainger J, et al. Evaluation of polymorphisms in the presenilin-1 gene and the butyrylcholinesterase gene as risk factors in sporadic Alzheimer’s disease. Eur J Hum Genet. 1999;7(6):659–63. doi: 10.1038/sj.ejhg.5200351. [DOI] [PubMed] [Google Scholar]
- 18.Wiebusch H, Poirier J, Sévigny P, Schappert K. Further evidence for a synergistic association between APOE epsilon4 and BCHE-K in confirmed Alzheimer’s disease. Hum Genet. 1999;104(2):158–63. doi: 10.1007/s004390050929. [DOI] [PubMed] [Google Scholar]
- 19.Yamamoto Y, Yasuda M, Mori E, Maeda K. Failure to confirm a synergistic effect between the K-variant of the butyrylcholinesterase gene and the epsilon4 allele of the apolipoprotein gene in Japanese patients with Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 1999;67(1):94–96. doi: 10.1136/jnnp.67.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee DW, Liu HC, Liu TY, et al. No association between butyrylcholinesterase K-variant and Alzheimer disease in Chinese. Am J Med Genet. 2000;96(2):167–69. [PubMed] [Google Scholar]
- 21.Mattila KM, Rinne JO, Röyttä M, et al. Dipeptidyl carboxypeptidase 1 (DCP1) and butyrylcholinesterase (BCHE) gene interactions with the apolipoprotein E epsilon4 allele as risk factors in Alzheimer’s disease and in Parkinson’s disease with coexisting Alzheimer pathology. J Med Genet. 2000;37(10):766–70. doi: 10.1136/jmg.37.10.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McIlroy SP, Crawford VL, Dynan KB, et al. Butyrylcholinesterase K variant is genetically associated with late onset Alzheimer’s disease in Northern Ireland. J Med Genet. 2000;37(3):182–85. doi: 10.1136/jmg.37.3.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim KW, Jhoo JH, Lee JH, et al. Neither the butyrylcholinesterase K variant nor transferrin C2 variant confers a risk for Alzheimer’s disease in Koreans. J Neural Transm. 2001;108(10):1159–66. doi: 10.1007/s007020170005. [DOI] [PubMed] [Google Scholar]
- 24.Prince JA, Feuk L, Sawyer SL, et al. Lack of replication of association findings in complex disease: an analysis of 15 polymorphisms in prior candidate genes for sporadic Alzheimer’s disease. Eur J Hum Genet. 2001;9(6):437–44. doi: 10.1038/sj.ejhg.5200651. [DOI] [PubMed] [Google Scholar]
- 25.Raygani AV, Zahrai M, Soltanzadeh A, et al. Analysis of association between butyrylcholinesterase K variant and apolipoprotein E genotypes in Alzheimer’s disease. Neurosci Lett. 2004;371(2–3):142–46. doi: 10.1016/j.neulet.2004.08.057. [DOI] [PubMed] [Google Scholar]
- 26.Combarros O, Riancho JA, Infante J, et al. Interaction between CYP19 aromatase and butyrylcholinesterase genes increases Alzheimer’s disease risk. Dement Geriatr Cogn Disord. 2005;20(2–3):153–57. doi: 10.1159/000087065. [DOI] [PubMed] [Google Scholar]
- 27.Cook LJ, Ho LW, Wang L, et al. Candidate gene association studies of genes involved in neuronal cholinergic transmission in Alzheimer’s disease suggests choline acetyltransferase as a candidate deserving further study. Am J Med Genet B Neuropsychiatr Genet. 2005;132B(1):5–8. doi: 10.1002/ajmg.b.30068. [DOI] [PubMed] [Google Scholar]
- 28.Déniz-Naranjo MC, Muñoz-Fernández C, Alemany-Rodríguez MJ, et al. Butyrylcholinesterase, ApoE and Alzheimer’s disease in a population from the Canary Islands (Spain) Neurosci Lett. 2007;427(1):34–38. doi: 10.1016/j.neulet.2007.08.059. [DOI] [PubMed] [Google Scholar]
- 29.Piccardi M, Congiu D, Squassina A, et al. Alzheimer’s disease: case-control association study of polymorphisms in ACHE, CHAT, and BCHE genes in a Sardinian sample. Am J Med Genet B Neuropsychiatr Genet. 2007;144B(7):895–99. doi: 10.1002/ajmg.b.30548. [DOI] [PubMed] [Google Scholar]
- 30.Mateo I, Llorca J, Infante J, et al. Gene-gene interaction between 14-3-3 zeta and butyrylcholinesterase modulates Alzheimer’s disease risk. Eur J Neurol. 2008;15(3):219–22. doi: 10.1111/j.1468-1331.2008.02059.x. [DOI] [PubMed] [Google Scholar]
- 31.Bizzarro A, Guglielmi V, Lomastro R, et al. BuChE K variant is decreased in Alzheimer’s disease not in fronto-temporal dementia. J Neural Transm. 2010;117(3):377–83. doi: 10.1007/s00702-009-0358-y. [DOI] [PubMed] [Google Scholar]
- 32.Simão-Silva DP, Bertolucci PH, de Labio RW, et al. Association analysis between K and -116A variants of butyrylcholinesterase and Alzheimer’s disease in a Brazilian population. Chem Biol Interact. 2013;203(1):358–60. doi: 10.1016/j.cbi.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 33.Carson KA, Geula C, Mesulam MM. Electron microscopic localization of cholinesterase activity in Alzheimer brain tissue. Brain Res. 1991;540(1–2):204–8. doi: 10.1016/0006-8993(91)90508-s. [DOI] [PubMed] [Google Scholar]
- 34.Guillozet AL, Smiley JF, Mash DC, Mesulam MM. Butyrylcholinesterase in the life cycle of amyloid plaques. Ann Neurol. 1997;42(6):909–18. doi: 10.1002/ana.410420613. [DOI] [PubMed] [Google Scholar]
- 35.Lopez OL, Becker JT, Wisniewski S, et al. Cholinesterase inhibitor treatment alters the natural history of Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2002;72(3):310–14. doi: 10.1136/jnnp.72.3.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Podoly E, Shalev DE, Shenhar-Tsarfaty S, et al. The butyrylcholinesterase K variant confers structurally derived risks for Alzheimer pathology. J Biol Chem. 2009;284(25):17170–79. doi: 10.1074/jbc.M109.004952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ballard C, Morris C, Kalaria R, et al. The k variant of the butyrylcholinesterase gene is associated with reduced phosphorylation of tau in dementia patients. Dement Geriatr Cogn Disord. 2005;19(5–6):357–60. doi: 10.1159/000084705. [DOI] [PubMed] [Google Scholar]
- 38.Alkalay A, Rabinovici GD, Zimmerman G, et al. Plasma acetylcholinesterase activity correlates with intracerebral β-amyloid load. Curr Alzheimer Res. 2013;10(1):48–56. [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang T, Jia Y. Meta-analysis of Ubiquilin1 gene polymorphism and Alzheimer’s disease risk. Med Sci Monit. 2015;21:2250–55. doi: 10.12659/MSM.891030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zimny A, Bladowska J, Neska M, et al. Quantitative MR evaluation of atrophy, as well as perfusion and diffusion alterations within hippocampi in patients with Alzheimer’s disease and mild cognitive impairment. Med Sci Monit. 2013;19:86–94. doi: 10.12659/MSM.883757. [DOI] [PMC free article] [PubMed] [Google Scholar]