Abstract
Background
This study aimed to evaluate the effects of lidocaine treatment on cognitive impairment in aged patients undergoing spine surgery and to explore the underlying mechanism.
Material/Methods
Patients were randomly divided into 2 treatment groups: (1) saline (control) and (2) lidocaine. After induction of anesthesia, the lidocaine group received lidocaine as a bolus of 1 mg/kg over 5 minutes, followed by a continuous infusion at 1.5 mg/kg/h until the end of the surgery. We examined the effects of lidocaine treatment on the improvement of cognitive function using the Mini-Mental State Examination (MMSE) at preoperation and 3 days postoperation. Serum samples were collected to assess the levels of IL-6, TNF-α, MDA, S100β, and NSE before inducing anesthesia, at the end of surgery, and 3 days after the end of surgery.
Results
We found that the MMSE scores in the lidocaine group were markedly higher than those in the control group at 3 days after surgery. Moreover, lidocaine treatment markedly suppressed the release of IL-6, S100β, and NSE into the serum at the end of surgery and 3 days after the end of surgery. In the control group, serum MDA levels increased by 3 days after the end of surgery. The lidocaine group had lower serum MDA levels than those in the control group.
Conclusions
Lidocaine may be an effective neuroprotective agent in treating early postoperative cognitive dysfunction in elderly patients undergoing spine surgery.
Keywords: Cognition, Lidocaine, Postoperative Complications
Background
Postoperative cognitive dysfunction (POCD) is a major complication characterized by disordered thinking and impaired consciousness following surgeries and anesthesia, especially in elderly individuals [1,2]. Early studies focused on POCD after cardiac surgery, but a recent study conducted by Evered et al. showed that it was associated with non-cardiac surgery and even with minor non-invasive procedures under sedation, such as coronary angiography [3]. A large multi-center study reported that the incidence of POCD was 25% 1 week after non-cardiac surgery and about 10% 3 months after non-cardiac surgery [4]. Cognitive changes after surgery prolong hospital stay, elevate perioperative medical cost, decrease postoperative quality of life, and increase surgical morbidity and mortality [5].
The underlying mechanisms of POCD are not yet fully understood. Clinical studies have consistently suggested that the main causes of this injury include an exaggerated systemic inflammation [6], endothelial dysfunction [7], cerebral hypoperfusion, and microembolism [8]. Others have specifically reported that POCD is indeed correlated with the serum protein levels of IL-6, TNF-α, S100β, neuron-specific enolase (NSE), and malonaldehyde (MDA) [9–11]. In addition, animal studies have also shown that neuroinflammation and brain cell death after surgery and anesthesia may contribute to the brain functional changes [12–14].
Although many drugs have been studied as neuroprotective during surgery and anesthesia, such as thiopental [15], propofol [15], ulinastatin [11], sevoflurane [9], and morphine [16], there is no agreement on the efficiency of prophylactic neuroprotectants in cardiac or non-cardiac surgery. Lidocaine, an inexpensive, widely available, and relatively safe compound, is a local anesthetic and class IB antiarrhythmic that readily crosses the blood-brain barrier [17]. Evans et al. initially demonstrated cerebral protection of lidocaine in a feline model of cerebral arterial gas embolism [18]. Later clinical studies have also demonstrated the effects of lidocaine on perioperative neuroprotection [19–21]. However, the mechanisms underlying lidocaine treatment-induced neuroprotection remain incompletely understood. Therefore, in the present study, we hypothesized that lidocaine ameliorated early cognitive damage in patients undergoing spine surgery. Also, we determined the effects of lidocaine treatment on the levels of IL-6, TNF-α, S100β, NSE, and MDA in serum.
Material and Methods
The study was approved by the Ethics Committee of Jining No. 1 People’s Hospital and written informed consent was obtained from all patients before the study.
Participants
The study included 87 American Society of Anesthesiologists (ASA) physical status I or II patients aged greater than 65 years scheduled for a spine surgery from September 2013 to February 2015. Seven patients were excluded because of refusal to neuropsychological evaluation after operation. Exclusion criteria included: Mini-Mental State Examination (MMSE) score <23 before surgery; history of neurological diseases (including Alzheimer’s disease and stroke history), psychological disorder, and drug or alcohol abuse; history of diabetes mellitus, severe hypertension, severe anemia, hepatic or renal dysfunction; unwillingness to comply with the protocol or procedures; inability to speak and read Chinese.
Protocol and general anesthesia
Patients were randomly allocated to 2 treatment groups: (1) lidocaine treatment group (n=40), a bolus of 1 mg/kg of lidocaine over 5 minutes administered after induction of anesthesia and followed by a continuous infusion at 1.5 mg/kg/h until the end of the surgery; or (2) control group (n=40), normal saline administered as a bolus and an infusion with the same volume and rate changes as the lidocaine group.
All patients were anesthetized using standard protocols, as follows. Anesthesia was induced with midazolam (0.03~0.05 mg/kg), sufentanil (0.2~0.3 μg/kg), cisatracurium (0.15~0.2 mg/kg) and propofol (2 mg/kg). Anesthesia was maintained by intravenous injection of propofol (4~12 mg/kg/h) and remifentanil (0.1~0.15 μg/kg/min). Depth of anesthesia was maintained to achieve a Bispectral Index Score of 40–60. Electrocardiogram, respiratory rate, pulse oximetry, PETCO2 and hemoglobin oxygen saturation (SpO2) were continuously monitored during surgery.
Serum samples
The blood samples were collected to observe changes in the levels of IL-6, TNF-α, S100β, NSE and MDA before inducing anesthesia (T1), at the end of surgery (T2) and three days after the end of surgery (T3). Blood samples (5 ml) were allowed to clot for 2 h at room temperature and were then centrifuged for 15 min at 2000×g at 4°C. The serum fraction was removed and stored at −80°C for further analysis. The levels of IL-6, TNF-α, S100β and NSE were measured using an Enzyme-linked immunosorbent assay (ELISA) kit (Neobioscience Technology Company, Beijing, China) according to the manufacturer’s protocol. MDA concentrations were determined using enzymatic methods following the manufacturer’s instructions (Jiancheng Biologic Project Company, Nanjing, China). All study personnel were blinded to the results of the laboratory analysis.
Measurement of neurocognitive function
Cognitive function was evaluated pre-operatively and three days post-operatively in a quiet room with only the patient and the experienced psychometrician. Subjects were examined using the MMSE [22]. The psychometrician trained in MMSE completed all data scoring and interpretation. Both patients and the psychometrician were blinded to the treatment and group.
Statistical analysis
All measurement data are presented as the means ±SD. Intergroup numerical data, including IL-6, TNF-α, MDA, S100β and NSE concentrations were analyzed with the Student’s t-test; intragroup numerical data were analyzed with repeated measures ANOVA. Nominal data were analyzed by χ2 test. The differences between the values were considered to be significant at P<0.05. All statistical tests and graphs were performed using Statistical Program for Social Sciences 20.0 software (SPSS, Inc., Chicago, IL, USA).
Results
Eighty-seven patients were enrolled in the study. Seven patients were excluded because of refusal to neuropsychological evaluation after operation, thus leaving eighty patients who completed the blood sample collection and neurocognitive tests. Demographic data of patients were shown in Table 1. There were no significant differences in age, sex, body weight, ASA classification, operation time, and hospital stay between groups (p>0.05).
Table 1.
Group demographics.
| Group control (n=40) | Group lidocaine (n=40) | p-Value | |
|---|---|---|---|
| Gender* (male/female) | 25/15 | 23/17 | 0.65 |
| ASA classification* (I/II) | 18/22 | 16/24 | 0.65 |
| Weight (kg)** | 63.8±4.3 | 64.7±4.3 | 0.37 |
| Age (year)** | 71.8±1.9 | 71.3±2.0 | 0.31 |
| Operation time (min)** | 128.3±7.3 | 129.2±7.4 | 0.61 |
| Hospital stay (day)** | 11.1±0.9 | 10.7±1.2 | 0.15 |
ASA – American Society of Anesthesiologists; p-values are based on * χ2 test; ** the Student’s t-test.
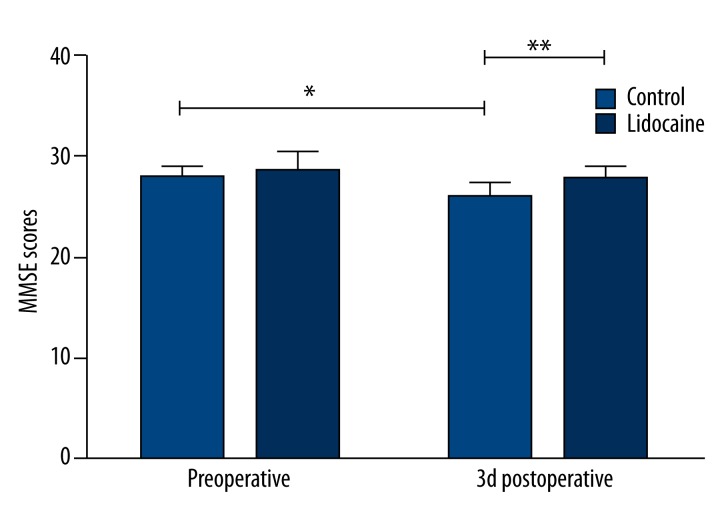
MMSE was performed at preoperation and 3 days postoperation. Compared with the preoperative MMSE scores, those on three days after surgery were significantly decreased in the control group. However, the MMSE scores in the lidocaine group were markedly higher than those in the control group at T3 (Figure 1, p<0.05). The MMSE scores did not differ between groups at T1 (p>0.05).
Figure 1.
MMSE scores in the 80 patients prior to and 3 days after surgery. All data are expressed as the means ±SD. n= 40 per group. * p<0.05 compared with preoperative MMSE scores; ** p<0.05 compared with control group.
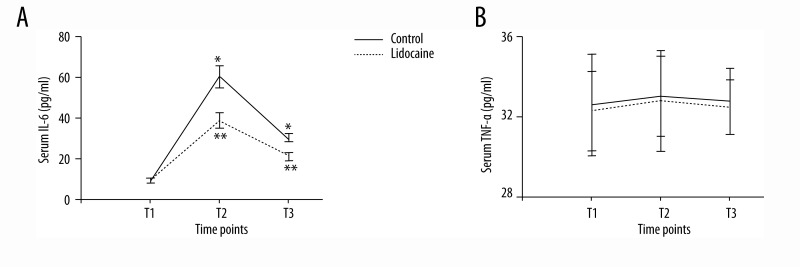
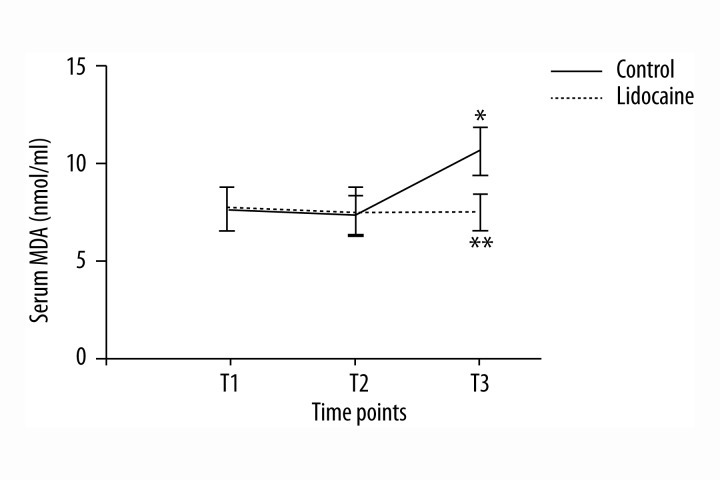
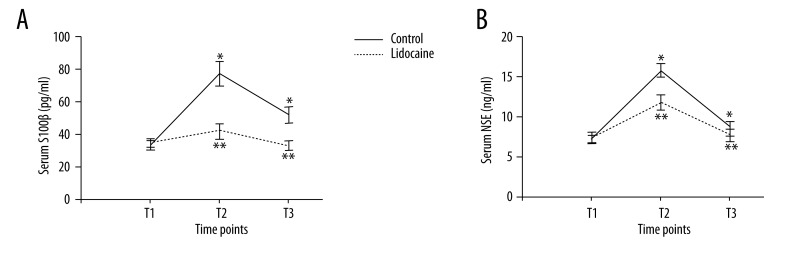
Serum assays were performed at 3 time points: preoperation (T1), at the end of surgery (T2), and 3 days after the end of surgery (T3). In the control group, serum IL-6 concentration increased at T2 and T3. However, the lidocaine group had lower serum IL-6 concentration than that in the control group (Figure 2A, p<0.05). There were no significant differences between groups in TNF-α concentration at 3 time points (Figure 2B, p>0.05). Serum levels of MDA were higher at 3 days after surgery when compared with preoperative levels in the control group. However, the elevated levels were dramatically attenuated by lidocaine treatment (Figure 3, p<0.05). Obviously, no significant differences in the level of MDA were observed between groups at T2 (p>0.05). In the control group, there was a marked increase in the serum concentrations of S100β and NSE at T2 and T3 compared with those at T1. However, lidocaine treatment clearly inhibited the up-regulation of S100β and NSE in serum (Figure 4, p<0.05).
Figure 2.
Serum IL-6 (A) and TNF-α (B) concentrations (pg/ml) at different time points. All data are expressed as the means ±SD. n=40 per group. * p<0.05 compared with preoperative IL-6 or TNF-α levels; ** p<0.05 compared with control group.
Figure 3.
Serum MDA concentrations (nmol/ml) at different time points. All data are expressed as the means ±SD. n=40 per group. * p<0.05 compared with preoperative MDA levels; ** p<0.05 compared with control group.
Figure 4.
Serum S100β (A, pg/ml) and NSE (B, ng/ml) concentrations at different time points. All data are expressed as the means ±SD. n=40 per group. * p<0.05 compared with preoperative S100β or NSE levels; ** p<0.05 compared with control group.
Discussions
The present study revealed that lidocaine can improve cognitive performance in elderly patients undergoing spine surgery. In addition, we reported for the first time that lidocaine can effectively decrease serum levels of IL-6, MDA, S100β, and NSE after non-cardiac surgery. Therefore, a potential mechanism underlying lidocaine treatment-induced neuroprotection may be to inhibit the release of IL-6, MDA, S100β, and NSE. These findings indicate that lidocaine may be a promising therapeutic approach for the treatment of POCD.
Lidocaine confers cerebral protection via many mechanisms, including reducing the cerebral metabolic rate, reducing the ischemic excitotoxin release, and decelerating the ischemic transmembrane ion shift [7]. Additionally, recent evidence has suggested that the neuroprotective effects of lidocaine may be associated with its anti-inflammatory and anti-apoptotic properties [12]. However, previous clinical trials on the effects of lidocaine on POCD are contradictory, with improvements [20] and no effect [8]. This variability may arise from different timing and dose of lidocaine administered and different patient selection [23]. In our study, the infusion protocol was designed to deliver a 1 mg/kg “bolus” over 5 minutes after induction of anesthesia, followed by a continuous infusion at 1.5 mg/kg/h until the end of the surgery. Our results suggested that the MMSE scores of patients in the lidocaine group were markedly higher than those in the control group 3 days after surgery, implying that lidocaine may attenuate cognitive function in patients undergoing spine surgery.
Studies suggest that the magnitude of inflammatory response is a risk factor for cognitive dysfunction after major surgery [6]. Surgical trauma activates the peripheral innate immune system, resulting in the release of inflammatory mediators, which impairs cognitive function [13,24]. Serum IL-6 is a sensitive marker of tissue damage. Xu and colleagues found that serum levels of IL-6 increased after general anesthesia in abdominal surgery and the increase may be associated with the occurrence of POCD [11]. Peng et al. [6] performed a meta-analysis to investigate the relationship between POCD and inflammatory markers. Their results showed that IL-6 might serve an indicator to guide the prevention and treatment of POCD. Serum TNF-α level is also suggested to be a critical mediator of inflammation-induced neuronal dysfunction. It is the first cytokine to be released following surgery and its peripheral blockade could limit the release of neuroinflammation and cognitive impairment in a mouse model of surgery-induced cognitive dysfunction [14]. However, the studies mentioned above did not demonstrate the relationship between POCD and the levels of serum TNF-α. In agreement, our results showed that concentration of serum IL-6 increased at the end of surgery and 3 days after the end of surgery. However, lidocaine may inhibit the release of IL-6. There were no significant differences in TNF-α level between the 2 groups at the 3 time points studied. Our results and those of others indicate that inflammatory response may be responsible for POCD and that lidocaine may prevent or reverse the cognitive deficits by inhibiting the response.
Numerous studies support that oxidative stress precedes the development of neurodegenerative diseases such as POCD, mild cognitive impairment (MCI), Parkinson’s disease, and Alzheimer’s disease (AD) [25,26]. Lipid peroxidation-induced oxidative DNA damage is believed to contribute to neuronal death and neurological dysfunction. MDA, a marker of lipid peroxidation, is diffusible and neurotoxic. Previous studies have provided experimental evidence that increased levels of MDA in peripheral blood are associated with development of AD and MCI [27,28]. Also, recent evidence suggests that elevated MDA levels are a risk factor for cognitive decline in aged patients after orthopedic surgery [25] and total hip-replacement surgery [10]. Therefore, intervention using serum MDA could be a primary prevention strategy for POCD. In the present study we have shown a steady increase in serum MDA levels in the control group but not in the lidocaine group. This suggests that lidocaine suppresses the release of MDA.
S100β and NSE are serum markers of neuronal injury and are increasingly used in diagnosis and prognosis of cognitive impairment after different kinds of surgery [9,29]. S100β, in astrocytes, is a calcium-binding protein that has been regarded as highly brain-specific. Normally, S100β cannot pass the blood-brain barrier to enter the blood stream. However, serum concentrations are increased after damage to central nervous cells as well as blood-brain barrier dysfunction [30]. Studies have confirmed the value of S100β in assessment of cognitive deficits after various surgeries [11,31]. Therefore, S100β protein release is a plausible candidate in assessment of incidence, course, and outcome of POCD. NSE is an isoenzyme of the glycolytic enzyme enolase, usually found in the cytoplasm of neurons and cells of neuro-endocrine differentiation [30]. Experimental findings have suggested that NSE corresponds with the degree of cognitive impairment [9,32]. Our study has demonstrated that both the serum S100β protein and NSE levels significantly increased at the end of the surgery and on postoperative day 3, suggesting that spine surgery may cause damage to brain tissue. Interestingly, S100β protein and NSE levels were reduced after the administration of lidocaine, suggesting the neuroprotective effect of lidocaine.
A limitation of this study is that we did not measure the serum concentration of lidocaine during or after surgery. The therapeutic and adverse effects of lidocaine are generally related to its serum concentration [8]. An accepted therapeutic range is 2 to 5 μg/mL, and detrimental effects, including arrhythmia, tremors, confusion, visual disturbances, impaired concentration, or even seizures and coma, usually occur at levels above 6 to 10 μg/mL [33]. However, we did not find any adverse effects caused by lidocaine in our study, which could be attributed to the relatively low lidocaine dose.
Conclusions
Our results indicate that lidocaine treatment attenuated cognitive impairment in elderly patients undergoing spine surgery. Furthermore, serum IL-6, S100β, NSE, and MDA were involved in the mechanism underlying the therapeutic effect of lidocaine on cognitive function. Therefore, the results indicated that lidocaine may be an effective neuroprotective agent for the treatment of POCD. Further clinical investigation is warranted to determine its potential on the subsequent development and progression of POCD.
Footnotes
Conflict of interest
Authors declare no competing interests exist concerning the content of this document.
Source of support: This work was supported by grants from the Shandong Province Science and Technology Program (Grant No. 2011GSF11801)
References
- 1.Kong F, Chen S, Cheng Y, et al. Minocycline attenuates cognitive impairment induced by isoflurane anesthesia in aged rats. PLoS One. 2013;8:e61385. doi: 10.1371/journal.pone.0061385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu B. Leptin: New hope for the treatment of post-operative cognitive dysfunction? Med Sci Monit. 2014;20:866–68. doi: 10.12659/MSM.890878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Evered L, Scott DA, Silbert B, Maruff P. Postoperative cognitive dysfunction is independent of type of surgery and anesthetic. Anesth Analg. 2011;112:1179–85. doi: 10.1213/ANE.0b013e318215217e. [DOI] [PubMed] [Google Scholar]
- 4.Moller JT, Cluitmans P, Rasmussen LS, et al. Long-term postoperative cognitive dysfunction in the elderly ISPOCD1 study. ISPOCD investigators. International Study of Post-Operative Cognitive Dysfunction. Lancet. 1998;351:857–61. doi: 10.1016/s0140-6736(97)07382-0. [DOI] [PubMed] [Google Scholar]
- 5.Xu T, Bo LL, Wang JF, et al. Risk factors for early postoperative cognitive dysfunction after non-coronary bypass surgery in Chinese population. J Cardiothorac Surg. 2013;8:204. doi: 10.1186/1749-8090-8-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peng L, Xu L, Ouyang W. Role of peripheral inflammatory markers in postoperative cognitive dysfunction (POCD): a meta-analysis. PLoS One. 2013;8:e79624. doi: 10.1371/journal.pone.0079624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Riedel B, Browne K, Silbert B. Cerebral protection: inflammation, endothelial dysfunction, and postoperative cognitive dysfunction. Curr Opin Anaesthesiol. 2014;27:89–97. doi: 10.1097/ACO.0000000000000032. [DOI] [PubMed] [Google Scholar]
- 8.Mathew JP, Mackensen GB, Phillips-Bute B, et al. Randomized, double-blinded, placebo controlled study of neuroprotection with lidocaine in cardiac surgery. Stroke. 2009;40:880–87. doi: 10.1161/STROKEAHA.108.531236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu J-H, Zhang T-Z, Peng X-F, et al. Effects of sevoflurane before cardiopulmonary bypass on cerebral oxygen balance and early postoperative cognitive dysfunction. Neurol Sci. 2013;34:2123–29. doi: 10.1007/s10072-013-1347-3. [DOI] [PubMed] [Google Scholar]
- 10.Ji M-H, Yuan H-M, Zhang G-F, et al. Changes in plasma and cerebrospinal fluid biomarkers in aged patients with early postoperative cognitive dysfunction following total hip-replacement surgery. J Anesth. 2012;27:236–42. doi: 10.1007/s00540-012-1506-3. [DOI] [PubMed] [Google Scholar]
- 11.Lili X, Zhiyong H, Jianjun S. A preliminary study of the effects of ulinastatin on early postoperative cognition function in patients undergoing abdominal surgery. Neurosci Lett. 2013;541:15–19. doi: 10.1016/j.neulet.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 12.Lin D, Cao L, Wang Z, et al. Lidocaine attenuates cognitive impairment after isoflurane anesthesia in old rats. Behav Brain Res. 2012;228:319–27. doi: 10.1016/j.bbr.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Terrando N, Eriksson LI, Kyu Ryu J, et al. Resolving postoperative neuroinflammation and cognitive decline. Ann Neurol. 2011;70:986–95. doi: 10.1002/ana.22664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Terrando N, Monaco C, Ma D, et al. Tumor necrosis factor-triggers a cytokine cascade yielding postoperative cognitive decline. Proc Natl Acad Sci USA. 2010;107:20518–22. doi: 10.1073/pnas.1014557107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bilotta F, Stazi E, Zlotnik A, et al. Neuroprotective effects of intravenous anesthetics: a new critical perspective. Curr Pharm Des. 2014;20:5469–75. doi: 10.2174/1381612820666140325110113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rambhia S, Mantione ML, Stefano GB, Cadet P. Morphine modulation of the ubiquitin-proteasome complex is neuroprotective. Med Sci Monit. 2005;11(11):BR386–96. [PubMed] [Google Scholar]
- 17.Butterworth J, Hammon JW. Lidocaine for neuroprotection: more evidence of efficacy. Anesth Analg. 2002;95:1131–33. doi: 10.1097/00000539-200211000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Evans DE, Kobrine AI, LeGrys DC, Bradley ME. Protective effect of lidocaine in acute cerebral ischemia induced by air embolism. J Neurosurg. 1984;60:257–63. doi: 10.3171/jns.1984.60.2.0257. [DOI] [PubMed] [Google Scholar]
- 19.Mitchell SJ, Pellett O, Gorman DF. Cerebral protection by lidocaine during cardiac operations. Ann Thorac Surg. 1999;67:1117–24. doi: 10.1016/s0003-4975(99)00057-0. [DOI] [PubMed] [Google Scholar]
- 20.Wang DX, Wu XM, Li J, et al. The effect of lidocaine on early postoperative cognitive dysfunction after coronary artery bypass surgery. Anesth Analg. 2002;95:1134–41. doi: 10.1097/00000539-200211000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Caracas HCPM, Maciel JVB, Martins PMReS, et al. The use of lidocaine as an anti-inflammatory substance: A systematic review. J Dent. 2009;37:93–97. doi: 10.1016/j.jdent.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 22.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 23.Xu SY, Pan SY. The failure of animal models of neuroprotection in acute ischemic stroke to translate to clinical efficacy. Med Sci Monit Basic Res. 2013;19:37–45. doi: 10.12659/MSMBR.883750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cao X-Z, Ma H, Wang J-K, et al. Postoperative cognitive deficits and neuroinflammation in the hippocampus triggered by surgical trauma are exacerbated in aged rats. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(8):1426–32. doi: 10.1016/j.pnpbp.2010.07.027. [DOI] [PubMed] [Google Scholar]
- 25.Cheng Q, Wang J, Wu A, et al. Can urinary excretion rate of 8-isoprostrane and malonaldehyde predict postoperative cognitive dysfunction in aging? Neurol Sci. 2013;34:1665–69. doi: 10.1007/s10072-013-1314-z. [DOI] [PubMed] [Google Scholar]
- 26.Riederer PF. Views on neurodegeneration as a basis for neuroprotective strategies. Med Sci Monit. 2004;10(12):RA287–90. [PubMed] [Google Scholar]
- 27.Keller JN, Schmitt FA, Scheff SW, et al. Evidence of increased oxidative damage in subjects with mild cognitive impairment. Neurology. 2005;64:1152–56. doi: 10.1212/01.WNL.0000156156.13641.BA. [DOI] [PubMed] [Google Scholar]
- 28.Schrag M, Mueller C, Zabel M, et al. Oxidative stress in blood in Alzheimer’s disease and mild cognitive impairment: A meta-analysis. Neurobiol Dis. 2013;59:100–10. doi: 10.1016/j.nbd.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 29.Rohan D, Buggy DJ, Crowley S, et al. Increased incidence of postoperative cognitive dysfunction 24 hr after minor surgery in the elderly. Can J Anaesth. 2005;52:137–42. doi: 10.1007/BF03027718. [DOI] [PubMed] [Google Scholar]
- 30.Linstedt U, Meyer O, Kropp P, et al. Serum concentration of S-100 protein in assessment of cognitive dysfunction after general anesthesia in different types of surgery. Acta Anaesthesiol Scand. 2002;46(4):384–89. doi: 10.1034/j.1399-6576.2002.460409.x. [DOI] [PubMed] [Google Scholar]
- 31.Li YC, Xi CH, An YF, et al. Perioperative inflammatory response and protein S-100β concentrations – relationship with post-operative cognitive dysfunction in elderly patients. Acta Anaesth Scand. 2012;56:595–600. doi: 10.1111/j.1399-6576.2011.02616.x. [DOI] [PubMed] [Google Scholar]
- 32.Gonçalves C-A, Concli Leite M, Nardin P. Biological and methodological features of the measurement of S100B, a putative marker of brain injury. Clin Biochem. 2008;41:755–63. doi: 10.1016/j.clinbiochem.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 33.Hsu Y-W, Somma J, Newman MF, Mathew JP. Population pharmacokinetics of lidocaine administered during and after cardiac surgery. J Cardiothorac Vasc Anesth. 2011;25:931–36. doi: 10.1053/j.jvca.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]