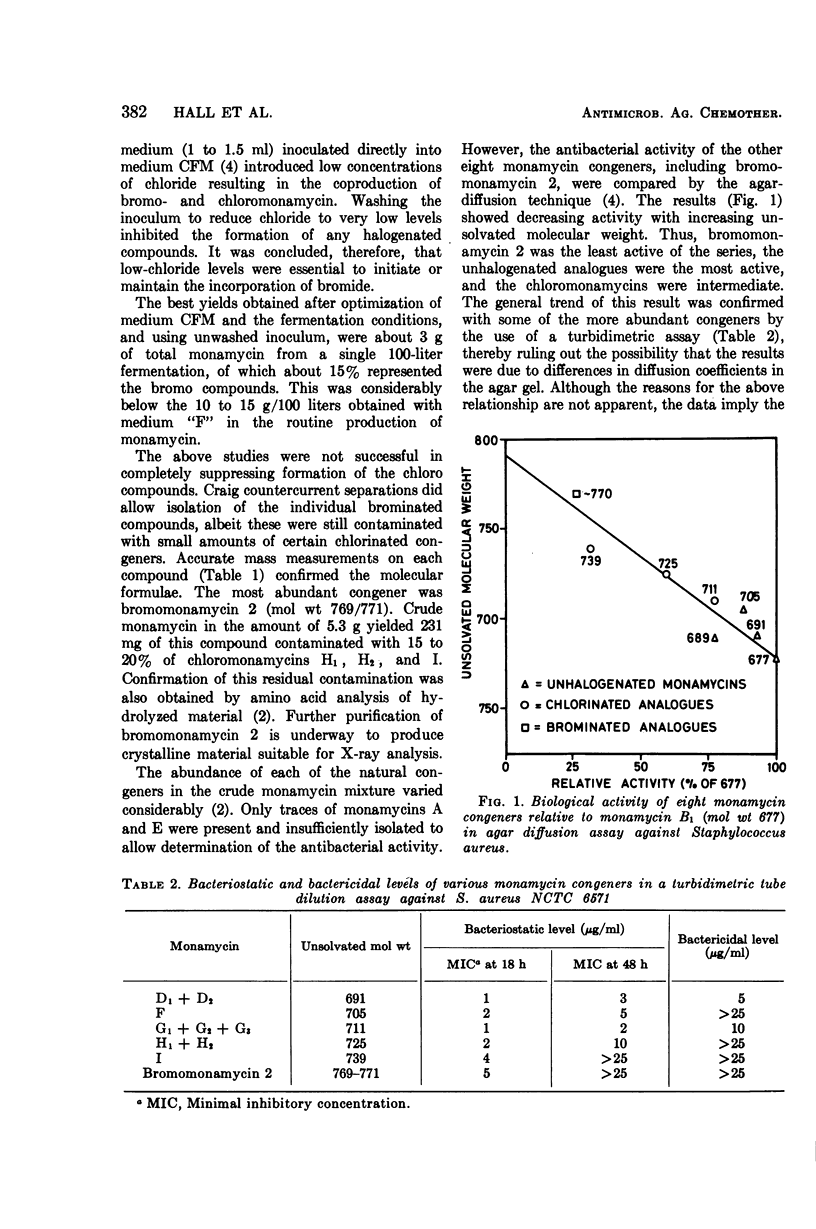
Abstract
Growth of Streptomyces jamaicensis in a low-chloride medium supplemented with NaBr resulted in the biosynthesis of brominated analogues of the natural chlorinated monamycins. Purification of these new compounds was undertaken by Craig countercurrent distribution studies. Diffusion and dilution assays indicated that antibiotic activity of a monamycin was inversely proportional to its unsolvated molecular weight. The bromo analogues were the least active of the series.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bevan K., Davies J. S., Hall M. J., Hassall C. H., Morton R. B., Phillips D. A., Ogihara Y., Thomas W. A. The monamycins, a new family of cyclodepsipeptide antibiotics. Experientia. 1970;26(2):122–123. doi: 10.1007/BF01895528. [DOI] [PubMed] [Google Scholar]
- Hall M. J., Hassall C. H. Production of the monamycins, novel depsipeptide antibiotics. Appl Microbiol. 1970 Jan;19(1):109–112. doi: 10.1128/am.19.1.109-112.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RHODES A., BOOTHROYD B., McGONAGLE P., SOMERFIELD G. A. Biosynthesis of griseofulvin: the methylated benzophenone intermediates. Biochem J. 1961 Oct;81:28–37. doi: 10.1042/bj0810028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH C. G., HINMAN J. W. CHLORAMPHENICOL. Prog Ind Microbiol. 1963;4:137–163. [PubMed] [Google Scholar]


