Abstract
AIM
To investigate the underlying relationship between obesity and the extent of emphysema depicted at CT.
METHODS AND MATERIALS
A dataset of 477 CT examinations was retrospectively collected from a study of chronic obstructive pulmonary disease (COPD). The low attenuation areas (LAAs; ≤950 HU) of the lungs were identified. The extent of emphysema (denoted as %LAA) was defined as the percentage of LAA divided by the lung volume. The association between log-transformed %LAA and body mass index (BMI) adjusted for age, sex, the forced expiratory volume in one second as percent predicted value (FEV1% predicted), and smoking history (pack years) was assessed using multiple linear regression analysis.
RESULTS
After adjusting for age, gender, smoking history, and FEV1% predicted, BMI was negatively associated with severe emphysema in patients with COPD. Specifically, one unit increase in BMI is associated with a 0.93-fold change (95% CI: 0.91–0.96, p < 0.001) in %LAA; the estimated %LAA for males was 1.75 (95% CI: 1.36–2.26, p < 0.001) times that of females; per 10% increase in FEV1% predicated is associated with a 0.72-fold change (95% CI: 0.69–0.76, p < 0.001) in %LAA.
CONCLUSION
Increasing obesity is negatively associated with severity of emphysema independent of gender, age, and smoking history.
Introduction
Obesity is a common, serious, and costly human condition associated with a number of health issues (e.g., pulmonary disease, heart disease, stroke, and diabetes).1–3 Body mass index (BMI) is used to quantify the level of obesity, and it is computed based on an individual’s mass and height.4 Obesity has been associated with numerous respiratory diseases that include asthma,5,6 chronic obstructive pulmonary disease (COPD), which includes individuals with emphysema and chronic bronchitis,7, 8 and sleep apnoea.9 As compared to asthma and sleep apnoea, there has been less information on the relationship between obesity and COPD; however, research on this topic has been increasing.10–12
Whereas respiratory mechanics (e.g., muscle strength) may be compromised with obesity, it is generally believed that obesity could accelerate the decline in lung function and increase the risk of death.13 However, several studies have paradoxically reported that obese patients with COPD have either better health and/or survival as compared with normal or underweight patients.14–25 This controversy, denoted the “obesity paradox”, has been observed in other diseases, including diabetes26 and heart disease.27 Celli et al.28,29 and Schols et al.30,31 independently investigated the effect of body mass on survival in COPD patients and both concluded that body mass could serve as an independent predictor of risk of death from COPD. In 1967, Vandenbergh et al.32 reported that the 5-year survival rate was significantly higher in normal-weight patients (80%) as compared with underweight patients (50%). Sava et al.10 reported that overweight patients had mild airflow obstruction compared to patients with normal weight. These observations, in part, led to the hypothesis that obesity could be a protective mechanism against a decline in airflow obstruction in COPD patients. However, the obesity paradox may represent an oversimplification in that it may ignore the fact that “optimal” weight could be a dynamic factor that changes during a person’s life cycle.33,34 Further investigations are necessary to understand the relationship between obesity and COPD better.
COPD is frequently diagnosed and assessed using pulmonary function testing because of its characteristic in terms of airflow limitation. As a heterogeneous disease, patients with COPD may have emphysema, airway involvement/chronic bronchitis, or both. In this study, CT examinations were used to quantify the presence (or absence) of emphysema, and investigate the association, if any, between obesity and COPD in patients with known COPD. In contrast to a symptom-based diagnosis,35 the utilization of CT could aid in quantifying emphysema thereby facilitating the investigation of the relationship between emphysema and obesity. Therefore, the relationship between obesity and extent of emphysema as depicted on CT images was analysed after controlling for BMI, pulmonary function, age, gender, and smoking history in a COPD screening cohort.
Methods and materials
Study population
The study cohort consisted of 477 participants in an National Institutes of Health (NIH)-sponsored specialized centre for COPD at the University of Pittsburgh. The inclusion criteria for enrolment were age >40 years, current or former smokers with at least a 10 pack-year history of tobacco exposure. The patients completed pre- and post-bronchodilator spirometry and plethysmography, measurement of lung diffusion capacity, a chest CT examination, and demographic and medical history questionnaires. The dataset included 301 participants with COPD as defined by the Global Initiative for Obstructive Lung Disease (GOLD),36 and 176 participants without airflow obstruction (Table 1). The patients represented the first 477 enrolled in the study without any selection criteria. All study procedures were approved by the University of Pittsburgh Institutional Review Board (#0612016). Written informed consent was obtained.
Table 1.
Patient demographics (n = 477).
| Parameter | Mean (±SD) or count (%) |
|---|---|
| Sex male | 265 (55.6%) |
| Age | 64.3 (± 5.3) |
| Pack years | 57.7 (± 32.9) |
| Height (cm) | 169.5 (± 9.4) |
| Weight (kg) | 79.6 (± 16.0) |
| BMI | 27.6 (± 4.4) |
| FEV1% predicted | 76.1 (± 27.3) |
| FEV1/FVC% (%) | 61.4 (± 16.6) |
| GOLD classification | |
| No airflow obstruction | 176 (36.9%) |
| GOLD I | 80 (16.8%) |
| GOLD II | 131 (27.5%) |
| GOLD III | 50 (10.5%) |
| GOLD IV | 40 (8.4%) |
BMI, body mass index; FEV1% predicted, the forced expiratory volume in one second as percent predicted value; FEV1/FVC%, ratio of forced expiratory volume in 1 s (FEV1) to forced vital capacity (FVC); GOLD, Global Initiative for Obstructive Lung Disease.
Acquisition of thin-section CT examinations
CT examinations were acquired using a 64-detector CT system (LightSpeed VCT, GE Healthcare, Waukesha, WI, USA) with patients holding their breath at end inspiration without the use of radiopaque contrast medium. Scans were acquired using a helical technique at the following parameters: 32 × 0.625 mm detector configuration, 0.969 pitch, 120 kVp tube energy, 250 mA tube current, and 0.4 s gantry rotation (or 100 mAs). Images were reconstructed to encompass the entire lung field in a 512 × 512 pixel matrix using the GE “bone” kernel at 0.625 mm section thickness and 0.625 mm interval. Pixel dimensions ranged from 0.549 to 0.738 mm, depending on participant body size. The “bone” kernel was used because of its ability to visualize and quantify both the parenchyma and airways.37
Quantifying the extent of emphysema depicted at CT
Emphysema depicted on CT images was quantified based on the application of a −950 HU threshold to the segmented lung, which is a common threshold used to identify areas of the lung with a low computed attenuation often associated with emphysema.38–40 The extent of emphysema was defined as the percentage of low attenuation areas (%LAA) relative to the total lung volume. To reduce overestimation of the percentage of emphysema possibly caused by image noise or artefact, small clusters of pixels below the −950 HU thresholds were removed. Considering that in-plane image pixel size ranges from approximately 0.55 to 0.74 mm, a relatively small threshold was selected, 3 mm2 (4–5 pixels), for discarding smaller clusters below this threshold. All CT image processing was performed using in-house software.
Statistical analysis
Emphysema severity was quantified in both the entire cohort and the pre-defined subgroups using medians and interquartile ranges (IQR). The distribution of %LAA was compared across the subgroups using the non-parametric Kruskal–Wallis test. Similarly, the Wilcoxon rank-sum test was used to compare the distributions of BMI between COPD and non-COPD patients. To determine whether BMI was independently associated with emphysema severity, a multiple linear regression analysis was conducted with log-transformed %LAA as the dependent variable and BMI, age, gender, FEV1% predicted, and smoking status as independent variables. The log-transformation was conducted due to the skewness of the %LAA distribution. For the regression coefficients 95% Wald-type confidence intervals (CI) were obtained. The results were also presented in terms of fold-changes, by transforming the coefficients back using an exponential function. Statistical significance was defined by p < 0.05 (two sided). The analyses were performed using SAS (SAS Institute, NC, USA), version 9.3.
Results
The median values for %LAA for the overweight (25 ≤ BMI ≤ 30) and obese (BMI > 30) subgroups were 1.8% (IQR: 0.6–6.3%) and 1.4% (IQR: 0.5–3.5%), respectively, which were both significantly lower than in the normal/underweight subgroup (p < 0.001 ; Table 2). Males had a median %LAA that was significantly higher than %LAA in females (p < 0.001). Patients younger than 60 years of age, 60–70 years of age, and older than 70 years of age, had median %LAAof 3.7% (IQR: 0.5–19.9%), 1.7% (IQR: 0.5–6.1%), and 2.7% (IQR: 0.7–5.5%), respectively. BMI distributions were normally distributed in patients with and without airflow obstruction based on GOLD classification (Fig 1). A boxplot in Fig 2 was used to demonstrate the relationship/match between emphysema extent quantified at CT and the clinical measures of COPD by GOLD. In patients with airflow obstruction (COPD), emphysema severity decreased with increasing BMI (Fig 3a, p< 0.001), whereas in patients without airflow obstruction there was no significant association between emphysema and BMI (Fig 3b, p = 0.3). Furthermore, patients in the “obstructed” subgroup had a significantly lower BMI (median 26.6, IQR: 23.6–30) than patients in the non-obstructed group (median BMI 28.9, IQR: 26.1–31.4, p < 0.001).
Table 2.
Radiographic emphysema (%LAA) according to patient characteristics.
| Characteristic | Categories | n | Emphysema extent %
|
p-Value |
|---|---|---|---|---|
| Median (Q1, Q3) | ||||
| Overall | Overall | 477 | 2.3 (0.6,7.8) | NA |
| Age group (years) | < 60 | 82 | 3.7 (0.5,19.9) | 0.04 |
| 60–70 | 267 | 1.7 (0.5,6.1) | ||
| >70 | 128 | 2.7 (0.7,5.5) | ||
| Gender | Male | 265 | 3.0 (1.1,8.6) | <0.001 |
| Female | 212 | 1.2 (0.3,5.8) | ||
| BMI (kg/m2) | <20 | 17 | 23.6 (15.2,34.2) | <0.001 |
| 20 to <25 | 121 | 3.6 (0.7,14.3) | ||
| 25 to <30 | 194 | 1.8 (0.6,6.3) | ||
| ≥30 | 145 | 1.4 (0.5,3.5) | ||
| Smoking (pack-year) | <30 | 64 | 1.3 (0.3,6.1) | 0.06 |
| 30 to 50 | 161 | 2.5 (0.5,8.5) | ||
| 50 to 75 | 137 | 2.2 (0.7,4.4) | ||
| >75 | 115 | 2.8 (0.9,10.2) | ||
| Disease severity | None COPD | 176 | 0.5 (0.2,1.4) | <0.001 |
| GOLD I | 80 | 2.4 (1.1,5.2) | ||
| GOLD II | 131 | 3.5 (1.5,6.1) | ||
| GOLD III | 50 | 15.1 (8.3,28.1) | ||
| GOLD IV | 40 | 29.8 (19.8,33.8) | ||
| FEV1/FVC% | <50 | 113 | 19.2 (10.6,30.3) | <0.001 |
| 50 to <60 | 71 | 3.6 (2.0,6.1) | ||
| 60 to <75 | 179 | 1.4 (0.6,3.3) | ||
| ≥75 | 114 | 0.5 (0.2,1.1) | ||
| FEV1% predicated | <50 | 90 | 22.2 (12.3,31.8) | <0.001 |
| 50 to <60 | 31 | 4.5 (1.7,11.6) | ||
| 60 to <75 | 76 | 3.4 (1.5,6.4) | ||
| ≥75 | 280 | 1.0 (0.3,2.8) |
%LAA, percentage of low attenuation areas; BMI, body mass index; FEV1% predicted, the forced expiratory volume in one second as percent predicted value; FEV1/FVC%, ratio of forced expiratory volume in 1 s (FEV1) to forced vital capacity (FVC); GOLD, Global Initiative for Obstructive Lung Disease.
Figure 1.
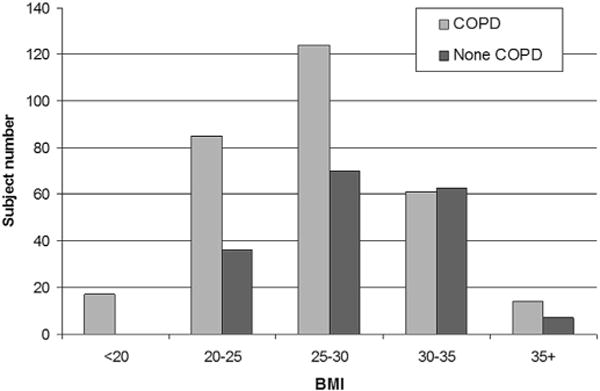
The distribution of BMI across all patients (n = 477).
Figure 2.
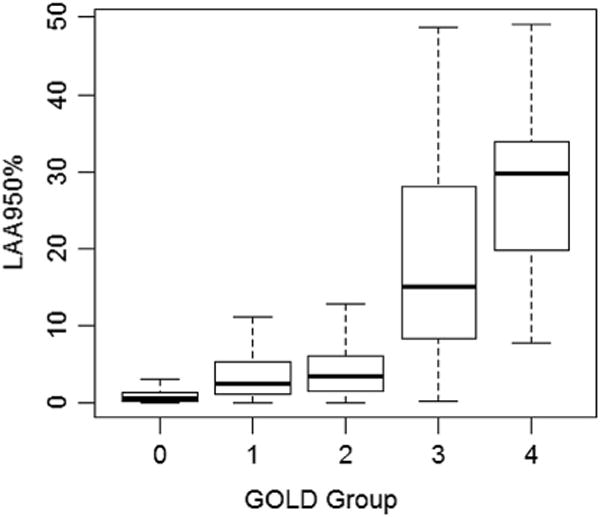
The emphysema extent quantified by CT in terms of GOLD.
Figure 3.
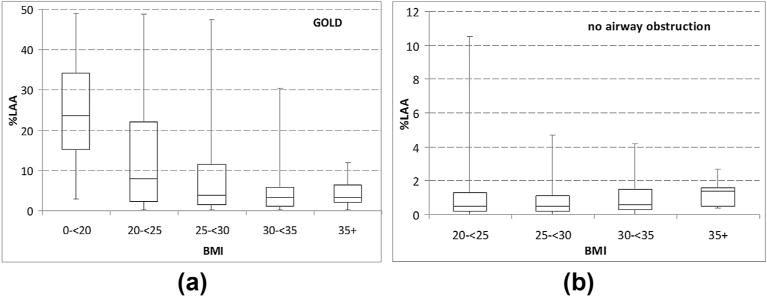
The percent of low attenuation areas (%LAA) stratified by BMI. (a) Patients with airflow obstruction based on GOLD. (b) Patients without airflow obstruction.
In patients with airflow obstruction (COPD), BMI, gender, and severity of obstruction were each independently associated with %LAA (Table 3). A one unit increase in BMI was associated with a 0.93-fold (95% CI: 0.91–0.96, p< 0.001) change in %LAA. The estimated %LAA in males was 1.75 times (95% CI: 1.36–2.26, p = 0.001) that in females. A 10% unit increase in FEV1% predicated was associated with a 0.72-fold (95% CI: 0.69–0.76, p< 0.001) change in %LAA. Age and smoking status were not significantly associated with %LAA in a multivariate analysis. In the non-obstructed group, gender was statistically significantly associated with %LAA (Table 4). The estimated %LAA in males was 2.24 times (95% CI: 1.52–3.3, p < 0.001) that in females. Age, smoking status, FEV1% predicted, and BMI were not statistically significantly associated with %LAA in a multivariate analysis.
Table 3.
Multiple linear regression for airflow obstructed subgroup (n = 301).
| Characteristics | Estimate | Wald | 95% CI: | Fold Change | 95% CI: for fold change | p-Value | ||
|---|---|---|---|---|---|---|---|---|
| Age | [60,70) | 0.02 | −0.32 | 0.35 | 1.02 | 0.73 | 1.42 | 0.9 |
| >70 | −0.01 | −0.38 | 0.37 | 0.99 | 0.68 | 1.45 | 1 | |
| Gender | Male | 0.56 | 0.31 | 0.82 | 1.75 | 1.36 | 2.26 | <0.001 |
| BMI | −0.07 | −0.10 | −0.05 | 0.93 | 0.91 | 0.96 | <0.001 | |
| Pack-year | 0.00 | 0.00 | 0.00 | 1.00 | 1.00 | 1.00 | 0.7 | |
| FEV1% predicted (per 10%) |
−0.32 | −0.37 | −0.27 | 0.72 | 0.69 | 0.76 | <0.001 | |
%LAA, percentage of low attenuation areas; BMI, body mass index; FEV1% predicted, the forced expiratory volume in one second as percent predicted value.
Table 4.
Multiple linear regression for non-airflow-obstructed subgroup (n = 176).
| Characteristics | Estimate | Wald | 95% CI: | Fold Change | 95% CI: for fold change | p-Value | ||
|---|---|---|---|---|---|---|---|---|
| Age | [60,70) | 0.25 | −0.28 | 0.79 | 1.29 | 0.75 | 2.21 | 0.4 |
| ≥70 | 0.44 | −0.22 | 1.09 | 1.54 | 0.80 | 2.99 | 0.2 | |
| Gender | Male | 0.81 | 0.42 | 1.19 | 2.24 | 1.52 | 3.30 | <0.001 |
| BMI | 0.01 | −0.04 | 0.06 | 1.01 | 0.96 | 1.06 | 0.7 | |
| Pack-year | 0.002 | −0.01 | 0.01 | 1.00 | 0.99 | 1.01 | 0.6 | |
| FEV1% predicted (per 10%) |
0.07 | −0.09 | 0.24 | 1.07 | 0.91 | 1.27 | 0.4 | |
%LAA, percentage of low attenuation areas; BMI, body mass index; FEV1% predicted, the forced expiratory volume in one second as percent predicted value.
Discussion
Chronic obstructive pulmonary disease is generally believed to be associated with weight loss. However, obesity is becoming increasingly common in the COPD population.7–12 Approximately 50% of the patients with COPD in the present cohort were either overweightor obese (Fig 1), a distribution that is similar to the results (ranging from 18–54%) reported in previous studies.14 Given the farranging negative effect of obesity on health and the high morbidity and mortality of COPD,41 the prevalence of obesity in individuals with COPD highlights the need for specific interventions aimed at avoiding complex health issues that may be caused by an interaction between COPD and obesity. The present cohort was mostly patients with normal to moderate airflow obstruction (Table 1) and considered to have a normal to obese body type based on BMI (Table 2). Therefore, the present results do not represent patient wasting that may indicate a more aggressive catabolic disease process, which the present cohort was not suited to investigate.
Emphysema severity was quantified using CT examinations and the relationship between emphysema and obesity was studied. In the present study, BMI was negatively associated with emphysema severity after adjusting for age, gender, lung function, and smoking status (p < 0.001). The present results are consistent with the studies of Ogawa et al.25 and Harik-Khan et al.42 in the Baltimore Longitudinal Study of Aging who reported that low BMI was a risk factor for COPD despite the differences in population. However, it cannot be safely concluded that obesity is a protective factor in COPD from the present results or the results of others. Emphysema was quantified using a single-threshold approach; this approach neglects the possible presence of other diseases related to airway obstruction (e.g., chronic bronchitis) which may impact obesity. As Guerra et al.35 discovered, patients with emphysema are more likely to be underweight, whereas patients with chronic bronchitis are more likely to be obese, suggesting that obesity could be a result rather than a cause of other diseases, such as chronic bronchitis. Hence, in order to address the apparent paradox, COPD patients should be stratified into subgroups according to the presence or absence of other diseases and a longitudinal investigation performed of the underlying relationship between obesity and COPD, specifically the impact of obesity on prognosis. In particular, a thorough investigation of airway tree morphology should be undertaken, which may aid in discriminating COPD patients into more specific subgroups and thus enable better understanding of the interaction between obesity and COPD.
Ogawa et al.25 divided COPD patients into four different groups according to emphysema and airway wall measurements based onCT images. Their study involved various metrics, including emphysema, airway wall thickness, serum markers, and subcutaneous fat measures. They found that %LAA (using a threshold of −960 HU) is inversely associated with BMI but no significant relationship between BMI and airway wall measures. When determining these complex relationships one needs to account for other variables such as gender, age, pack-years, and lung function; otherwise, the underlying relationship could be confounded. Also, both males and females were included in the present study population, which was also a milder cohort with two subgroups (i.e., with obstruction/without obstruction). The use of a multivariate analysis enabled the investigation of whether other potentially important variables affected the results. The present results showed that age and smoking status are not significantly associated with %LAA after adjusting for gender, BMI, and FEV1% predicted. Airway data were not included in the analysis, which limits the ability to capture and comment on the chronic bronchitis COPD phenotype in the present cohort. As others did not find a significant relationship between BMI and airway metrics,25 it is unclear whether airway metrics would significantly contribute to BMI in the present cohort. However, this does not necessarily weaken the findings of the present as the overall relationship between BMI and emphysema was comprehensively investigated.
Unlike previous biochemical-based investigations,43,44 the present study quantitatively assessed the impact of tobacco exposure on the development of emphysema based on imaging. Emphysema in men was overall more severe than in women (p <0.001). This finding is consistent with previous studies.45 At the same time, it was also reported that adult females made more emergency department visits for COPD than adult males, and female smokers are more likely to have a reduced lung function as compared with their male counterparts.46 These observations may suggest that airway obstruction may lead to more severe symptoms than emphysema; however, additional longitudinal effort is needed to verify and explain these gender differences in terms of susceptibility of developing COPD. Finally, BMI, a commonly used index of obesity, does not provide an exact quantification of the distribution of fat and lean muscle mass that could affect assessments of the impact of obesity on disease severity. Hence, an imaging-based index of obesity computed from chest CT or dual x-ray absorptiometry may be a better method as it may have a direct impact on respiratory muscle strength and thus on ventilation.47 However, as Ogawa et al.25 reported that the area of subcutaneous fat correlated significantly with BMI, it may prove to be a sufficient index of obesity.
There are some limitations related to the present study. A method of removing clusters of pixels below the emphysema cut-off and smaller than 3 mm2 (4 or 5 pixels) is related to patient size because larger patients will have larger reconstruction FOV (i.e., pixel dimension) compared to smaller patients. However, whether the cluster size is 4 versus 5 pixels because of different pixel dimensions had no effect on emphysema quantification in previous investigations, and it was not expected to affect the current study. A possible limitation is that the CT examinations were reconstructed using a high-spatial frequency kernel, which creates CT images with a low signal-to-noise ratio compared to low-frequency kernels. In an effort to adjust (correct) for the increased image noise, small clusters (3 mm2) below the threshold for emphysema were removed. This correction may or may not have produced results that were close to the actual percentage of emphysema present in patients. Nevertheless, the CT examinations for all patients were performed and analysed under the same CT protocol, and, therefore, the quantitative emphysema metric, on a relative scale, reproducibly ranked patients according to CT emphysema. The same tube current-rotation time product (mAs) was used for all patients. This could result in more photon starvation (i.e., increased image noise) in large patients as compared to small patients, which may incorrectly increase the number of pixels below the emphysema threshold. As patient BMI increased, the % LAA decreased; however, in the present study, the association between BMI and %LAA was simply stronger than the association between photon starvation and emphysema quantification. The cluster-removal approach may have, in part, corrected for photon starvation. The present findings are from a single institution and may or may not be generalizable to other institutions or cohorts.
In conclusion, in patients with COPD, BMI is inversely associated with emphysema and is independent of age, gender, and smoking history. Additionally, emphysema severity in men was higher than that in women. Although a causal relationship between obesity and emphysema cannot be established, the present findings strengthen the understanding of the link between the two in a well-defined, diverse, tobacco-exposed cohort.
Acknowledgments
This work is supported in part by a grant R01 HL096613 from National Institutes of Health, a grant No. 201402013 from National Health and Family Planning Commission of the People’s Republic of China, and a grant No. 2012KTCL0307 from Shaanxi Science and Technology Innovation Program.
References
- 1.Ogden CL, Carroll MD, Kit BK, et al. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311:806–14. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haslam DW, James WP. Obesity. Lancet. 2005;366:1197–209. doi: 10.1016/S0140-6736(05)67483-1. [DOI] [PubMed] [Google Scholar]
- 3.Finkelstein EA, Trogdon JG, Cohen JW, et al. Annual medical spending attributable to obesity: payer- and service-specific estimates. Health Aff (Millwood) 2009;28:w822–31. doi: 10.1377/hlthaff.28.5.w822. [DOI] [PubMed] [Google Scholar]
- 4.Eknoyan G. Adolphe Quetelet (1796–1874)—the average man and indices of obesity. Nephrol Dial Transplant. 2008;23:47–51. doi: 10.1093/ndt/gfm517. [DOI] [PubMed] [Google Scholar]
- 5.Dixon AE. Obesity: changing asthma in the 21st century. Am J Respir Crit Care Med. 2012;186:395–6. doi: 10.1164/rccm.201206-1092ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holguin F, Comhair SA, Hazen SL, et al. An association between L-arginine/asymmetric dimethyl arginine balance, obesity, and the age of asthma onset phenotype. Am J Respir Crit Care Med. 2013;187:153–9. doi: 10.1164/rccm.201207-1270OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Donnell DE, Deesomchok A, Lam YM, et al. Effects of BMI on static lung volumes in patients with airway obstruction. Chest. 2011;140:461–8. doi: 10.1378/chest.10-2582. [DOI] [PubMed] [Google Scholar]
- 8.Leone N, Courbon D, Thomas F, et al. Lung function impairment and metabolic syndrome: the critical role of abdominal obesity. Am J Respir Crit Care Med. 2009;179:509–16. doi: 10.1164/rccm.200807-1195OC. [DOI] [PubMed] [Google Scholar]
- 9.Banerjee D, Leong WB, Arora T, et al. The potential association between obstructive sleep apnea and diabetic retinopathy in severe obesity-the role of hypoxemia. PLoS One. 2013;8:e79521. doi: 10.1371/journal.pone.0079521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sava F, Laviolette L, Bernard S, et al. The impact of obesity on walking and cycling performance and response to pulmonary rehabilitation in COPD. BMC Pulm Med. 2010;10:55. doi: 10.1186/1471-2466-10-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greening NJ, Evans RA, Williams JE, et al. Does body mass index influence the outcomes of a Waking-based pulmonary rehabilitation programme in COPD? Chron Respir Dis. 2012;9:99–106. doi: 10.1177/1479972312439317. [DOI] [PubMed] [Google Scholar]
- 12.Schols AM. Nutritional and metabolic modulation in chronic obstructive pulmonary disease management. Eur Respir J Suppl. 2003;46:81s–6s. doi: 10.1183/09031936.03.00004611. [DOI] [PubMed] [Google Scholar]
- 13.Pistelli F, Bottai M, Carrozzi L, et al. Changes in obesity status and lung function decline in a general population sample. Respir Med. 2008;102:674–80. doi: 10.1016/j.rmed.2007.12.022. [DOI] [PubMed] [Google Scholar]
- 14.Franssen FM, O’Donnell DE, Goossens GH, et al. Obesity and the lung: 5. Obesity and COPD. Thorax. 2008;63:1110–7. doi: 10.1136/thx.2007.086827. [DOI] [PubMed] [Google Scholar]
- 15.Steuten LM, Creutzberg EC, Vrijhoef HJ, et al. COPD as a multicomponent disease: inventory of dyspnoea, underweight, obesity and fat free mass depletion in primary care. Prim Care Respir J. 2006;15:84–91. doi: 10.1016/j.pcrj.2005.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramachandran K, McCusker C, Connors M, et al. The influence of obesity on pulmonary rehabilitation outcomes in patients with COPD. Chron Respir Dis. 2008;5:205–9. doi: 10.1177/1479972308096711. [DOI] [PubMed] [Google Scholar]
- 17.BMI Classification. Global Database on body mass Index. World Health Organization; 2006. www.who.int/bmi/index.jsp [accessed 05.09.2014. [Google Scholar]
- 18.van den Bemt L, van Wayenburg CA, Smeele IJ, et al. Obesity in patients with COPD, an undervalued problem? Thorax. 2009;64:640. doi: 10.1136/thx.2008.111716. author reply 640–1. [DOI] [PubMed] [Google Scholar]
- 19.Fimognari FL, Scarlata S, Pastorelli R, et al. Visceral obesity and different phenotypes of COPD. Am J Respir Crit Care Med. 2009;180:192–3. doi: 10.1164/ajrccm.180.2.192a. author reply 193. [DOI] [PubMed] [Google Scholar]
- 20.Ora J, Laveneziana P, Wadell K, et al. Effect of obesity on respiratory mechanics during rest and exercise in COPD. J Appl Physiol (1985) 2011;111:10–9. doi: 10.1152/japplphysiol.01131.2010. [DOI] [PubMed] [Google Scholar]
- 21.McNamara RJ, McKeough ZJ, McKenzie DK, et al. Obesity in COPD: the effect of water-based exercise. Eur Respir J. 2013;42:1737–9. doi: 10.1183/09031936.00103613. [DOI] [PubMed] [Google Scholar]
- 22.Zutler M, Singer JP, Omachi TA, et al. Relationship of obesity with respiratory symptoms and decreased functional capacity in adults without established COPD. Prim Care Respir J. 2012;21:194–201. doi: 10.4104/pcrj.2012.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Monteiro F, Camillo CA, Vitorasso R, et al. Obesity and physical activity in the daily life of patients with COPD. Lung. 2012;190:403–10. doi: 10.1007/s00408-012-9381-0. [DOI] [PubMed] [Google Scholar]
- 24.Laviolette L, Sava F, O’Donnell DE, et al. Effect of obesity on constant workrate exercise in hyperinflated men with COPD. BMC Pulm Med. 2010;10:33. doi: 10.1186/1471-2466-10-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ogawa E, Nakano Y, Ohara T, et al. Body mass index in male patients with COPD: correlation with low attenuation areas on CT. Thorax. 2009;64:20–5. doi: 10.1136/thx.2008.097543. [DOI] [PubMed] [Google Scholar]
- 26.Carnethon MR, Rasmussen-Torvik LJ, Palaniappan L. The obesity paradox in diabetes. Curr Cardiol Rep. 2014;16:446. doi: 10.1007/s11886-013-0446-3. [DOI] [PubMed] [Google Scholar]
- 27.Lavie CJ, McAuley PA, Church TS, et al. Obesity and cardiovascular diseases: implications regarding fitness, fatness, and severity in the obesity paradox. J Am Coll Cardiol. 2014;63:1345–54. doi: 10.1016/j.jacc.2014.01.022. [DOI] [PubMed] [Google Scholar]
- 28.Celli BR, Cote CG, Marin JM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:1005–12. doi: 10.1056/NEJMoa021322. [DOI] [PubMed] [Google Scholar]
- 29.Celli BR. Predictors of mortality in COPD. Respir Med. 2010;104:773–9. doi: 10.1016/j.rmed.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 30.Schols AM, Slangen J, Volovics L, et al. Weight loss is a reversible factor in the prognosis of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157:1791–7. doi: 10.1164/ajrccm.157.6.9705017. [DOI] [PubMed] [Google Scholar]
- 31.Schols AM, Broekhuizen R, Weling-Scheepers CA, et al. Body composition and mortality in chronic obstructive pulmonary disease. Am J Clin Nutr. 2005;82:53–9. doi: 10.1093/ajcn.82.1.53. [DOI] [PubMed] [Google Scholar]
- 32.Vandenbergh E, Van de Woestijne KP, Gyselen A. Weight changes in the terminal stages of chronic obstructive pulmonary disease. Relation to respiratory function and prognosis. Am Rev Respir Dis. 1967;95:556–66. doi: 10.1164/arrd.1967.95.4.556. [DOI] [PubMed] [Google Scholar]
- 33.Poulain M, Doucet M, Major GC, et al. The effect of obesity on chronic respiratory diseases: pathophysiology and therapeutic strategies. CMAJ. 2006;174:1293–9. doi: 10.1503/cmaj.051299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dixon JB, Egger GJ, Finkelstein EA, et al. “Obesity Paradox” misunderstands the biology of optimal weight throughout the life cycle. Int J Obes (Lond) 2015;39:82–4. doi: 10.1038/ijo.2014.59. [DOI] [PubMed] [Google Scholar]
- 35.Guerra S, Sherrill DL, Bobadilla A, et al. The relation of body mass index to asthma, chronic bronchitis, and emphysema. Chest. 2002;122:1256–63. doi: 10.1378/chest.122.4.1256. [DOI] [PubMed] [Google Scholar]
- 36.Rabe KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176:532–55. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 37.Pauls S, Gulkin D, Feuerlein S, et al. Assessment of COPD severity by computed tomography: correlation with lung functional testing. Clin Imaging. 2010;34:172–8. doi: 10.1016/j.clinimag.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 38.Wang Z, Gu S, Leader JK, et al. Optimal threshold in CT quantification of emphysema. Eur Radiol. 2013;23(4):975–84. doi: 10.1007/s00330-012-2683-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gevenois PA, de Maertelaer V, De Vuyst P, et al. Comparison of computed density and macroscopic morphometry in pulmonary emphysema. Am J Respir Crit Care Med. 1995;152(2):653–7. doi: 10.1164/ajrccm.152.2.7633722. [DOI] [PubMed] [Google Scholar]
- 40.Ju J, Li R, Gu S, et al. Impact of emphysema heterogeneity on pulmonary function. PLoS One. 2014;9(11):e113320. doi: 10.1371/journal.pone.0113320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Franssen FM, Rochester CL. Comorbidities in patients with COPD and pulmonary rehabilitation: do they matter? Eur Respir Rev. 2014;23:131–41. doi: 10.1183/09059180.00007613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harik-Khan RI, Fleg JL, Wise RA. Body mass index and the risk of COPD. Chest. 2002;121:370–6. doi: 10.1378/chest.121.2.370. [DOI] [PubMed] [Google Scholar]
- 43.Alam S, Li Z, Atkinson C, et al. Z α1-antitrypsin confers a proinflammatory phenotype that contributes to chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2014;189:909–31. doi: 10.1164/rccm.201308-1458OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kratzer A, Salys J, Nold-Petry C, et al. Role of IL-18 in second-hand smoke-induced emphysema. Am J Respir Cell Mol Biol. 2013;48:725–32. doi: 10.1165/rcmb.2012-0173OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Martinez FJ, Curtis JL, Sciurba F, et al. National Emphysema Treatment Trial Research Group Sex differences in severe pulmonary emphysema. Am J Respir Crit Care Med. 2007;176:243–52. doi: 10.1164/rccm.200606-828OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.COPD International. COPD statistics. Available at: http://www.copdinternational.com/library/statistics.htm. [accessed 05.09.2014.
- 47.Lazarus R, Sparrow D, Weiss ST. Effects of obesity and fat distribution on ventilatory function: the normative aging study. Chest. 1997;111:891–8. doi: 10.1378/chest.111.4.891. [DOI] [PubMed] [Google Scholar]


