Abstract
Aim
This nested case-control study sought to determine whether an accelerated rate of leukocyte telomere length (LTL) shortening over six years was associated with chronic periodontitis.
Materials and Methods
We sampled cases (n=178) with severe chronic periodontitis and controls (n=178) with no/mild chronic periodontitis from the Atherosclerosis Risk in Communities study. Controls were frequency-matched to cases by study site, age, sex, and race. Age ranged from 53 to 73 years. Severe chronic periodontitis was defined using the CDC-AAP case classification. LTL was measured from DNA collected at two time points, six years apart, with quantitative polymerase chain reaction relative to a single-copy control gene. Multiple linear regression evaluated associations between LTL measured at baseline, follow-up and change scores with severe chronic periodontitis, adjusting for potential confounders.
Results
Cases had shorter LTL than controls at baseline (p=0.03) and follow-up (p=0.04) after adjusting for confounding. Overall there was a net reduction in LTL over time (p=0.02). The rate of LTL did not differ between cases and controls (p=0.80).
Conclusions
LTL shortening occurred at the same rate among adults with and without severe chronic periodontitis. This suggests that LTL shortening may have occurred earlier in the life course.
Keywords: epidemiology, telomere shortening, cell aging, health status disparities, oral health
INTRODUCTION
Chronic periodontitis is an inflammatory polymicrobial disease characterized by episodic progressive destruction of gingivae, periodontal ligament and bone. In 2009–2010, 38.5% of the U.S. population aged 30 years or older had moderate or severe periodontitis (Eke et al., 2012a). Older age, male sex, African American race, and low socioeconomic status are characteristics associated with earlier onset, higher prevalence and greater severity of the disease, whereas psychological stress, alcohol consumption and obesity are risk indicators for periodontitis and cigarette smoking and diabetes mellitus are established causal risk factors (Genco and Borgnakke, 2013). Recent large-scale genetic studies have provided new insights into possible genetic factors and pathways underlying the disease (Divaris et al., 2013, Teumer et al., 2013). Models of disease pathogenesis are congruent in emphasizing the role of oral microbial dysbiotic shifts (Berezow and Darveau, 2011, Hajishengallis, 2014) and deregulated host immune response (Darveau, 2010) which, beyond clinical disease, result in chronic systemic inflammation and oxidative stress (D'Aiuto et al., 2010).
Molecular mechanisms may also shed light onto reasons for heterogeneity in chronic periodontitis prevalence between population groups. Levels of oxidative stress, for example, are higher in African Americans than whites (Morris et al., 2012). Oxidative stress is involved in the pathogenesis of chronic periodontitis and it accelerates cellular aging. Chronic activation of the stress response system—more common in low income and minority racial groups (Geronimus et al., 2006) also accelerates cellular aging (Tomiyama et al., 2012) which in turn mediates immune function deregulation (Montoya-Ortiz, 2013) impairing immune resistance to oral pathogens. Aging itself alters expression of genes in gingival fibroblasts (Domon et al., 2013). Central to each of these mechanistic pathways is the putative effect on of cellular aging.
Leukocyte telomere length (LTL) is a widely used biological marker of cellular age. Telomeres are nucleoprotein caps located at the end of chromosomes that protect them from degradation and fusion (de Lange, 2009). With mitotic DNA replication during cell division the telomere progressively shortens. Attrition also occurs as a consequence of exposure to oxidative stress, secondary to cumulative inflammatory load. Critically short telomeres induce cellular senescence, at which time senescent cells secrete proinflammatory proteins (Rock and Kono, 2008).
Numerous cross-sectional studies report that shorter LTL is associated with a variety chronic inflammatory disorders, including chronic periodontitis (Masi et al., 2011). To date no study has examined the association of longitudinal changes in LTL and chronic periodontitis. To address this gap, we investigated longitudinal LTL changes and chronic periodontitis, hypothesizing that severe periodontitis would be associated with increased LTL attrition.
MATERIALS & METHODS
Study Design and Setting
This nested case-control study is ancillary to the biracial Atherosclerosis Risk in Communities (ARIC) study. ARIC is a prospective investigation of the etiology of atherosclerosis, its natural history and its relationship with cardiovascular risk factors. Between 1987 and 1989, ARIC recruited 15,795 men and women aged 45–64 years from four U.S. communities: Forsyth County, North Carolina; Jackson, Mississippi; suburban Minneapolis, Minnesota; and Washington County, Maryland. Interviews and clinical examinations collected information at Visit 1, and three follow-up visits (Visits 2 to 4) conducted at 3-year intervals.
Periodontal Status Case Definitions
In 1996–1998, the Dental ARIC ancillary study evaluated periodontal health in 6,691 dentate adults of the 11,656 cohort members who participated in Visit 4. Four trained and calibrated dental examiners measured probing pocket depth (PD) and gingival recession on six sites for all teeth to calculate clinical attachment loss (CAL). In this analysis, controls were participants with mild or no periodontitis according to the case definition developed jointly by Centers for Disease Control and Prevention and American Academy of Periodontology (CDC/AAP)(Eke et al., 2012b) . Cases had severe CP defined as presence of ≥4 interproximal sites with ≥6 mm CAL (not on the same tooth) and ≥2 interproximal sites pocket depth ≥ 5 mm. This case definition is more stringent than that of the CDC/AAP by doubling the number of sites, without changing the millimeter thresholds. The rationale for a more stringent definition was that this cohort, aged 54-73 years old at the time of diagnosis, had a much greater percentage of people who met the CDC/AAP classification for severe periodontitis (17%) than the U.S. adult population where prevalence based on the CDC/AAP classification in NHANES III was 1.8% (Eke et al., 2010).
Sampling Design
Sampling of Dental ARIC participants for this study was stratified by race, selecting equal numbers of cases and controls within each racial group: all participants in the Jackson study site were African American and participants in the other field centers were white. To maximize statistical efficiency and control for potential confounding, cases and controls were frequency matched by sex, 5-year age group and field center to yield 356 participants. A sample size requirement of 160 cases and 160 controls was calculated based on a magnitude of difference in relative LTL for periodontitis cases (mean = 1.12) and controls (mean = 1.23) with a pooled standard deviation of 0.35, (Masi et al., 2011) assuming 80% power and a two-tailed type I error of 5%.
DNA Isolation and Extraction
At ARIC Visits 1–4, fasting blood was drawn and stored under standardized conditions. DNA from Visit 1 was extracted using phenol chloroform and from Visits 2, 3, and 4 was extracted using the QIAGEN Gentra Puregene Kit. Because the use of two different methods of DNA isolation was a potential threat to the validity of LTL measurement, this analysis considered LTL attrition over a 6-year period between ARIC visits 2 (“baseline”) and 4 (“follow-up) where only Puregene extraction was used. DNA obtained at ARIC visits 2 and 4, 6 years apart, was used to measure LTL.
Telomere Length Measurement
DNA was shipped to the Cytometry and Telomere Center, Department of Pathology, University of Washington where telomere length was measured by quantitative polymerase chain reaction (qPCR) using the method described by Cawthon (Cawthon, 2002). For each sample, two PCRs were performed: the first one to amplify the telomeric DNA and the second one to amplify a single-copy control gene (36B4, acidic ribosomal phosphoprotein P0). This provided an internal control to normalize the starting amount of DNA. A four-point standard curve (2-fold serial dilutions from 5 to 0.625ng of DNA) was included in all PCRs to allow the transformation of Ct (cycle threshold) into nanograms of DNA. All PCR reactions were performed in a Rotor Gene Q (in 100-well discs). All samples were run in triplicate and the median was used for subsequent calculations. The amount of telomeric DNA was divided by the amount of control-gene DNA, producing a relative measurement of the telomere length of the sample. Two control samples were run in each experiment to allow for normalization between experiments and periodical reproducibility experiments were performed. The median inter-assay variability was 5%, consistent with published data (Martin-Ruiz, 2014).
Covariates
Covariates included the selection and matching characteristics (age, sex, race, and ARIC study center), socioeconomic characteristics (education and income), behaviors (smoking and alcohol consumption), body mass index (BMI), comorbidities [hypertension, diabetes mellitus (DM)], as well as the number of remaining natural teeth excluding third molars. Socioeconomic status was determined by education (assessed at Visit 1) and income (Visit 4). Education was classified as basic (<12 years) intermediate (12-16 years) or advanced (17-21 years). Income was classified as a 7-level ordinal variable. Participants were classified as never, former, or current smokers and alcohol drinkers based on self-report at Visit 2. Additional covariates measured at Visit 2 included body mass index (BMI), diabetes mellitus, and hypertension. Weight was measured with participants wearing underwear and examination suit. Both weight and standing height were recorded to the nearest 0.5 cm, rounding down. BMI was calculated using the formula: BMI = (Weight (lbs)/2.20 / Height (cm)/100)2 and categorized as normal (<25.0), overweight (25.0-29.9), and obese (≥30.0). Participants were asked to fast for 12 hours before the clinic visit. Serum glucose was assessed by the hexokinase method. Prevalent diabetes mellitus was a derived variable defined as a fasting glucose of ≥126 mg/dL, nonfasting glucose of ≥200 mg/dL, a self-reported history of diabetes, or treatment for diabetes. Systolic and diastolic blood pressure were measured three times using a random zero sphygmomanometer in the right arm of seated participants. The mean of the last two measurements were used. Hypertension was considered present if: systolic blood pressure was ≥140 mmHg; if diastolic blood pressure was ≥ 90 mmHg; or if antihypertensive medications were being used.” Number of remaining natural teeth (excluding third molars, 0-28) was determined at the Visit 4 dental examination. To conduct additional exploratory analyses we considered the following biomarkers that were available at Visit 4 for a subset of ARIC participants: gingival crevicular fluid interleukin 1B (GCF-IL1B), GCF-prostaglandin (PG) E2, and serum C-reactive protein (CRP).
Analytical strategy
Descriptive methods were used for initial data presentation. The distribution of covariates across strata of sex and race was examined using X2 tests of equivalence for categorical variables and analysis of variance for continuous ones. Descriptive tabular and graphical methods were used to summarize LTL characteristics in visits 2 and 4, as well as changes in LTL during the 6-year study period. Change in LTL between the 2 visits was tested using a paired t test. For subsequent analyses, LTL values were normalized using age and sex-specific Z-scores. To examine the association between CP and LTL, we employed multivariate regression modeling adjusting for study design characteristics and potential confounders. Multivariate models were constructed to examine contemporaneous associations between CP and sex- and race-standardized LTL scores in visits 2 and 4, as well as changes in LTL. Age, sex, race, and their interactions with CP were included a priori in all models, while the inclusion of additional covariates was determined by a 10% change-in-CP beta coefficient criterion. We used a conventional p<0.05 statistical significance threshold and present p values rounded to one significant digit. Because a “regression to the mean” bias has been reported in previous studies of LTL change, we conducted additional analyses using a correction equation recently proposed by Verhulst (Verhulst et al., 2013). Further, we explored the inclusion of biomarker variables in the final models, acknowledging the limitation of substantial data missingness in some strata. Finally, to enable comparisons with previous studies in the field, we computed Spearman's rank correlation coefficients for cross-sectional LTL associations with study covariates, and Pearson's correlation coefficients between ARIC visit 2, visit 4, and LTL-change measurements. All analyses were conducted with Stata 13.1 (StataCorp LP, College Station, TX) statistical software.
RESULTS
The sampling design ensured that cases and controls were balanced with respect to sex, race, and age. The descriptive characteristics of the study sample in ARIC visit 4 are presented in Table 1 and Table 1 in the online supplement. Overall, the mean age of participants was 63 years (range: 53-73), approximately 20% were current smokers, 39% were obese, 17% had diabetes mellitus, and 36% had hypertension. The mean number of teeth excluding third molars was 23 (range: 2-28). As expected, a greater proportion of chronic periodontitis cases reported smoking, lower income, and few remaining teeth. Whites had higher educational attainment and higher income, lower BMI, lower prevalence of DM and hypertension, and retained more teeth than African Americans. Whites had also lower LTL compared with African Americans, as had men compared to women (Table 2). Apart from race, age, and sex, bivariate associations of LTL were also found with alcohol consumption and CRP in the entire sample, as well as education, income, BMI, DM, hypertension, and GCF-PGE2 in certain race-sex strata (Table 2 in online supplement).
Table 1.
Sociodemographic, behavioral, anthropometric, clinical, and biomarker characteristics of the analytical sample of 356 Dental Atherosclerosis Risk In Communities participants, stratified by chronic periodontitis case status.
| Cases | Controls | P † | |||
|---|---|---|---|---|---|
| n | %* | n | %* | ||
| Entire sample | 178 | 100 | 178 | 100 | |
| Race | 0.8 | ||||
| African American | 86 | 48 | 83 | 47 | |
| White | 92 | 52 | 95 | 53 | |
| Sex | 1.0 | ||||
| Female | 91 | 51 | 91 | 51 | |
| Male | 87 | 49 | 87 | 49 | |
| Education | 0.002 | ||||
| Basic | 47 | 26 | 24 | 13 | |
| Intermediate | 72 | 40 | 69 | 39 | |
| Advanced | 59 | 33 | 85 | 48 | |
| Income | 0.004 | ||||
| < $12,000 | 32 | 19 | 13 | 8 | |
| $12,000 - $15,999 | 23 | 13 | 9 | 5 | |
| $16,000 - $24,999 | 25 | 15 | 30 | 18 | |
| $25,000 - $34,999 | 25 | 15 | 34 | 20 | |
| $35,000 - $49,999 | 25 | 15 | 25 | 15 | |
| $50,000 - $74,999 | 23 | 14 | 35 | 21 | |
| ≥ $75,000 | 18 | 11 | 23 | 14 | |
| Smoking status | <0.0005 | ||||
| Never | 69 | 39 | 86 | 48 | |
| Former | 55 | 31 | 77 | 43 | |
| Current | 52 | 30 | 15 | 8 | |
| Drinking status | 0.7 | ||||
| Never | 35 | 20 | 41 | 23 | |
| Former | 39 | 22 | 36 | 20 | |
| Current | 102 | 58 | 101 | 57 | |
| BMI category‡ | 0.6 | ||||
| Normal (<25.0) | 34 | 20 | 31 | 18 | |
| Overweight (25-29.9) | 68 | 39 | 78 | 45 | |
| Obese (≥30.0) | 71 | 41 | 66 | 38 | |
| Diabetes mellitus | 0.9 | ||||
| No | 113 | 64 | 115 | 65 | |
| Yes | 64 | 36 | 63 | 35 | |
| Hypertension§ | 0.9 | ||||
| No | 113 | 64 | 115 | 65 | |
| Yes | 64 | 36 | 63 | 35 | |
| Natural teeth◇ (n) | 0.02 | ||||
| 26-28 | 42 | 24 | 63 | 35 | |
| 19-25 | 73 | 41 | 52 | 29 | |
| 2-18 | 63 | 35 | 63 | 35 | |
| Mean | SD | Mean | SD | ||
| Age (years; mean, SD) | 62.6 | 5.5 | 62.4 | 5.4 | 0.7 |
| LTL∥ in visit 4 (length, SD) | 1.05 | 0.17 | 1.06 | 0.17 | 0.6 |
| LTL∥ in visit 2 (length, SD) | 1.07 | 0.18 | 1.08 | 0.18 | 0.6 |
| LTL∥ change (SD) | −0.02 | 0.14 | −0.02 | 0.14 | 1.0 |
Column percentages;
Corresponding to X2 tests of equivalence for categorical variables and t test for age and LTL;
One participant had BMI in the underweight category;
Diabetes mellitus was defined as was defined as fasting plasma glucose ≥ 126 mg/dL, non-fasting plasma glucose ≥ 200 mg/dL, self-reported-history of physician-diagnosed diabetes, or current medication for diabetes;
Hypertension was defined as a previous diagnosis of hypertension, taking hypertensive medication, or having a current systolic blood pressure ≥140 mmHg or a diastolic blood pressure ≥ 90 mmHg;
Excluding 3rd molars;
Relative leukocyte telomere length was determined according to quantitative polymerase chain reaction
Table 2.
Leucocyte telomere length* (LTL) distribution of the analytical sample of 356 Dental Atherosclerosis Risk In Communities participants, overall and stratified by race and sex.
| African American | White | |||||
|---|---|---|---|---|---|---|
| Overall Mean (SD) | Women Mean (SD) | Men Mean (SD) | Women Mean (SD) | Men Mean (SD) | P † | |
| LTL at visit 2 | 1.08 (0.18) | 1.13 (0.18) | 1.07 (0.19) | 1.07 (0.16) | 1.03 (0.17) | 0.006 |
| missing, n | 6 | 2 | 1 | 1 | 2 | |
| LTL at visit 4 | 1.06 (0.17) | 1.10 (0.15) | 1.10 (0.19) | 1.03 (0.16) | 1.01 (0.16) | 0.0001 |
| missing, n | 0 | 0 | 0 | 0 | 0 | |
| LTL change (visits 2-4) | −0.02 (0.14) | −0.03 (0.15) | 0.03 (0.17) | −0.04 (0.13) | −0.03 (0.11) | 0.008 |
| missing, n | 6 | 2 | 1 | 1 | 2 | |
| LTL change P‡ | 0.02 | 0.07 | 0.1 | 0.005 | 0.03 | |
Measured using quantitative-polymerase chain reaction relative to a single-copy control gene, 36B4 (acidic ribosomal phosphoprotein P0);
Corresponding to analysis of variance
corresponding to paired t tests for LTL change between visits 2 and 4
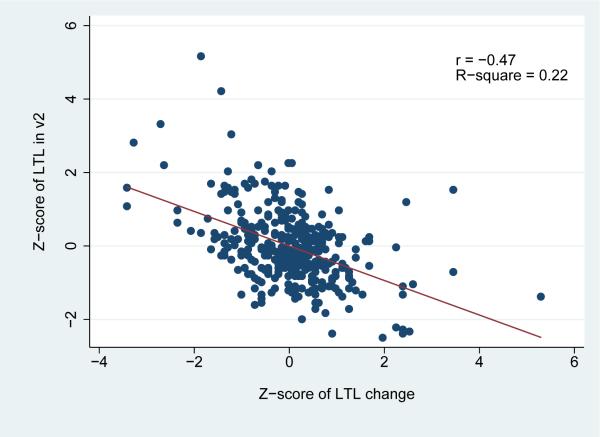
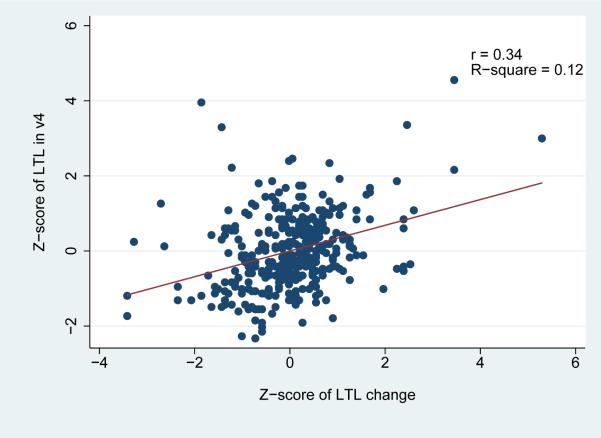
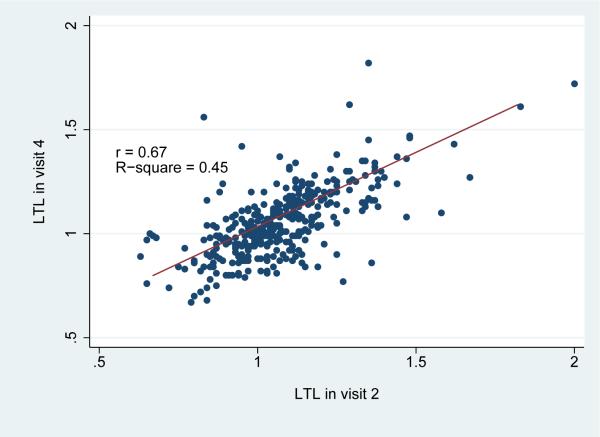
Over the 6-year study period, a statistically significant net decrease in LTL was observed in the study population overall [LTL change=-0.02; standard deviation (SD)=0.14; paired t test p=0.02] (Table 2). Telomere attrition was more pronounced among white participants, whereas a non-significant increase [(LTL change=0.03 (SD=0.17)] was noted among African American males. Changes in LTL were correlated with both visit 2 (r=-0.47) and visit 4 (r=0.34) LTL (Figure 2). As illustrated in Figure 3, LTL in visits 2 and in visit 4 was highly inter-correlated (r=0.67). Notably, when the LTL change scores were corrected for the “regression to the mean” effect, the effective true change observed during the 6-year study period was calculated to be virtually zero.
Figure 2.
Correlation of Leucocyte Telomere Length (LTL) Change by LTL at ARIC visit 2 (a) and visit 4 (b) among the 356 participants of the Atherosclerosis Risk in Communities Study, illustrated with scatter plots.
Figure 3.
Correlation of Leucocyte Telomere Length (LTL) in visits 2 and 4 among the 356 participants of the Atherosclerosis Risk in Communities Study, illustrated with a scatter plot.
Table 4 presents the results of the final multivariate regression modeling results for visit 2, visit 4, and change LTL scores. In addition to sex, race, and age, the final model specification included terms for income, smoking, BMI category, and number of retained natural teeth. We found no association between LTL attrition over the 6-year period and chronic periodontitis risk using either the crude (b=0.05; p=0.8) or corrected LTL change (b=−0.004; p=0.8). However, our findings provide support for an association between a contemporaneous LTL and chronic periodontitis, with shorter telomeres associated with increased risk for chronic periodontitis: visit 4—b=−0.43, p=0.04; visit 2—b=−0.44, p=0.03. Further exploratory adjustment for inflammatory markers (GCF-IL1B and CRP) did not result in any substantial changes (<10%) in the estimates of association between LTL and periodontitis.
Table 4.
Multiple linear regression modeling* results of leucocyte telomere length† (LTL) at visits 2 and 4 and change between visits 2 and 4‡ among the 356 Dental Atherosclerosis Risk in Communities participants.
| Leukocyte telomere length |
||||||
|---|---|---|---|---|---|---|
| Model: Visit 2 | Model 2: Visit 4 | Model 3: Change between visits 2 and 4 | ||||
| b (95% CI) | P | b (95% CI) | P | b (95% CI) | P | |
| Chronic periodontitis | ||||||
| None/mild | referent | referent | referent | |||
| Severe | −0.44 (−0.85, −0.04) | 0.03 | −0.43 (−0.83, −0.02) | 0.04 | 0.05 (−0.37, 0.47) | 0.8 |
| Number of teeth | −0.01 (−0.02, 0.01) | 0.5 | 0.01 (−0.01, 0.02) | 0.4 | 0.02 (−0.00, 0.32) | 0.08 |
| Sex | ||||||
| Female | referent | referent | referent | |||
| Male | −0.78 (−1.22, −0.34) | 0.001 | −0.62 (−1.05, −0.18) | 0.005 | 0.27 (−0.18, 0.72) | 0.2 |
| Race | ||||||
| African American | 0.27 (−0.23, 0.76) | 0.3 | 0.36 (−0.13, 0.85) | 0.1 | 0.12 (−0.39, 0.64) | 0.6 |
| White | referent | referent | referent | |||
| Smoking | −0.04 (−0.19, 0.11) | 0.6 | 0.01 (−0.14, 0.16) | 0.7 | 0.08 (−0.08, 0.23) | 0.3 |
| Age | −0.02 (−0.04, −0.00) | 0.1 | −0.02 (−0.04, −0.00) | 0.03 | −0.00 (−0.03, 0.02) | 0.7 |
| BMI category | 0.10 (−0.04, 0.25) | 0.2 | 0.06 (−0.09, 0.20) | 0.5 | −0.06 (−0.22, 0.09) | 0.4 |
| Income | ||||||
| < $12,000 | referent | referent | referent | |||
| $12,000 - $15,999 | −0.01 (−0.48, 0.45) | 0.9 | 0.01 (−0.44, 0.46) | 0.9 | 0.06 (−0.42, 0.54) | 0.8 |
| $16,000 - $24,999 | −0.30 (−0.72, 0.12) | 0.2 | −0.38 (−0.79, 0.03) | 0.07 | −0.08 (−0.51, 0.36) | 0.7 |
| $25,000 - $34,999 | 0.11 (−0.32, 0.54) | 0.6 | −0.08 (−0.50, 0.34) | 0.7 | −0.21 (−0.65, 0.23) | 0.3 |
| $35,000 - $49,999 | 0.39 (−0.07, 0.84) | 0.09 | 0.03 (−0.42, 0.47) | 0.9 | −0.44 (−0.91, 0.03) | 0.07 |
| $50,000 - $74,999 | 0.42 (−0.04, 0.87) | 0.07 | −0.02 (−0.46, 0.43) | 0.9 | −0.53 (−0.99, −0.06) | 0.03 |
| ≥ $75,000 | 0.51 (−0.02, 1.04) | 0.06 | 0.15 (−0.37, 0.67) | 0.6 | −0.43 (−0.98, 0.12) | 0.1 |
The multivariate linear regression models included terms for ARIC study examination center and a three-way interaction for the selection and matching variables (chronic periodontitis diagnosis*race*sex) the coefficients of which are suppressed in this table
LTL was measured using quantitative-polymerase chain reaction relative to a single-copy control gene, 36B4 (acidic ribosomal phosphoprotein P0)
LTL and change scores were normalized via Z-score transformation prior to analysis
DISCUSSION
This study found no evidence of greater LTL attrition among chronic periodontitis cases compared to controls over six years, either with or without adjustment for baseline LTL. Neither was LTL attrition greater among African Americans than whites. In fact, counter to a notion of accelerated cell aging in disadvantaged social status, LTL attrition was apparent for whites but not for African Americans. Furthermore greater LTL attrition was observed in high-income relative to low-income participants. Nonetheless, the overall net reduction in LTL over six years in both cases and controls was in the expected direction. Consistent with a review of previous studies (Sanders and Newman, 2013), LTL was shorter in men than in women and shorter in whites than African Americans. The observation of shorter LTL in cases than in controls at both visits concurs with the cross-sectional findings of shorter LTL in association with chronic periodontitis (Masi et al., 2011, Masi et al., 2014). Our findings build on these earlier studies by measuring LTL in the same subjects at two points in time. Compared to the strength of associations reported earlier by these authors, correlation analysis of pairwise associations between clinical parameters and LTL revealed only weak relationships. However the strength of association between age and LTL in our study (rho=-0.183) was similar to that reported by Masi et al. (r-0.2) (Masi et al., 2011). Differences in study design might account for differences in the strength of associations between LTL and clinical parameters between both studies. For instance, our study was restricted to adults with severe periodontitis. The rationale was to maximize the phenotypic separation between cases and controls and thereby optimize statistical power to detect differences. More pragmatically, moderate periodontitis was the norm in this middle-aged to elderly population. Yet our decision to select the severe case classification may have limited the potential to find a significant effect if one exists. Unlike moderate chronic periodontitis that increases in prevalence in the U.S. adult population from 10% at 30 years of age to 70% at 75 years, on average, prevalence of severe periodontitis is relatively stable, with estimates less than 15% across all age groups (Eke et al., 2012a) possibly due to tooth loss as a consequence of the disease.
One explanation for the null association between LTL change over six years and periodontitis in the presence of significant contemporaneous, i.e. cross-sectional, associations of LTL with chronic periodontitis is that much of the reduction in LTL has already occurred, earlier in life (Aviv et al., 2011). At the time of the ARIC study periodontal examination, participants were aged from 53 to 73 years. National NHANES data show that prevalence estimates of severe periodontitis between 30 and 49 years of age increases 10-fold before reaching a plateau that is constant across older age groups. This suggests a time of rapid change in the natural history of periodontitis in early midlife that predates our study. Accordingly, the reduction of LTL may have occurred in the early stages of the disease or alternatively, individuals with short telomeres were more likely to develop chronic periodontitis.
An unexpected finding was of a dose-response increase in LTL shortening across successive categories of higher household income. It is possible that exposures associated with higher income confer protection against LTL attrition earlier in the life course, and that now in middle age, more affluent adults were losing that advantage. This “postponement” of LTL attrition might also explain why we saw greater LTL attrition in whites than African Americans. Interestingly, LTL shortening has been associated with social deprivation in early childhood (Drury et al., 2012) and recent experimental data indicate that telomere in early life may predict life expectancy (Heidinger et al., 2012).
The association between contemporaneous LTL and periodontitis in both study visits persisted after adjustment for markers of inflammation, including GCF-IL1B and CRP. This observation was based on a small subset of approximately 210 study participants, and should be regarded with caution. Although inflammatory load has been shown to be associated with short LTL (O'Donovan et al., 2011) our findings indicate that the observed association with periodontitis was not explained by these inflammatory markers and other pathways may be in play. For example, oxidative stress is associated with both shorter telomeres (Demissie et al., 2006, Houben et al., 2008) and chronic periodontitis (Bullon et al., 2014) and is likely important in both conditions.
Recent evidence indicates that LTL dynamics are complex, with patterns of change being more consistent with a constant oscillation rather than a monotonic linear decrease (Svenson et al., 2011). Since LTL shows large inter-individual variability, cross-sectional investigations with a single LTL measurement are less informative. Our study has a major advantage over those cross-sectional designs with its measurement of change in LTL over six years. Another design strength was the frequency-matching of cases and controls on age, sex and race characteristics within ARIC study field centers. This causes cases and controls to have balanced distributions across confounding variables, providing greater efficiency for stratified analysis of race and sex while simultaneously reducing a source of confounding bias. Other strengths were the full mouth periodontal examination protocol which results in less bias than partial mouth protocols and the conservative case classification.
Recognizing that our study used a different quantification method than Masi et al., (2011), we also calculated power post hoc using our study's observed mean (1.07) and standard deviation (0.18) of LTL at baseline, and applying type I error protection of P<0.05. The results showed power >0.99 to detect a difference in mean LTL of 9%, which is the percentage difference in mean LTL between periodontal cases and controls reported by Masi et al.”
We draw attention to several limitations in this study. Firstly our failure to detect differential amounts of LTL attrition between cases and controls over six years may reflect inadequate statistical power. To investigate this possibility, we conducted a post-hoc power calculation for LTL attrition assuming our observed pooled standard deviation of 0.14 and a conventional alpha of 0.05. For this sample size of 356 subjects, there was 77% power to detect a minimum of difference in LTL attrition of −0.04 between cases and controls: e.g. reduction in LTL of −0.01 in controls versus reduction of −0.05 in cases. Therefore the study was adequately powered to detect change of this magnitude. However we did not observe this magnitude of differential LTL attrition. In fact, cases and controls did not vary at all in their amount of LTL attrition. It is possible that six years of follow-up may be insufficient to detect differential LTL attrition giving the high population variance in LTL and the slow rate of LTL with age. On the other hand, a recent study of 611 breast cancer survivors found that greater telomere shortening over a follow-up period as short as 24 months predicted breast cancer–specific and all-cause mortality (Duggan et al., 2014). Of note, in the breast cancer study, telomere length was analyzed by our laboratory with the same methods as the present periodontitis study and the variability was comparable (average intra and inter-assay CV of 6% and 7%, respectively). This indicates that the LTL assay that we used and the time span between measurements in our study should have been sufficient to detect longitudinal changes in telomere length. Another limitation is that although LTL was measured at two time-points, a periodontal examination was conducted only once, limiting our understanding of change in periodontitis in relation to LTL dynamics. Finally, although the nested case control design was efficient, by using a rigid case classification for periodontitis, it does preclude a more comprehensive examination of LTL dynamics across a spectrum of periodontal parameters.
In conclusion, our findings did not reveal any association between LTL attrition over a 6-year period and chronic periodontitis; however, we detected an association between contemporaneous shorter LTL and the disease. Future investigations of LTL dynamics across the life course may provide additional insights into systemic influences, cellular markers and operating pathways underlying biological aging in oral health and disease.
Supplementary Material
Clinical Relevance.
Scientific rationale for study: Chronic periodontitis is strongly associated with age and leukocyte telomere length is a measure of cellular age. It is plausible that people with chronic periodontitis are undergoing cellular aging at a faster rate. Principal findings: Severe chronic periodontitis cases had shorter telomere lengths than age-matched controls, indicative of greater cellular aging. However the rate of telomere shortening over 6 years was the same in periodontitis cases and healthy controls. Practical implications: People with severe chronic periodontitis may be born with shorter telomere lengths, or may have experienced greater telomere shortening earlier in life.
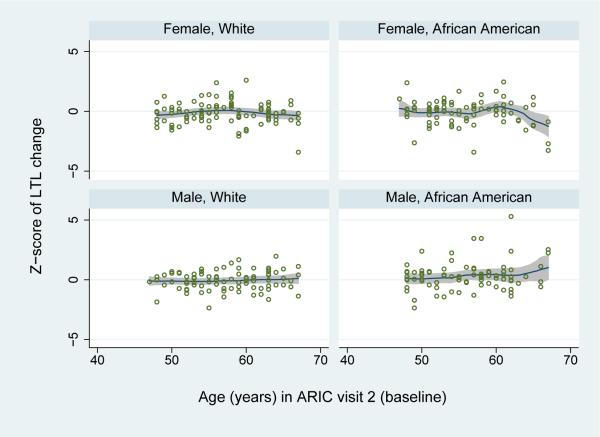
Figure 1.
Changes in leukocyte telomere length (LTL) between visits 2 and 4 by age among the 356 participants of the Atherosclerosis Risk in Communities Study, illustrated with polynomial smoothing functions with Epanechnikov kernel, stratified by sex and race. Dots represent normalized (Z-scores of) individual LTL changes. The solid line and grey areas represent the moving mean and 95% confidence interval according to participants’ ages.
Table 3.
Pairwise associations between leukocyte telomere length (LTL) and socio-demographic, health behavior, comorbidity, and biomarker characteristics, among the 356 participants of the Atherosclerosis Risk In Communities Study, based on Spearman's rank correlation.
| Visit 4 |
Visit 2 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| White | African American | White | African American | |||||||
| All | Female | Male | Female | Male | All | Female | Male | Female | Male | |
| Total, n (row %) | 356 (100) | 97 (27) | 90 (25) | 85 (24) | 84 (24) | 350 (100) | 96 (27) | 88 (25) | 83 (24) | 83 (24) |
| Race (ref: white) | 0.247** | 0.138** | ||||||||
| Sex (ref: female) | 0.062 | −0.137* | ||||||||
| Age | −0.183** | −0.168 | −0.210* | −0.150 | −0.093 | − 0.161** | −0.128 | − 0.246* | −0.014 | −0.204 |
| Periodontitis | −0.050 | − 0.251* | 0.157 | −0.009 | −0.093 | −0.062 | − 0.231* | 0.141 | −0.086 | −0.034 |
| Education (ref: basic) | −0.033 | 0.054 | −0.019 | 0.055 | −0.007 | 0.047 | 0.024 | 0.014 | 0.050 | 0.217* |
| Income | −0.029 | 0.096 | 0.171 | 0.097 | 0.033 | 0.097 | 0.204 | 0.203 | 0.112 | 0.273* |
| Smoking status (0: never, 1: former, 2: current) | −0.006 | −0.074 | 0.011 | −0.054 | 0.111 | −0.012 | −0.068 | −0.006 | 0.005 | 0.150 |
| Alcohol drinking status (0: never, 1: former, 2: current) | − 0.136* | −0.116 | 0.106 | 0.135 | − 0.284** | −0.094 | −0.152 | 0.203 | 0.014 | −0.100 |
| Body Mass Index (kg/m2) | −0.009 | − 0.222* | 0.154 | −0.039 | 0.032 | 0.029 | −0.038 | 0.081 | −0.083 | 0.012 |
| Diabetes mellitus | −0.027 | −0.056 | 0.034 | − 0.242* | 0.042 | 0.11 | −0.043 | 0.043 | −0.055 | 0.010 |
| Hypertension | −0.017 | − 0.200* | 0.075 | −0.145 | −0.008 | 0.010 | 0.127 | 0.004 | −0.114 | 0.012 |
| GCF- IL1B | −0.108 | −0.066 | 0.083 | −0.045 | −0.085 | −0.078 | −0.182 | −0.014 | 0.030 | 0.052 |
| GCF- PGE2 | 0.067 | 0.303** | 0.093 | 0.100 | 0.005 | −0.011 | 0.027 | 0.002 | 0.092 | −0.045 |
| CRP | 0.141* | 0.068 | −0.029 | 0.071 | 0.159 | 0.116 | −0.029 | −0.020 | 0.080 | 0.033 |
| Number of remaining teeth | −0.022 | 0.027 | 0.176 | 0.069 | 0.112 | −0.048 | −0.110 | 0.094 | −0.034 | 0.061 |
Denotes p<0.05
denotes p<0.01
ACKNOWLEDGEMENTS
This research was supported by the National Institute of Dental and Craniofacial Research (R03-DE022555) as an ancillary study to the Atherosclerosis Risk in Communities (ARIC) Study (AS #2010.18). The ARIC Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, HHSN268201100012C and grant R01-DE11551 from the National Institute of Dental and Craniofacial Research). The authors thank staff and participants of the ARIC Study for their important contributions. Likewise we thank Dr. Megan L. Grove-Gaona and colleagues in the Human Genetics Center, the University of Texas Health Science Center at Houston for extracting DNA from stored ARIC Visit 4 samples. We also thank Calvin Ngo and Donna Prunkard in the Rabinovitch Laboratory, Department of Pathology, University of Washington for technical assistance with the telomere length analysis.
Footnotes
DISCLOSURE OF CONFLICTS OF INTERESTS
The authors declare that they have no conflicts of interest.
REFERENCES
- Aviv A, Hunt SC, Lin J, Cao X, Kimura M, Blackburn E. Impartial comparative analysis of measurement of leukocyte telomere length/DNA content by Southern blots and qPCR. Nucleic Acids Res. 2011;39:e134. doi: 10.1093/nar/gkr634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berezow AB, Darveau RP. Microbial shift and periodontitis. Periodontol 2000. 2011;55:36–47. doi: 10.1111/j.1600-0757.2010.00350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullon P, Newman HN, Battino M. Obesity, diabetes mellitus, atherosclerosis and chronic periodontitis: a shared pathology via oxidative stress and mitochondrial dysfunction? Periodontol 2000. 2014;64:139–153. doi: 10.1111/j.1600-0757.2012.00455.x. [DOI] [PubMed] [Google Scholar]
- Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30:e47. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Aiuto F, Nibali L, Parkar M, Patel K, Suvan J, Donos N. Oxidative stress, systemic inflammation, and severe periodontitis. J Dent Res. 2010;89:1241–1246. doi: 10.1177/0022034510375830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darveau RP. Periodontitis: a polymicrobial disruption of host homeostasis. Nat Rev Microbiol. 2010;8:481–490. doi: 10.1038/nrmicro2337. [DOI] [PubMed] [Google Scholar]
- de Lange T. How telomeres solve the end-protection problem. Science. 2009;326:948–952. doi: 10.1126/science.1170633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demissie S, Levy D, Benjamin EJ, Cupples LA, Gardner JP, Herbert A, Kimura M, Larson MG, Meigs JB, Keaney JF, Aviv A. Insulin resistance, oxidative stress, hypertension, and leukocyte telomere length in men from the Framingham Heart Study. Aging Cell. 2006;5:325–330. doi: 10.1111/j.1474-9726.2006.00224.x. [DOI] [PubMed] [Google Scholar]
- Divaris K, Monda KL, North KE, Olshan AF, Reynolds LM, Hsueh WC, Lange EM, Moss K, Barros SP, Weyant RJ, Liu Y, Newman AB, Beck JD, Offenbacher S. Exploring the genetic basis of chronic periodontitis: a genome-wide association study. Hum Mol Genet. 2013;22:2312–2324. doi: 10.1093/hmg/ddt065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domon H, Tabeta K, Nakajima T, Yamazaki K. Age-related alterations in gene expression of gingival fibroblasts stimulated with Porphyromonas gingivalis. J Periodontal Res. 2013 doi: 10.1111/jre.12134. [DOI] [PubMed] [Google Scholar]
- Drury SS, Theall K, Gleason MM, Smyke AT, De Vivo I, Wong JY, Fox NA, Zeanah CH, Nelson CA. Telomere length and early severe social deprivation: linking early adversity and cellular aging. Mol Psychiatry. 2012;17:719–727. doi: 10.1038/mp.2011.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggan C, Risques R, Alfano C, Prunkard D, Imayama I, Holte S, Baumgartner K, Baumgartner R, Bernstein L, Ballard-Barbash R, Rabinovitch P, McTiernan A. Change in peripheral blood leukocyte telomere length and mortality in breast cancer survivors. J Natl Cancer Inst. 2014;106:dju035. doi: 10.1093/jnci/dju035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eke PI, Dye BA, Wei L, Thornton-Evans GO, Genco RJ. Prevalence of periodontitis in adults in the United States: 2009 and 2010. J Dent Res. 2012a;91:914–920. doi: 10.1177/0022034512457373. [DOI] [PubMed] [Google Scholar]
- Eke PI, Page RC, Wei L, Thornton-Evans G, Genco RJ. Update of the case definitions for population-based surveillance of periodontitis. J Periodontol. 2012b;83:1449–1454. doi: 10.1902/jop.2012.110664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eke PI, Thornton-Evans GO, Wei L, Borgnakke WS, Dye BA. Accuracy of NHANES periodontal examination protocols. J Dent Res. 2010;89:1208–1213. doi: 10.1177/0022034510377793. [DOI] [PubMed] [Google Scholar]
- Genco RJ, Borgnakke WS. Risk factors for periodontal disease. Periodontol 2000. 2013;62:59–94. doi: 10.1111/j.1600-0757.2012.00457.x. [DOI] [PubMed] [Google Scholar]
- Geronimus AT, Hicken M, Keene D, Bound J. “Weathering” and age patterns of allostatic load scores among blacks and whites in the United States. Am J Public Health. 2006;96:826–833. doi: 10.2105/AJPH.2004.060749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajishengallis G. Immunomicrobial pathogenesis of periodontitis: keystones, pathobionts, and host response. Trends Immunol. 2014;35:3–11. doi: 10.1016/j.it.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidinger BJ, Blount JD, Boner W, Griffiths K, Metcalfe NB, Monaghan P. Telomere length in early life predicts lifespan. Proc Natl Acad Sci U S A. 2012;109:1743–1748. doi: 10.1073/pnas.1113306109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houben JM, Moonen HJ, van Schooten FJ, Hageman GJ. Telomere length assessment: biomarker of chronic oxidative stress? Free Radic Biol Med. 2008;44:235–246. doi: 10.1016/j.freeradbiomed.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Masi S, Gkranias N, Li K, Salpea KD, Parkar M, Orlandi M, Suvan JE, Eng HL, Taddei S, Patel K, Darbar U, Donos N, Deanfield JE, Hurel S, Humphries SE, D'Aiuto F. Association between short leukocyte telomere length, endotoxemia, and severe periodontitis in people with diabetes: a cross-sectional survey. Diabetes Care. 2014;37:1140–1147. doi: 10.2337/dc13-2106. [DOI] [PubMed] [Google Scholar]
- Masi S, Salpea KD, Li K, Parkar M, Nibali L, Donos N, Patel K, Taddei S, Deanfield JE, D'Aiuto F, Humphries SE. Oxidative stress, chronic inflammation, and telomere length in patients with periodontitis. Free Radic Biol Med. 2011;50:730–735. doi: 10.1016/j.freeradbiomed.2010.12.031. [DOI] [PubMed] [Google Scholar]
- Montoya-Ortiz G. Immunosenescence, aging, and systemic lupus erythematous. Autoimmune Dis. 2013;2013:267078. doi: 10.1155/2013/267078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris AA, Zhao L, Patel RS, Jones DP, Ahmed Y, Stoyanova N, Gibbons GH, Vaccarino V, Din-Dzietham R, Quyyumi AA. Differences in systemic oxidative stress based on race and the metabolic syndrome: the Morehouse and Emory Team up to Eliminate Health Disparities (META-Health) study. Metab Syndr Relat Disord. 2012;10:252–259. doi: 10.1089/met.2011.0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donovan A, Pantell MS, Puterman E, Dhabhar FS, Blackburn EH, Yaffe K, Cawthon RM, Opresko PL, Hsueh WC, Satterfield S, Newman AB, Ayonayon HN, Rubin SM, Harris TB, Epel ES. Cumulative inflammatory load is associated with short leukocyte telomere length in the Health, Aging and Body Composition Study. PLoS One. 2011;6:e19687. doi: 10.1371/journal.pone.0019687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock KL, Kono H. The inflammatory response to cell death. Annu Rev Pathol. 2008;3:99–126. doi: 10.1146/annurev.pathmechdis.3.121806.151456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders JL, Newman AB. Telomere Length in Epidemiology: A Biomarker of Aging, Age-Related Disease, Both, or Neither? Epidemiol Rev. 2013 doi: 10.1093/epirev/mxs008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenson U, Nordfjall K, Baird D, Roger L, Osterman P, Hellenius ML, Roos G. Blood cell telomere length is a dynamic feature. PLoS One. 2011;6:e21485. doi: 10.1371/journal.pone.0021485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teumer A, Holtfreter B, Volker U, Petersmann A, Nauck M, Biffar R, Volzke H, Kroemer HK, Meisel P, Homuth G, Kocher T. Genome-wide association study of chronic periodontitis in a general German population. J Clin Periodontol. 2013;40:977–985. doi: 10.1111/jcpe.12154. [DOI] [PubMed] [Google Scholar]
- Tomiyama AJ, O'Donovan A, Lin J, Puterman E, Lazaro A, Chan J, Dhabhar FS, Wolkowitz O, Kirschbaum C, Blackburn E, Epel E. Does cellular aging relate to patterns of allostasis? An examination of basal and stress reactive HPA axis activity and telomere length. Physiol Behav. 2012;106:40–45. doi: 10.1016/j.physbeh.2011.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhulst S, Aviv A, Benetos A, Berenson GS, Kark JD. Do leukocyte telomere length dynamics depend on baseline telomere length? An analysis that corrects for 'regression to the mean'. Eur J Epidemiol. 2013;28:859–866. doi: 10.1007/s10654-013-9845-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.