Abstract
BACKGROUND
Wireless pH and pressure motility capsule (wireless motility capsule) technology provides a method to assess regional gastrointestinal transit times.
AIMS
Data from a multi-center study of gastroparetic patients and healthy controls was analyzed to: compare regional transit times measured by wireless motility capsule in healthy controls and gastroparetics (GP).
METHODS
66 healthy controls and 34 patients with GP [15 diabetic and 19 idiopathic] swallowed wireless motility capsule together with standardized meal (255 kcal). Gastric emptying time (GET), small bowel transit time (SBTT), colon transit time (CTT), and whole gut transit time (WGTT) were calculated using the wireless motility capsule.
RESULTS
GET, CTT and WGTT but not SBTT were significantly longer in GP than in controls. Eighteen percent of gastroparetic patients had delayed WGTT. Both diabetic and idiopathic etiologies of gastroparetics had significantly slower WGTT (p<0.0001) in addition to significantly slower GET than healthy controls. Diabetic gastroparetics additionally had significantly slower CTT than healthy controls (p = 0.0054).
CONCLUSIONS
1) In addition to assessing gastric emptying, regional transit times can be measured using wireless motility capsule. 2) The prolongation of CTT in gastroparetic patients indicates dysmotility beyond the stomach in GP is present and could be contributing to symptom presentation.
Keywords: gastric emptying, small intestine, large intestine, motility, device, colonic transit, wireless motility capsule, gastroparesis, Stomach and duodenum, Constipation, Functional GI diseases
INTRODUCTION
Motility disorders of the alimentary tract pose major challenges in the daily practice of gastroenterology1–5. Gastroparesis is a common motility disorder that can be related to neuropathy or myopathy and is associated with such conditions as advanced diabetes, post-vagotomy complications, as well as idiopathic etiologies3, 6–9. In addition to measuring gastric emptying, assessment of intestinal and colon transit may be useful in gastroparetics in that symptoms of intestinal dysmotility may overlap with those of gastroparesis and complaints of lower gastrointestinal tract dysfunction are often present in patients with gastroparesis.
Commonly employed methodologies for assessing regional gut transit (gastric, small bowel, and colon) include scintigraphy, radio-opaque markers, and breath tests10. Although gastric emptying scintigraphy studies are widely available, the method is not standardized at the community hospital level in regards to meal composition, monitoring times, the endpoints reported, and normal values. This lack of standardization limits the sensitivity and specificity of the test and often results in repeat testing upon referral to an academic center, adding to the overall cost, radiation exposure, and potential for conflicting test results11–13. Recently, The American Neurogastroenterology and Motility Society and the Society of Nuclear Medicine established consensus guidelines for gastric emptying scintigraphy. Adoption of the consensus guidelines, however, will require interest and renewed effort by community hospital nuclear medicine physicians to update their techniques, hopefully with encouragement by their local gastroenterologist14. How effectively the guidelines will be adopted remains uncertain.
Whole gut scintigraphy assesses small bowel and colonic transit in addition to providing gastric emptying time. However, the test is available in only a handful of specialized motility centers and requires patients to return for scintigraphic scans on at least two sequential days after the start of the test11–13.
Assessing gastrointestinal transit with radio opaque markers (ROM) requires exposure to radiation during follow-up abdominal X-rays. The ROM transit studies used in clinical practice lack standardization of the test protocol including: number of markers, dietary intake and diagnostic endpoints, and measure whole gut transit rather than colonic transit15. Breath tests address cecal arrival time of lactulose, but are not accurate tools for determining motility abnormalities within the small bowel16–21. Further, small bowel bacterial overgrowth may interfere with the interpretation of the test. Therefore, the search for a more standardized, safe and accessible diagnostic test to detect upper and the lower alimentary tract motility disorders continues22–24. In addition, a standardized, convenient test that avoids radiation exposure provides an ideal tool for safe evaluation of pharmacologic agents to treat GI motility disorders and to assess the effects of any pharmacologic agents on the GI tract as part of standard drug safety profiling.
The assessment of regional gut transit times using a motility capsule and requiring no exposure to radiation is an attractive and practical approach. A wireless motility capsule system (SmartPill GI Monitoring System, The Smart Pill Corporation, Buffalo, NY) was approved by the Food and Drug Administration in 2006 for the assessment of gastric emptying and whole gut transit time. In this manuscript, we report a secondary analysis of regional and whole gut transit times from data collected in a previous study, which compared gastric emptying of the capsule to that of a standard radio-labeled low fat eggbeater meal measured by gastric emptying scintigraphy (GES) in gastroparetic patients and healthy controls25. The aims of the current analysis were to compare regional transit times as measured by the capsule in healthy controls and patients with gastroparesis (GP).
METHODS
In a study described by Kuo et al.25, gastric emptying time as assessed by a non-digestible solid (wireless motility capsule) and gastric emptying as measured by standard scintigraphy (GES) were found to exhibit comparable sensitivity and specificity for detection of gastroparesis. Healthy controls and patients with previously confirmed gastroparesis (diabetic and idiopathic) were enrolled at 7 academic medical centers and this study on gastric emptying is the source database for this analyses for regional, and whole gut transit times25. The study was approved by the Institutional Review Board of each participating center and each subject gave informed consent before entering the study. The Clinical Trial is registered with: clinicaltrials.gov, registry number: NCT001282884
This investigation assessed gastric, small bowel, colonic and whole gut transit times. Eligibility was limited to subject data containing the four physiological landmarks necessary to assess regional transit (ingestion, gastric emptying, ileocecal arrival and body exit). The physiological landmarks used are discernable by changes in the pH or temperature profiles and are described later in this section.
Study Subjects
General Exclusion Criteria – all subjects
Subjects with previous GI abdominal surgery were excluded except those with uncomplicated appendectomy and/or laparoscopic cholecystectomy. Prescription medications such as lipid lowering agents, antidepressants, or birth control pills were permitted if the condition and the dose were stable for six months prior to enrollment in the study. NSAIDs and narcotic drugs were stopped one week prior to the study and other over the counter drugs were stopped 3 days before.
Healthy Controls
Males and females between ages 18 to 65 years with no gastrointestinal disease as screened by the Mayo GI Disease Screening Questionnaire26 and no cardiovascular, endocrine, renal or chronic disease were recruited as healthy volunteers. Additional criteria included: average bowel movement frequency of at least one per 48 hours, no pregnancy, no surgery within the past 3 months, no clinical evidence of diverticulitis demonstrated by the absence of chronic or acute abdominal pain, no medications or over-the-counter agents that could influence GI motility, no tobacco use within eight hours before and after capsule ingestion, no alcohol use 24 hours before capsule ingestion and during the monitoring period, and a BMI < 35.
Gastroparesis Patients
Males and females between ages 18 and 66 years with history of nausea and vomiting, early satiety, epigastric pain or discomfort for at least 6 months, and documented abnormal scintigraphy as defined by local medical center standards within two years were enrolled as gastroparetic subjects. Gastroparetics with excessively delayed gastric emptying time (> 90% of a standard egg meal retained after 2 hours), average bowel movement intervals exceeding 72 hours, evidence of gastric bezoar within the last 3 years, stricture, peptic ulcer, severe dysphagia to solid food and pills, severe vomiting, severe abdominal pain, severe weight loss (>10 lbs in last 2 months), or diabetes with a hemoglobin A1C greater than 10 were excluded. Proton pump inhibitors were stopped for 1 week, histamine2 receptor blockers for 2 days and antacids for 1 day. Medications that affect gastric motility were stopped 48 hours before the start of the study unless the subject was on the medication during the previous scintigraphy test.
Experimental Procedure
Following adequate screening and on the day of the study, a urine pregnancy test for females of child-bearing age and glucose level test for all diabetic subjects were obtained. After an overnight fast, all subjects swallowed the wireless motility capsule, which is equipped with 3 sensors for continuous measurement of luminal pH, pressure, and temperature. Immediately after ingestion of the capsule with 50cc of water, subjects ate a standardized meal of 120 g Eggbeaters (60 kcal) radio-labeled with Technetium-99m-sulfur colloid, 2 pieces of bread (120 kcal) with jam (74 kcal) and an additional 120cc of water11, 12. Total caloric content of the meal was 255 kcal (72% carbohydrate, 24% protein, 2% fiber, and 2% fat). Subjects completed the meal within 10 minutes of capsule ingestion and underwent a 6 hour gastric emptying scintigraphy study as previous reported25.
Six hours after ingestion of the capsule and scintigraphy test meal, subjects were provided a second meal of 237 ml (8 fl oz) of Ensure (Abbott Laboratories, Abbott Park, Ill). This second meal was given because, apart from the radio-labeled 255 kcal test meal, diabetic patients would have been fasting since midnight and the prolonged fast risked hypoglycemia. Provision of the Ensure meal, however, imposed a 6 hour upper limit cap for the evaluation of the gastric emptying of the test meal25. Two hours after the Ensure meal subjects were allowed to go home and resume normal daily activities and diet. Restrictions included no strenuous exercise (sit-ups, abdominal crunches, prolonged aerobic activity), alcohol use, and use of gastrointestinal medications (bowel cathartics, anti-diarrhea remedies, and prescription medications previously described) that could affect motility.
During and after completing the scintigraphic gastric emptying test, subjects had pH, pressure, and temperature continuously measured by the capsule and recorded by a portable receiver worn on the waist or suspended on a lanyard placed around the neck. Subjects maintained an activity diary to record times of bowel movements and meals, gastrointestinal symptoms (pain/discomfort, nausea, vomiting), and supine/sleeping times. Subjects used the receiver event button to mark these events in the electronic record.
At 2–3 days post capsule ingestion, subjects returned to the study center with the diary and the receiver. If no signal was detected from the capsule, an abdominal radiograph was taken to confirm capsule exit from the body. If a signal was detected, the subject was asked to return on day 3 to 5 post ingestion for additional follow-up. Capsule exit was confirmed in all subjects either by the return of the excreted capsule or abdominal radiograph.
pH, Pressure and Temperature Monitoring
Measures of luminal pH, pressure, and temperature were made using the SmartPill GI Monitoring System (The Smart Pill Corporation, Buffalo, NY, USA). The capsule contains three sensors (pressure, pH, and temperature) and after ingestion, wirelessly transmits sensed data at 434MHz to a data receiver worn by the subject. pH is measured from 0.5 to 9.0 pH units and has an accuracy of ±0.5 pH units; pressure is accurate to ± 5mmHg up to 100mmHg and ±10% between 100 and 350mmHg and temperature is accurate within ±1.0°C. The capsule measures 15 by 35 mm and is nearly identical in size to the Given Imaging Ltd. (Yoqneam, Israel) capsule used for endoscopy. Data is downloaded from the receiver using a docking station/battery charger via USB connection to a Windows PC compatible computer (Dell Corporation, Round Rock, TX, USA).
Determination of Regional Transit Times
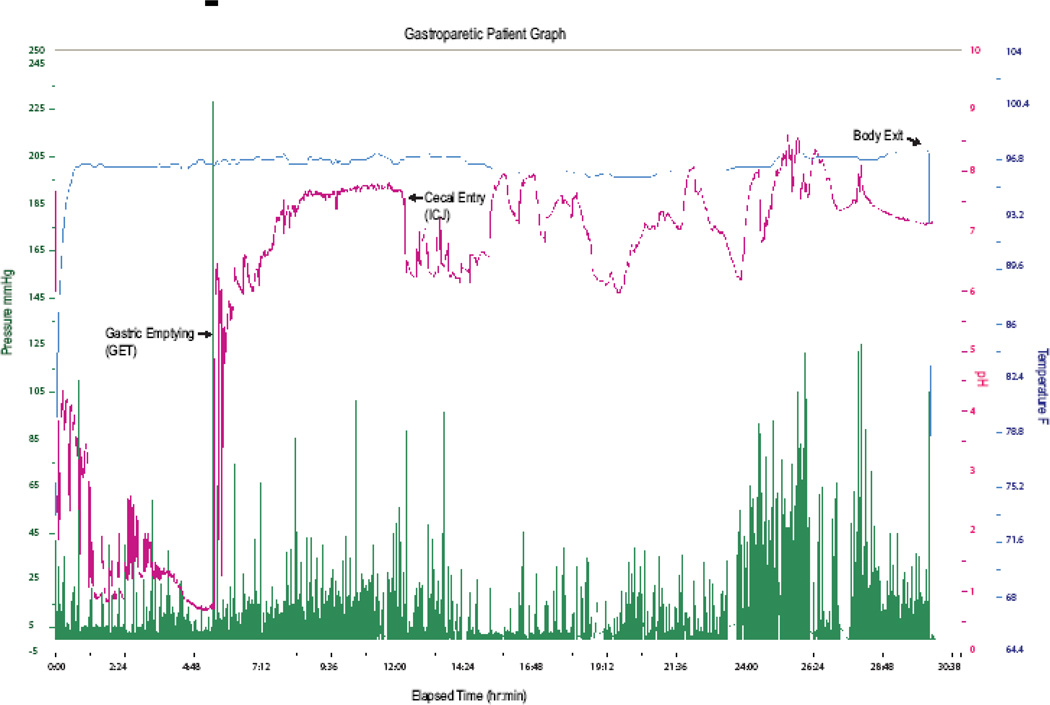
The locations of regional GI physiological landmarks (gastric emptying, cecal arrival, and body exit) within the electronic data record were determined by two independent investigators. All discrepancies in landmark times were resolved by further review. If a landmark was absent or discrepancy could not be resolved by the review, the subject data was ineligible for inclusion in the analysis. Figure 1 shows the capsule data tracing from a gastroparetic patient graph with physiological landmarks at gastric emptying, cecal entry, and body exit indicated. The regional transit times (elapsed time between physiological pH landmarks) derived from the electronic data are defined as follows:
Figure 1.
Wireless Motility Capsule Graph from a patient with gastroparesis. pH (magenta), Pressure (green), and Temperature (blue) profile showing Gastric Emptying signified by a >4 unit sharp pH rise at 5 hours, cecal arrival indicated by a 1 unit drop in pH at approximately 12 hours into the test and capsule body exit at 30 hours after the test started accompanied by a temperature drop.
Gastric Emptying Time (GET)(Figure 1)
GET (or gastric transit time) is defined as the elapsed time between the ingestion of capsule and an abrupt, sustained rise in pH (greater than 2 pH units) as the capsule enters the more alkaline duodenum from the acidic stomach.
Small Bowel Transit Time (SBTT) (Figure 1)
SBTT is defined as the elapsed time from GET until the capsule’s arrival at the cecum as determined by a sudden drop of approximately 1 pH unit after a gradual, sustained rise in pH as the capsule passes through the small bowel. The pH drop, indicative of the capsule’s arrival at the ileocecal region, was reported by Evans et al., in a study of 72 healthy volunteers who ingested a radiotelemetry capsule27.
Colonic Transit Time (CTT) (Figure 1)
CTT is defined as the elapsed time from the capsule’s arrival at the ileocecal junction until capsule exit from the body. The exit of the capsule from the body is determined in one of two ways 1) Cessation of capsule data coinciding with a bowel movement entry in the subject’s activity diary or 2) Presence of a distinct pressure pattern caused by the pressure sensor’s intrinsic sensitivity to temperature change as the capsule exits the body.
Whole Gut Transit Time (WGTT) (Figure 1)
WGTT is defined as the elapsed time from ingestion to body exit of the capsule exit.
Statistical analysis
SBTT, CTT, and WGTT endpoints are expressed as medians, and 25th and 75th percentiles. To statistically assess differences between groups, the Wilcoxon rank sum test was used. Reported p-values were obtained from the permutation distributions of the test statistics based on 10,000 Monte Carlo simulations. Associations were characterized using Spearman correlation. With the given sample sizes in our two groups we have 80% power in detecting differences of 0.6 standard deviations.
Due to the Ensure meal administered at 6 hours, subjects with GET values greater than 6 hours were capped at 6 hours. Reported estimates of median GET, therefore, are based on inversion of the Kaplan-Meier curve. To accommodate the capping of GET, the rank based procedure proposed by Gehan28 was utilized in the statistical comparison of groups.
A nominal significance level of 0.05 was used in all testing. All analyses were done using SAS (version 9.1).
RESULTS
Of the 125 subjects included in the analyses of the core study, 106 had confirmed body exit of the capsule. Body exit was missing in 19 subjects due to data loss resulting primarily from subject’s failure to keep the receiver attached to their body throughout the test. Each of the 106 subjects with a confirmed body exit had a pH increase indicative of gastric emptying. Six subjects had no discernible pH decrease landmark in the ileocecal region and were excluded from the analysis dataset. Confirmation of pH drop at cecal entry was required for 4 of the remaining subjects by expert observers. The median value (interquartile range) of the pH drop associated with cecal entry was 1.3 (1.1–1.6). Thus, data from 100 subjects (66 healthy controls [26 females]; and 34 gastroparetic patients [25 females]) was included in the subset analysis. Subject demographics are summarized in Table 1.
Table 1.
Age and Gender Breakdown for Healthy and Gastroparetic Groups.
| Demographic | Healthy Controls | Gastroparetic Patients |
|---|---|---|
| n | 66 | 34 |
| Gender F / M | 26/40 | 25/9 |
| Age Mean (Range) | 31 (19–57) | 43 (20–66) |
Regional Transit Times in Healthy Controls
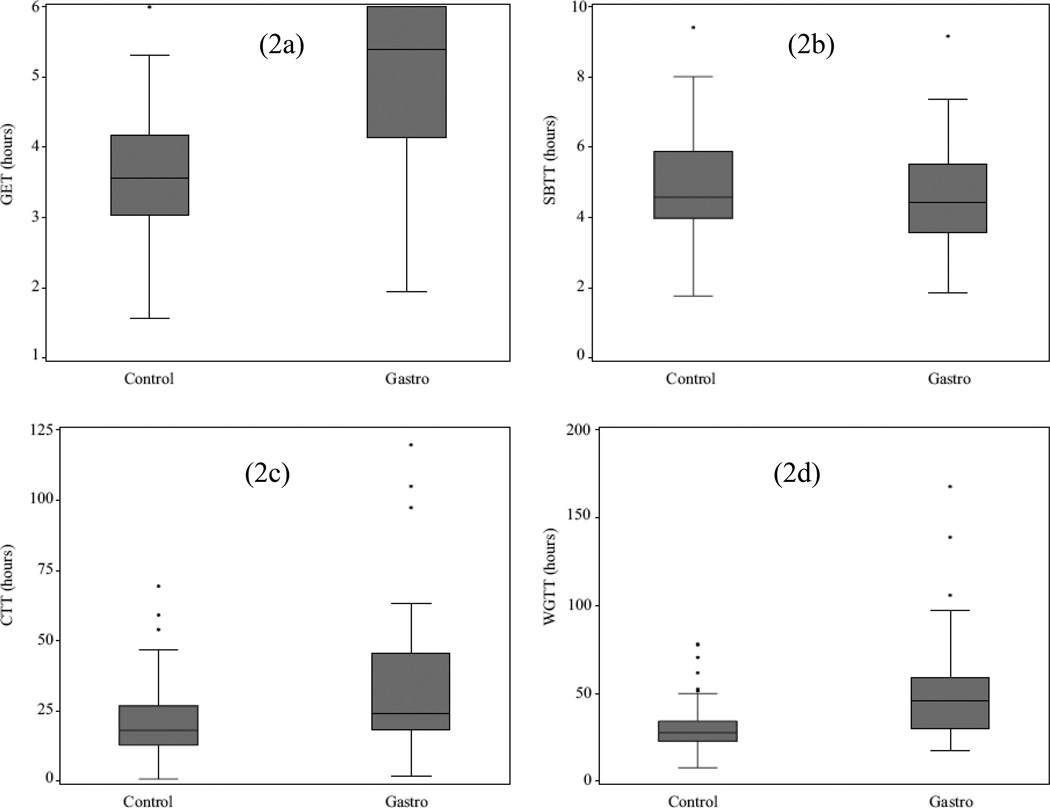
Regional transit times in healthy controls are summarized in Table 2 and Figure 2. The median value for WGTT was 27.7 hours with 3.6 hours for GET, 4.6 hours for SBTT, and 18.1 hours for CTT.
Table 2.
Median (25th and 75th percentiles) Transit Times in hours for Gastric Emptying Time, Small Bowel Transit Time, Colonic Transit Time, and Whole Gut Transit Time for Healthy Controls and Gastroparetic Patients
| Transit Parameter (hours) |
Healthy Controls | Gastroparesis Patients |
p |
|---|---|---|---|
| n | 66 | 34 | |
| GET Median (percentiles) | 3.6 (3.0, 4.2) | 5.4 (4.1, †) | p<0.0001 |
| SBTT Median (percentiles) | 4.6 (4.0, 5.9) | 4.5 (3.6, 5.5) | p = 0.615 |
| CTT Median (percentiles) | 18.1 (12.8, 26.8) | 24.3 (18.4, 45.7) | p = 0.004 |
| WGTT Median (percentiles) | 27.7 (22.9, 34.3) | 45.9 (30.0, 59.0) | p = <0.0001 |
75% percentile was not observed in gastroparetic patients because of capping at 6 hours.
Figure 2.
Box and Whisker plots of healthy controls and gastroparesis patients. Gastric Emptying Time (2a), Colonic Transit Time (2c), and Whole Gut Transit Time (2d) are significantly prolonged in gastroparetics. Small Bowel Transit Time was not significantly different. Medians are shown as the lines within the box, the box boundaries are the 25th and 75th percentiles. Outliers are depicted by asterisks. The Y-axes are transit times in hours.
Regional Transit Times in Gastroparetic Patients Compared to Healthy Controls
Transit times in gastroparetic patients were significantly longer in the stomach, colon, and whole gut when compared with those in healthy controls. Small bowel transit (SBTT) in both groups was similar. Idiopathic and diabetic gastroparetic subgroups had significantly slower WGTT (p=0.02 and p< 0.0001) along with significantly slower GET than the healthy control group; diabetics had significantly slower CTT. Regional transit time data and comparisons are summarized in Table 2 and in Figure 2.
Relationship of regional transit times
The relationships between transit parameters of the different regions were analyzed using Spearman correlation. Strong, significant correlations were found for WGTT to CTT in all subjects (r=0.92, p<0.0001), in healthy controls (r=0.90, p<0.0001), and in gastroparetic patients (r=0.93, p<0.0001). A strong, significant correlation (r=0.53, p=0.001) between GET and WGTT was found in gastroparetic patients29.
DISCUSSION
In this report we explore the use of wireless motility capsule for assessing regional and whole gut transit times as indicators of GI tract motility in healthy controls and patients with diabetic (DGP) and idiopathic (IGP) gastroparesis. Current approaches for assessing transit in both the upper gut and lower gut are poorly standardized leading to difficulty in interpretation of results and often the need for repeat testing. The wireless motility capsule provides regional transit measures throughout the entire GI tract in a single test. Small bowel and colonic transit times in addition to gastric emptying times measured by the wireless motility capsule are reported here including the prevalence of slow colonic transit in gastroparetic subjects.
In addition to delayed gastric emptying, colonic and whole gut transits were significantly delayed in patients with gastroparesis. Both idiopathic and diabetic gastroparetic subgroups had significantly slower WGTT along with delayed GET, a finding consistent with reports from Sadik et al.30 using radio-opaque markers (ROM), and Bonapace et al.31 using whole gut scintigraphy methods to assess whole gut transit in subjects with upper GI symptoms. Eighteen percent of the gastroparetics in our study had delayed whole gut transit. Sadik et al. reported 17% of his subjects with the primary upper GI symptom of nausea had whole gut transit delay, and Bonapace et al. reported 31% of his subjects with upper GI symptoms (abdominal discomfort, early satiety, nausea and bloating) were delayed. Within our diabetic population, delayed gastric emptying and significantly prolonged CTT were identified, consistent with the broad neuropathy changes reported in diabetes22. Iida et al.32 also reported significant delays in colonic and whole gut transit in type II diabetics without symptoms of neuropathy using ROM to characterize whole gut transit. The significantly prolonged CTT and WGTT detected confirm the importance of evaluating regional and whole gut transit in gastroparesis.
Few studies using either scintigraphy or radio-opaque markers for determining small bowel transit have been reported. As in the Degen and Phillips study33, we saw no significant difference in SBTT in males and females. Graff et al.34 and Sadik et al.30 reported differences and suggested that the conflicting results reported in the literature could be attributed to differences in methods used to measure SBTT, most of which rely on short duration scintigraphic estimates of isotope in the stomach. Furthermore, gender differences observed in gastric emptying30, 34 could influence the calculation of small bowel transit time. Our method, relying on pH landmarks within the gastrointestinal lumen to determine SBTT, is not influenced by gastric emptying and has the potential to yield a more exact measure.
Because of concerns for hypoglycemia in our diabetic subjects, a second meal was given 6 hours after the scintigraphy egg meal & capsule ingestion. The second meal returns the subject to the gastric fed state, delaying capsule exit until the second meal exits. Therefore, the GET values greater than 6 hours were capped at 6 hours since the values greater than 6 hours no longer reflect the emptying of the initially administrated standard test meal. The core study determined that GET values in excess of 5 hours are considered prolonged25. The introduction of the second meal at 6 hours does limit the understanding of the duration, and hence severity of gastric emptying delay.
The start of colonic transit was determined by the observation of a rapid drop of approximately 1.3 pH units after a gradual, sustained rise in pH as the wireless motility capsule transits the small bowel. This physiological pH landmark corresponds to the start of the cecum as reported by Evans et al.27 using a radio-telemetry pH capsule. Fallingborg et al. reported the same pH drop observation in a study using a radiotelemetry pH capsule and reported a gradual rise in pH through the duodenum and mid small bowel terminating in a pH of 7.4 at the ileum before dropping to pH 5.9 in the cecum35, 36. The significant increase in microflora population in the cecum is suspected to cause the observed pH drop. In ninety-four percent of eligible core study subjects a discernible drop in pH at the cecum was observed. In the absence of the pH drop, colonic transit cannot be determined and is a potential limitation of the procedure. WGTT can be used as a surrogate measure, given the high degree of correlation between the WGTT and CTT parameters. Changes in pressure patterns between the ileum and cecum associated with this pH drop have also been reported37.
We confirmed body exit by corroboration of a temperature drop with a bowel movement diary entry in 80 subjects. In 26 subjects lacking a diary entry, wireless motility capsule body exit was confirmed by the presence of a distinct pressure pattern that results from the intrinsic sensitivity of the pressure sensor to a temperature change as the capsule exits the body. Thus, body exit was confirmed in 106 of the 125 subjects (85%) analyzed in the core study. Because the core study focused on upper gut function (gastric emptying) and contained a safety requirement that all capsule body exits had to be confirmed by either retrieval of the capsule from stool or abdominal x-ray, we were not overly concerned about subject compliance with diary entries or diligence in keeping the receiver near the body beyond the initial two days of study. Product refinements have been implemented to enhance confirmation of capsule exit based on the electronic data record.
The relevance of transit times obtained with the wireless motility capsule to transit times of physiological food may be questioned because of the artificial nature of wireless motility capsule, and because food and wireless motility capsule are propelled through the stomach by different mechanisms. For instance, digestible food undergoes mixing and breakdown followed by a slow continuous propulsion into the duodenum during the post-prandial state. Wireless motility capsule empties in the fasted state following near complete emptying of the meal with short bursts of either migrating motor complexes or isolated high amplitude antral contractions, as reported by Cassilly et al38. The strong correlation between the gastric emptying of the radiolabeled meal and the wireless motility capsule reported by Kuo et al.25 and the dependence of the wireless motility capsule emptying on the near complete emptying of the meal suggest that the two different mechanistic events are related. Characteristics of wireless motility capsule movement in the small bowel and colon are not precisely understood. Rao et al.29, reported a strong correlation in colonic transit times measure by wireless motility capsule to those measured using ROM. Recently, Maqbool et al.39 reported a strong correlation between geometric mean values of whole gut scintigraphy and wireless motility capsule, which suggests that wireless motility capsule has whole gut and colonic transit patterns similar to a meal. Further studies are needed to more fully understand and characterize wireless motility capsule movement. However the correlations observed with ROM and scintigraphy and the mechanistic studies of Cassilly suggests that transit times derived using wireless motility capsule are at least as clinically relevant as times derived using other clinically accepted methodologies.
In conclusion, we report that regional gut transit times (GET, CTT, and WGTT) in patients with gastroparesis are significantly longer than in healthy controls. Numerous authors40, 41, 42 have reported the prevalence of transit delays distal to the main symptom focus in functional motility disorders and this is borne out by our data indicating a significant prevalence of delay in the colon in subjects with gastroparesis. The ability to characterize discreet transit times for each region of the GI tract suggests the potential for this technique to assess drug efficacy and drug effects on the different regions of the GI tract in a single test without exposure to radiation providing a methodology for investigating new gut pharmacology. Since there are limited methods available for evaluation of regional transit, the efficiency, convenience, safety and precision of results makes wireless motility capsule an attractive choice for assessing regional gut transit and motility.
Acknowledgments
Drs. Chey, Hasler, Kuo, Lackner, McCallum, Parkman, Koch, and Katz serve as speakers, consultants, or advisory board members for The Smart Pill Corporation and have received research funding from The Smart Pill Corporation. Dr. Wilding serves as a consultant to The Smart Pill Corporation. Dr. Semler and Ms. Selover are employees of The Smart Pill Corporation; Dr. Semler owns stock in The Smart Pill Corporation.
Dr. I Sarosiek authored the manuscript; all other listed authors provided insightful edits, feedback, and approval of the final manuscript.
The study was sponsored by The Smart Pill Corporation, Buffalo, New York manufacturer of the SmartPill GI Monitoring System, and partially supported by NYSTAR grant C020118 and NIH grant DK069614.
Footnotes
STATEMENT OF INTERESTS:
Declaration of personal interests: Drs. I and J Sarosiek and Dr. M Sitrin have no financial interest in The SmartPill Corporation.
REFERENCES
- 1.Prather CM, Camilleri M, Zinsmeister AR, McKinzie S, Thomforde G. Tegaserod accelerates orocecal transit in patients with constipation-predominant irritable bowel syndrome. Gastroenterology. 2000;118(3):463–468. doi: 10.1016/s0016-5085(00)70251-4. [DOI] [PubMed] [Google Scholar]
- 2.Quigley EMM. Review article: gastric emptying in functional gastrointestinal disorders. Aliment Pharmacol Ther. 2004;20(7):56–60. doi: 10.1111/j.1365-2036.2004.02186.x. [DOI] [PubMed] [Google Scholar]
- 3.Buckles DC, Sarosiek I, McMillan C, McCallum RW. Delayed gastric emptying in gastroesophageal reflux disease: reassessment with new methods and symptomatic correlations. Am J Med Sci. 2004;327(1):1–4. doi: 10.1097/00000441-200401000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Quigley EMM. Functional dyspepsia (FD) and non-erosive reflux disease (NERD): overlapping or discrete entities? Best Pract Res Clin Gastroenterol. 2004;18(4):695–706. doi: 10.1016/j.bpg.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 5.Quigley EMM. Functional gastrointestinal disease: has the genomic era arrived? Gastroenterology. 2004;126(4):1193–1195. doi: 10.1053/j.gastro.2004.02.028. [DOI] [PubMed] [Google Scholar]
- 6.Park MI, Camilleri M. Gastroparesis: clinical update. Am J Gastroenterol. 2006;101(5):1129–1139. doi: 10.1111/j.1572-0241.2006.00640.x. [DOI] [PubMed] [Google Scholar]
- 7.Reddymasu SC, Bonino J, McCallum RW. Gastroparesis secondary to a demyelinating disease: a case series. BMC Gastroenterol. 2007;7:3. doi: 10.1186/1471-230X-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rao SS, McCallum RW, Parkman HP, et al. A comparison study of Smartpill and radiopaque markers for the assessment of colonic transit time in humans. Gastroenterology. 2007;132(4):A458. [Google Scholar]
- 9.Samsom M, Vermeijden JR, Smout AJPM, et al. Prevalence of delayed gastric emptying in diabetic patients and relationship to dyspeptic symptoms: a prospective study in unselected diabetic patients. Diabetes Care. 2003;26(11):3116–3122. doi: 10.2337/diacare.26.11.3116. [DOI] [PubMed] [Google Scholar]
- 10.Lin HC, Prather C, Fisher RS, et al. Measurement of gastrointestinal transit. Dig Dis Sci. 2005;50(6):989–1004. doi: 10.1007/s10620-005-2694-6. [DOI] [PubMed] [Google Scholar]
- 11.Camilleri M, Bharucha AE, Ueno R, et al. Effect of a selective chloride channel activator, lubiprostone, on gastrointestinal transit, gastric sensory, and motor functions in healthy volunteers. Am J Physiol - Gastrointest Liver Physiol. 2006;290(5):G942–G947. doi: 10.1152/ajpgi.00264.2005. [DOI] [PubMed] [Google Scholar]
- 12.Tougas G, Eaker EY, Abell TL, et al. Assessment of gastric emptying using a low fat meal: establishment of international control values. Am J Gastroenterol. 2000;95(6):1456–1462. doi: 10.1111/j.1572-0241.2000.02076.x. [DOI] [PubMed] [Google Scholar]
- 13.Tougas G, Chen Y, Coates G, et al. Standardization of a simplified scintigraphic methodology for the assessment of gastric emptying in a multicenter setting. Am J Gastroenterol. 2000;95(1):78–86. doi: 10.1111/j.1572-0241.2000.01703.x. [DOI] [PubMed] [Google Scholar]
- 14.Abell TL, Camilleri M, Donohoe K, et al. Consensus recommendations for gastric emptying scintigraphy: a joint report of the American Neurogastroenterology and Motility Society and the Society of Nuclear Medicine. J Nuc Med Tech. 2008;36(1):44–54. doi: 10.2967/jnmt.107.048116. [DOI] [PubMed] [Google Scholar]
- 15.Rao SSC, Ozturk R, Laine L. Clinical utility of diagnostic tests for constipation in adults: a systematic review. Am J Gastroenterol. 2005;100:1605–1615. doi: 10.1111/j.1572-0241.2005.41845.x. [DOI] [PubMed] [Google Scholar]
- 16.Arce DA, Ermocilla CA, Costa H. Evaluation of constipation. Am Fam Physician. 2002;65(11):2283–2290. [PubMed] [Google Scholar]
- 17.Remes-Troche JM, Rao SS. Diagnostic testing in patients with chronic constipation. Cur Gastroenterol Rep. 2006;8(5):416–424. doi: 10.1007/s11894-006-0028-2. [DOI] [PubMed] [Google Scholar]
- 18.Rao SS. Constipation: evaluation and treatment. Gastroenterol Cl of North America. 2003;32(2):659–683. doi: 10.1016/s0889-8553(03)00026-8. [DOI] [PubMed] [Google Scholar]
- 19.Corazza GR, Menozzi MG, Strocchi A, et al. The diagnosis of small bowel bacterial overgrowth. Reliability of jejunal culture and inadequacy of breath hydrogen testing. Gastroenterology. 1990;98(2):302–309. doi: 10.1016/0016-5085(90)90818-l. [DOI] [PubMed] [Google Scholar]
- 20.Quigley EMM. From comic relief to real understanding; how intestinal gas causes symptoms. Gut. 2003;52(12):1659–1661. doi: 10.1136/gut.52.12.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O'Leary C, Quigley EM. Small bowel bacterial overgrowth, celiac disease, and IBS: what are the real associations? Am J Gastroenterol. 2003;98(4):720–722. doi: 10.1111/j.1572-0241.2003.07395.x. [comment]. [DOI] [PubMed] [Google Scholar]
- 22.Camilleri M. Clinical practice. Diabetic gastroparesis. N Engl J Med. 2007;356(8):820–829. doi: 10.1056/NEJMcp062614. [DOI] [PubMed] [Google Scholar]
- 23.Smith DS, Ferris CD. Current concepts in diabetic gastroparesis. Drugs. 2003;63(13):1339–1358. doi: 10.2165/00003495-200363130-00002. [DOI] [PubMed] [Google Scholar]
- 24.Dickman R, Fass R. Ambulatory esophageal pH monitoring: new directions. Dig Dis. 2006;24(3–4):313–318. doi: 10.1159/000092885. [DOI] [PubMed] [Google Scholar]
- 25.Kuo B, McCallum RW, Koch KL, et al. Comparison of gastric emptying of a nondigestible capsule to a radio-labeled meal in healthy and gastroparetic subjects. Aliment Pharmacol Ther. 2008;27:186–196. doi: 10.1111/j.1365-2036.2007.03564.x. [DOI] [PubMed] [Google Scholar]
- 26.Locke GR, Talley NJ, Weaver AL, Zinsmeister AR. A new questionnaire for gastroesophageal reflux disease. Mayo Clin Proc. 1994;69(6):539–547. doi: 10.1016/s0025-6196(12)62245-9. [DOI] [PubMed] [Google Scholar]
- 27.Evans DF, Pye G, Bramley R, Clark AG, Dyson TJ, Hardcastle JD. Measurement of gastrointestinal ph profiles in normal ambulant human subjects. Gut. 1988;29:1035–1041. doi: 10.1136/gut.29.8.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gehan E. A generalized Wilcoxin test for comparing arbitrarily singly-censored samples. Biometrika. 1965;52:203–223. [PubMed] [Google Scholar]
- 29.Rao SSC, Kuo B, McCallum RW, et al. Investigation of Colonic and Whole-Gut Transit With Wireless Motility Capsule and Radiopaque Markers in Constipation. Clinical Gastroenterology and Hepatology. 2009;7:537–544. doi: 10.1016/j.cgh.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 30.Sadik R, Abrahamsson H, Stotzer PO. Gender differences in gut transit shown with a newly developed radiological procedure. Scand J Gastroenterol. 2003;38(1):36–42. doi: 10.1080/00365520310000410. [DOI] [PubMed] [Google Scholar]
- 31.Bonapace ES, Maurer AH, Davidoff S, Krevsky B, Fisher RS, Parkman HP. Whole Gut Transit Scintigraphy in the Clinical Evaluation of Patients With Upper and Lower Gastrointestinal Symptoms. Am J Gastroenterol. 2000;95(10):2838–2847. doi: 10.1111/j.1572-0241.2000.03195.x. [DOI] [PubMed] [Google Scholar]
- 32.Iida M, Ikeda M, Kishimoto M, et al. Evaluation of Gut Motility in type II Diabetes by Radiopaque Marker Method. J Gastroenterology and Hepatology. 2000;15:381–385. doi: 10.1046/j.1440-1746.2000.02076.x. [DOI] [PubMed] [Google Scholar]
- 33.Degen LP, Phillips SF. Variability of gastrointestinal transit in healthy women and men. Gut. 1996;39:299–305. doi: 10.1136/gut.39.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Graff J, Brinch K, Madsen JL. Gastrointestinal mean transit times in young and middle aged healthy subjects. Clinical Physiology. 2001;21(2):253–259. doi: 10.1046/j.1365-2281.2001.00308.x. [DOI] [PubMed] [Google Scholar]
- 35.Fallingborg J, Pedersen P, Jacobsen BA. Small intestine transit time and intraluminal pH in ileocecal resected patients with Crohn’s disease. Dig Dis Sci. 1998;43(4):702–705. doi: 10.1023/a:1018893409596. [DOI] [PubMed] [Google Scholar]
- 36.Fallingborg J, Christensen LA, Ingeman-Neilsen M. Measurement of gastrointestinal pH and regional transit times in normal children. J Ped Gastroenterol Nutr. 1990;11(2):211–214. doi: 10.1097/00005176-199008000-00010. [DOI] [PubMed] [Google Scholar]
- 37.Gaman A, Hutson A, Hwang J, et al. A motility device discriminates motility patterns with a luminal acidic pH change within the distal small bowel and proximal colon. Gastroenterology. 2007;32(4) suppl.2:A-460. [Google Scholar]
- 38.Cassilly D, Kantor S, Knight LC, et al. Gastric emptying of a non-digestible solid: assessment with simultaneous SmartPill pH and pressure capsule, antroduodenal manometry, gastric emptying scintigraphy. Neurogastroenterol Motil. 2008;20(4):311–319. doi: 10.1111/j.1365-2982.2007.01061.x. [DOI] [PubMed] [Google Scholar]
- 39.Maqbool S, Parkman HP, Friedenberg FK. Wireless capsule motility: Comparison of the SmartPill® GI monitoring system with scintigraphy for measuring whole gut transit. Dig Dis Sci. 2009 Aug 5; doi: 10.1007/s10620-009-0899-9. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 40.Zarate N, Knowles CH, Yazaki E, Lunnis PJ, Scott SM. Clinical presentation and patterns of slow transit constipation do not predict coexistent upper gut dysmotility. Dig Dis Sci. 2009;54(1):122–131. doi: 10.1007/s10620-008-0324-9. [DOI] [PubMed] [Google Scholar]
- 41.van der Sijp JR, Kamm MA, Nightingale JM, et al. Disturbed gastric and small bowel transit in severe idiopathic constipation. Dig Dis Sci. 1993;38:837–844. doi: 10.1007/BF01295909. [DOI] [PubMed] [Google Scholar]
- 42.Mollen RM, Hopman WP, Kuijpers HH, Jansen JBMJ. Abnormalities of upper gut motility in patients with slow-transit constipation. Eur J Gastroenterol Hepatol. 1999;11:701–708. doi: 10.1097/00042737-199907000-00003. [DOI] [PubMed] [Google Scholar]