Abstract
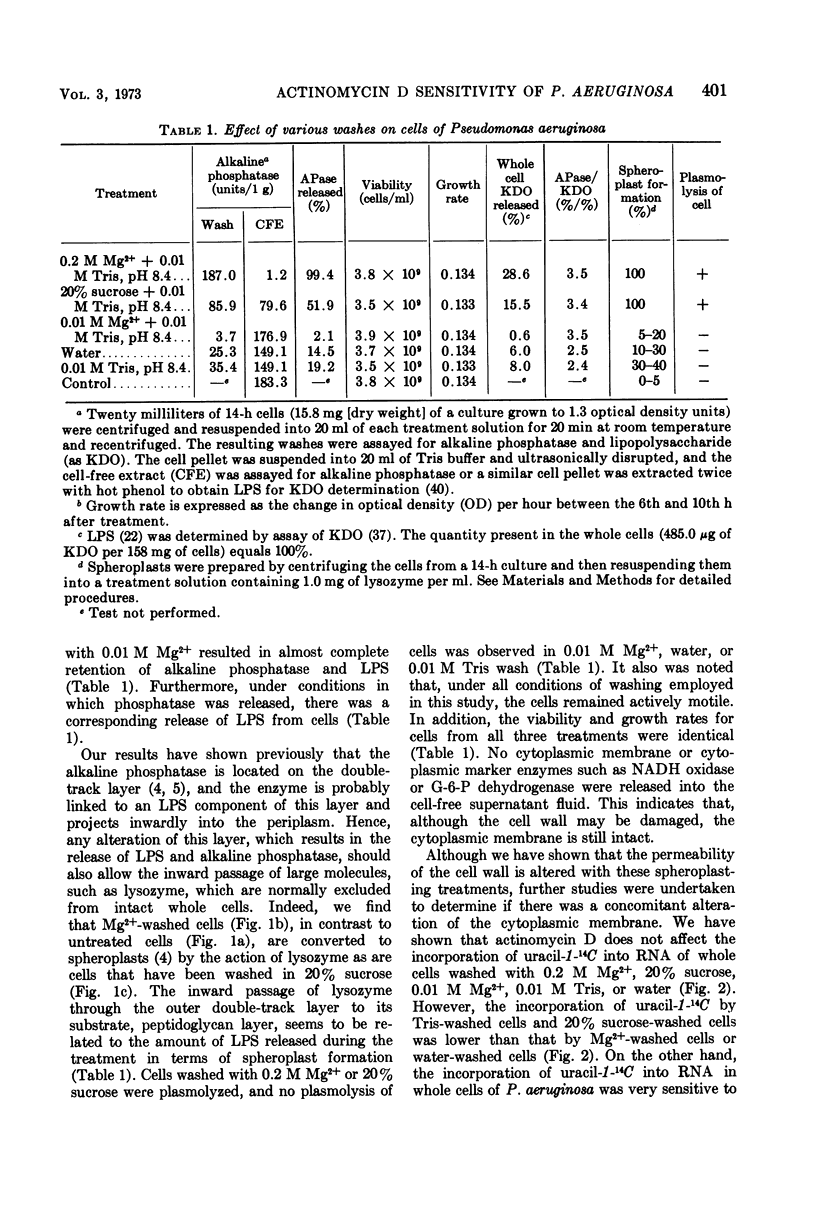
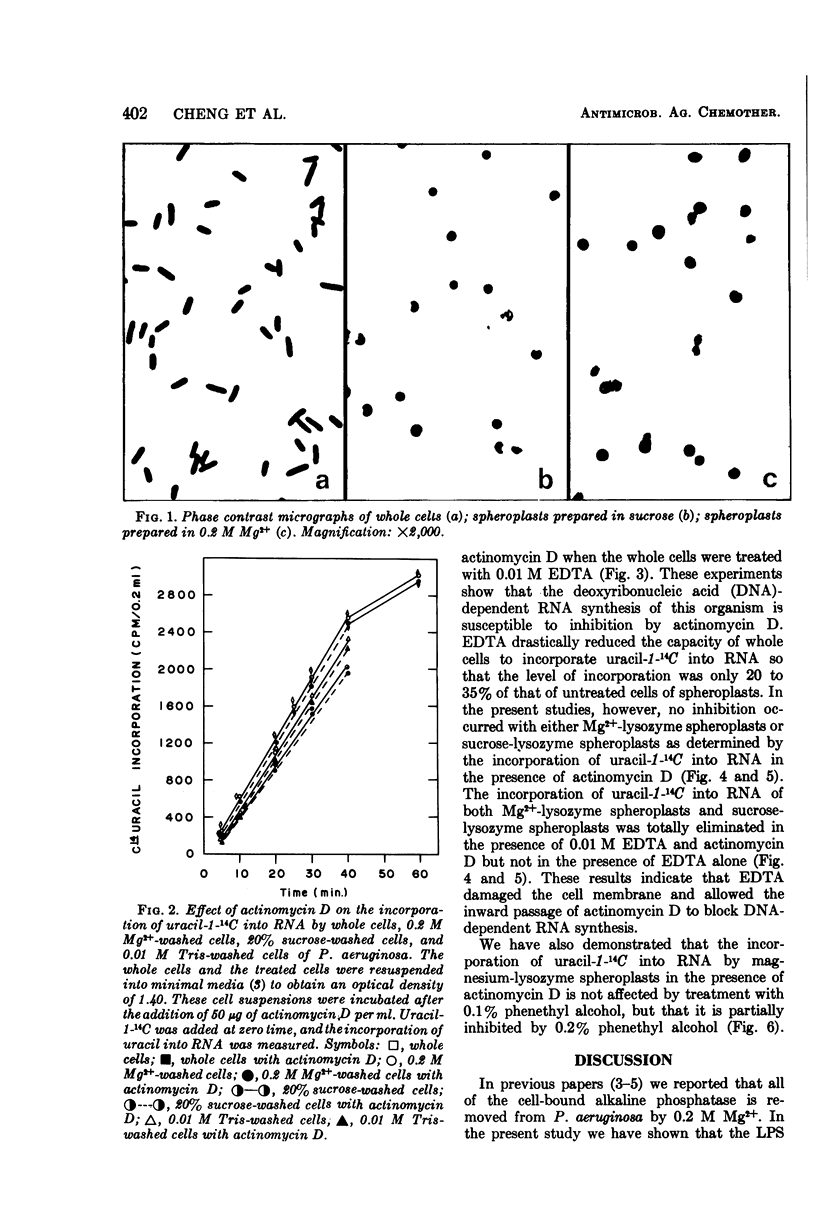
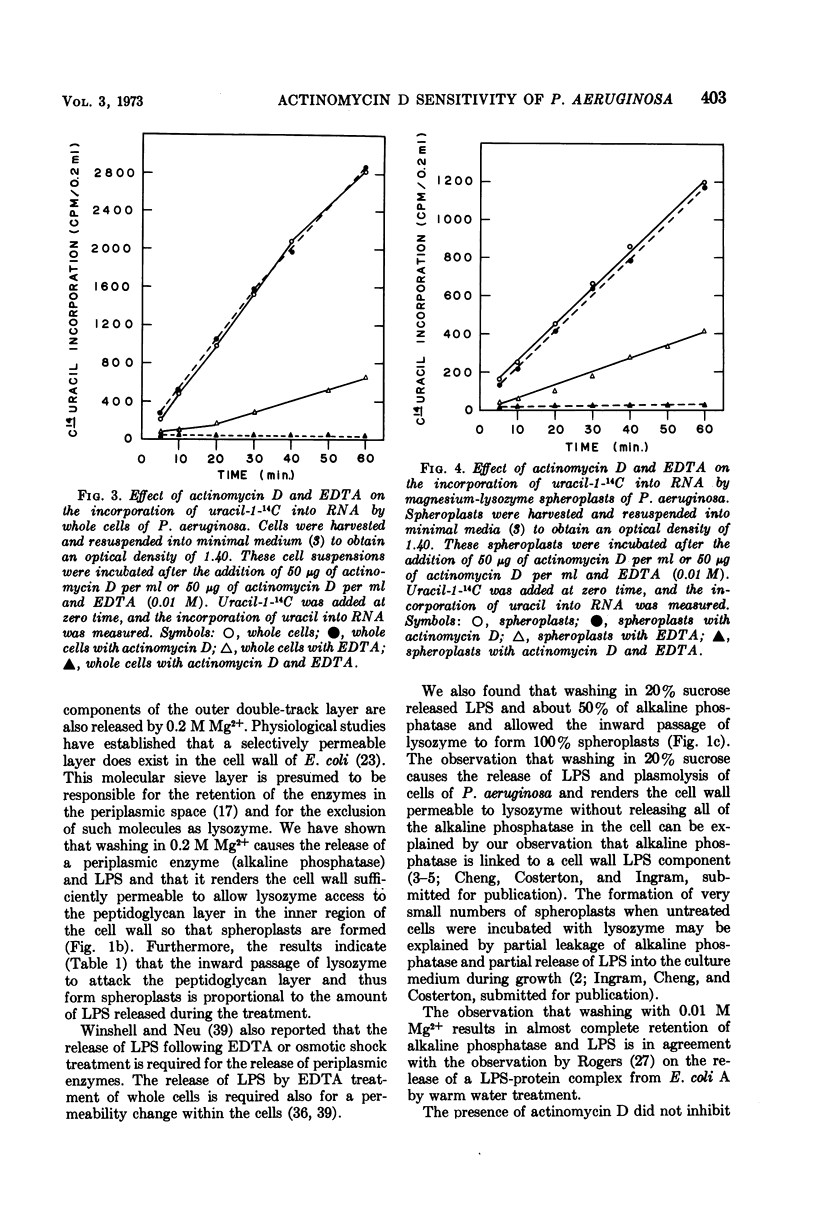
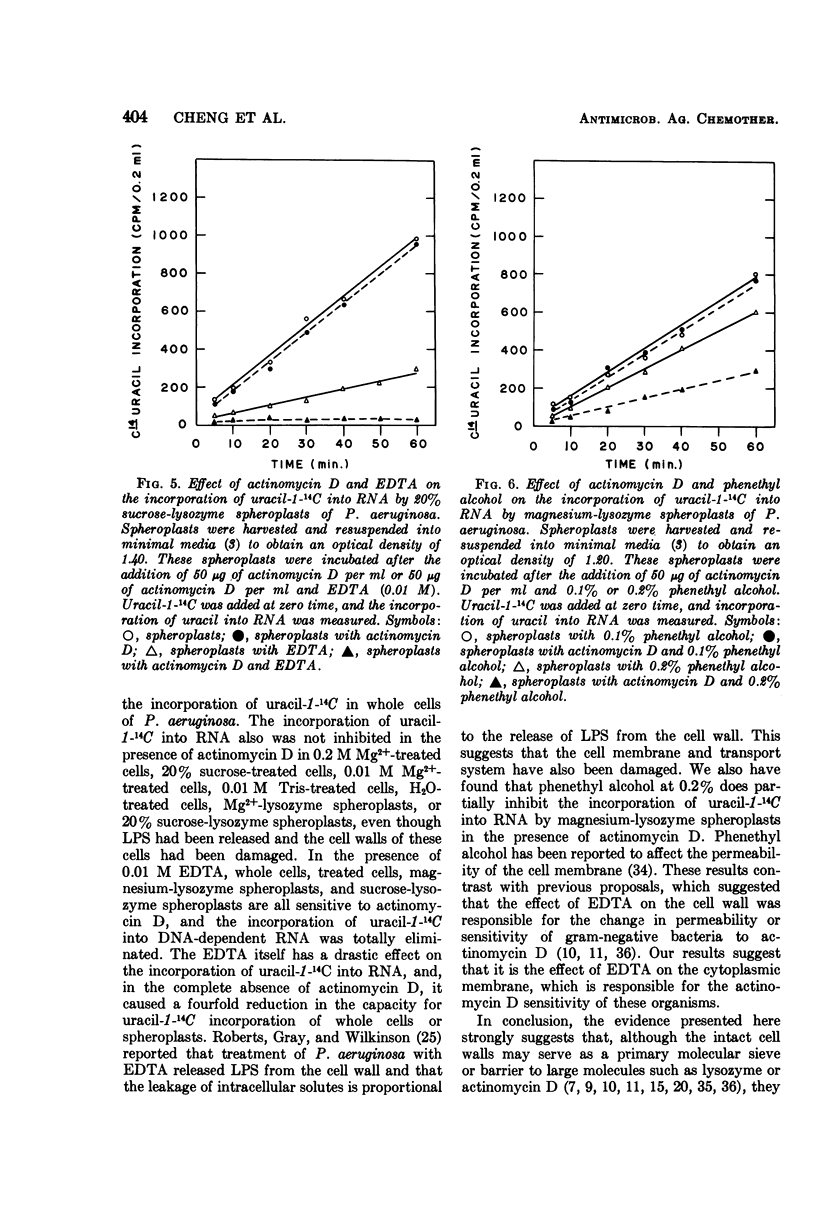
Cells of Pseudomonas aeruginosa suspended in 0.2 M Mg2+, 20% sucrose, 0.01 M tris(hydroxymethyl)aminomethane, or water partially release lipopolysaccharide. The release of alkaline phosphatase from the periplasmic space and the ability to form spheroplasts on lysozyme treatment is directly related to the lipopolysaccharide released during treatment with 0.2 M Mg2+, 20% sucrose, or other agents. The synthesis of ribonucleic acid (RNA) by intact cells, magnesium-lysozyme spheroplasts, or 20% sucrose-lysozyme spheroplasts is not sensitive to actinomycin D, whereas RNA synthesis by intact cells or spheroplasts in the presence of ethylene-diaminetetraacetic acid (EDTA) is sensitive to actinomycin D. EDTA alone has an inhibitory effect on RNA synthesis by whole cell, by magnesium-lysozyme spheroplasts, and by 20% sucrose-lysozyme spheroplasts. The experimental data indicate that, although the cell wall is damaged by 0.2 M Mg2+ or 20% sucrose treatment in the presence of lysozyme, the treated cells or spheroplasts are still resistant to actinomycin D. These results suggest that the cytoplasmic membrane should be considered as the final and determinative barrier to this antibiotic in this organism.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cheng K. J., Day D. F., Costerton J. W., Ingram J. M. Alkaline phosphatase subunits in the culture filtrate of Pseudomonas aeruginosa. Can J Biochem. 1972 Mar;50(3):268–276. doi: 10.1139/o72-038. [DOI] [PubMed] [Google Scholar]
- Cheng K. J., Ingram J. M., Costerton J. W. Alkaline phosphatase localization and spheroplast formation of Pseudomonas aeruginosa. Can J Microbiol. 1970 Dec;16(12):1319–1324. doi: 10.1139/m70-218. [DOI] [PubMed] [Google Scholar]
- Cheng K. J., Ingram J. M., Costerton J. W. Interactions of alkaline phosphatase and the cell wall of Pseudomonas aeruginosa. J Bacteriol. 1971 Jul;107(1):325–336. doi: 10.1128/jb.107.1.325-336.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng K. J., Ingram J. M., Costerton J. W. Release of alkaline phosphatase from cells of Pseudomonas aeruginosa by manipulation of cation concentration and of pH. J Bacteriol. 1970 Nov;104(2):748–753. doi: 10.1128/jb.104.2.748-753.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray G. W., Wilkinson S. G. The effect of ethylenediaminetetra-acetic acid on the cell walls of some gram-negative bacteria. J Gen Microbiol. 1965 Jun;39(3):385–399. doi: 10.1099/00221287-39-3-385. [DOI] [PubMed] [Google Scholar]
- HAYWOOD A. M., SINSHEIMER R. L. Inhibition of protein synthesis in E. coli protoplasts by actinomycin-D. J Mol Biol. 1963 Mar;6:247–249. doi: 10.1016/s0022-2836(63)80074-1. [DOI] [PubMed] [Google Scholar]
- HURWITZ J., FURTH J. J., MALAMY M., ALEXANDER M. The role of deoxyribonucleic acid in ribonucleic acid synthesis. III. The inhibition of the enzymatic synthesis of ribonucleic acid and deoxyribonucleic acid by actinomycin D and proflavin. Proc Natl Acad Sci U S A. 1962 Jul 15;48:1222–1230. doi: 10.1073/pnas.48.7.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heppel L. A. Selective release of enzymes from bacteria. Science. 1967 Jun 16;156(3781):1451–1455. doi: 10.1126/science.156.3781.1451. [DOI] [PubMed] [Google Scholar]
- LEIVE L. ACTINOMYCIN SENSITIVITY IN ESCHERICHIA COLI PRODUCED BY EDTA. Biochem Biophys Res Commun. 1965 Jan 4;18:13–17. doi: 10.1016/0006-291x(65)90874-0. [DOI] [PubMed] [Google Scholar]
- Leive L., Kollin V. Controlling EDTA treatment to produce permeable Escherichia coli with normal metabolic processes. Biochem Biophys Res Commun. 1967 Jul 21;28(2):229–236. doi: 10.1016/0006-291x(67)90434-2. [DOI] [PubMed] [Google Scholar]
- Leive L., Kollin V. Synthesis, utilization and degradation of lactose operon mRNA in Escherichia coli. J Mol Biol. 1967 Mar 14;24(2):247–259. doi: 10.1016/0022-2836(67)90330-0. [DOI] [PubMed] [Google Scholar]
- Leive L., Shovlin V. K., Mergenhagen S. E. Physical, chemical, and immunological properties of lipopolysaccharide released from Escherichia coli by ethylenediaminetetraacetate. J Biol Chem. 1968 Dec 25;243(24):6384–6391. [PubMed] [Google Scholar]
- Leive L. Studies on the permeability change produced in coliform bacteria by ethylenediaminetetraacetate. J Biol Chem. 1968 May 10;243(9):2373–2380. [PubMed] [Google Scholar]
- MacAlister T. J., Costerton J. W., Cheng K. J. Effect of the removal of outer cell wall layers on the actinomycin susceptibility of a gram-negative bacterium. Antimicrob Agents Chemother. 1972 May;1(5):447–449. doi: 10.1128/aac.1.5.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno S., Matsuzawa H., Nagata Y., Shibuya I., Takahashi H., Maruo B. Synthesis of protein and nucleic acid by disrupted spheroplasts of Pseudomonas schuylkilliensis. J Bacteriol. 1971 Feb;105(2):538–552. doi: 10.1128/jb.105.2.538-552.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C., Chou J. Release of surface enzymes in Enterobacteriaceae by osmotic shock. J Bacteriol. 1967 Dec;94(6):1934–1945. doi: 10.1128/jb.94.6.1934-1945.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C., Heppel L. A. The release of enzymes from Escherichia coli by osmotic shock and during the formation of spheroplasts. J Biol Chem. 1965 Sep;240(9):3685–3692. [PubMed] [Google Scholar]
- Nisman B., Pelmont J. De novo protein synthesis in vitro. Prog Nucleic Acid Res Mol Biol. 1964;3:235–297. doi: 10.1016/s0079-6603(08)60743-6. [DOI] [PubMed] [Google Scholar]
- OSBORN M. J., ROSEN S. M., ROTHFIELD L., ZELEZNICK L. D., HORECKER B. L. LIPOPOLYSACCHARIDE OF THE GRAM-NEGATIVE CELL WALL. Science. 1964 Aug 21;145(3634):783–789. doi: 10.1126/science.145.3634.783. [DOI] [PubMed] [Google Scholar]
- OSBORN M. J. STUDIES ON THE GRAM-NEGATIVE CELL WALL. I. EVIDENCE FOR THE ROLE OF 2-KETO- 3-DEOXYOCTONATE IN THE LIPOPOLYSACCHARIDE OF SALMONELLA TYPHIMURIUM. Proc Natl Acad Sci U S A. 1963 Sep;50:499–506. doi: 10.1073/pnas.50.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne J. W., Gilvarg C. Size restriction on peptide utilization in Escherichia coli. J Biol Chem. 1968 Dec 10;243(23):6291–6299. [PubMed] [Google Scholar]
- REICH E., FRANKLIN R. M., SHATKIN A. J., TATUM E. L. Effect of actinomycin D on cellular nucleic acid synthesis and virus production. Science. 1961 Aug 25;134(3478):556–557. doi: 10.1126/science.134.3478.556. [DOI] [PubMed] [Google Scholar]
- Rogers D. Osmotic pools in Escherichia coli. Science. 1968 Feb 2;159(3814):531–532. doi: 10.1126/science.159.3814.531. [DOI] [PubMed] [Google Scholar]
- Rogers D. Release of a lipopolysaccharide-protein complex from Escherichia coli A by warm-water treatment. Biochim Biophys Acta. 1971 Jan 26;230(1):72–81. doi: 10.1016/0304-4165(71)90055-9. [DOI] [PubMed] [Google Scholar]
- Rogers S. W., Gilleland H. E., Jr, Eagon R. G. Characterization of a protein-lipopolysaccharide complex released from cell walls of Pseudomonas aeruginosa by ethylenediaminetetraacetic acid. Can J Microbiol. 1969 Jul;15(7):743–748. doi: 10.1139/m69-130. [DOI] [PubMed] [Google Scholar]
- Roy A., Mitra S. Susceptibility of E. coli K-12 to actinomycin D after infection with phage M13. Nature. 1970 Oct 24;228(5269):365–366. doi: 10.1038/228365a0. [DOI] [PubMed] [Google Scholar]
- SWEENEY D. Dopamine: its occurrence in molluscan ganglia. Science. 1963 Mar 15;139(3559):1051–1051. doi: 10.1126/science.139.3559.1051. [DOI] [PubMed] [Google Scholar]
- Schnaitman C. A. Protein composition of the cell wall and cytoplasmic membrane of Escherichia coli. J Bacteriol. 1970 Nov;104(2):890–901. doi: 10.1128/jb.104.2.890-901.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiguchi M., Iida S. Mutants of Escherichia coli permeable to actinomycin. Proc Natl Acad Sci U S A. 1967 Dec;58(6):2315–2320. doi: 10.1073/pnas.58.6.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver S., Wendt L. Mechanism of action of phenethyl alcohol: breakdown of the cellular permeability barrier. J Bacteriol. 1967 Feb;93(2):560–566. doi: 10.1128/jb.93.2.560-566.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A. P., Cheng K. J., Costerton J. W., Idziak E. S., Ingram J. M. Sensitivity of normal and mutant strains of Escherichia coli to actinomycin-D. Can J Microbiol. 1972 Jun;18(6):909–915. doi: 10.1139/m72-139. [DOI] [PubMed] [Google Scholar]
- Voll M. J., Leive L. Release of lipopolysaccharide in Escherichia coli resistant to the permeability increase induced by ethylenediaminetetraacetate. J Biol Chem. 1970 Mar 10;245(5):1108–1114. [PubMed] [Google Scholar]
- WEISSBACH A., HURWITZ J. The formation of 2-keto-3-deoxyheptonic acid in extracts of Escherichia coli B. I. Identification. J Biol Chem. 1959 Apr;234(4):705–709. [PubMed] [Google Scholar]
- Wilkinson S. G. The sensitivity of pseudomonads to ethylenediaminetetra-acetic acid. J Gen Microbiol. 1967 Apr;47(1):67–76. doi: 10.1099/00221287-47-1-67. [DOI] [PubMed] [Google Scholar]
- Winshell E. B., Neu H. C. Relation of lipopolysaccharide and fatty acid ester release to the ethylenediaminetetraacetic acid alteration of permeability in enterobacteriaceae. J Bacteriol. 1970 May;102(2):537–539. doi: 10.1128/jb.102.2.537-539.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]




