Abstract
This 2011 Peripheral Nerve Society plenary lecture reviews the role of axonal transport in neuroimmune communication following peripheral nerve injury, linking focal changes in Schwann cell activation and release of the proinflammatory cytokine tumor necrosis factor-alpha (TNF-α) with subsequent activation and sensitization of ascending sensory neurons and glia which culminate in the neuropathic pain state. New data demonstrate that axonally transported (biotinylated) TNF-α activates and localizes with dorsal horn astrocytes within 96 h after injection into sciatic nerve, and that glial fibrillary acidic protein (GFAP) activation in these glial cells is diminished in TNF receptor 1 knockout mice. The pathophysiology, neuropathology and molecular biology of Wallerian degeneration are also reviewed from a perspective that links it to upregulation of proinflammatory cytokines and the development of neuropathic pain states. Finally, insights into neuroimmune communication provide rationale for new therapy based on interference with the processes of Wallerian degeneration, cytokine signaling and TNF-α protein sequestration.
Keywords: axonal transport, glia, nerve, neuropathy, pain, Schwann cell, spinal cord, TNF-α
Introduction
This plenary lecture reviews the scientific study of neuropathy, the ology of neuropathy if you will, in the context of our recent work on the cytokine mechanisms of degeneration, regeneration, and pain. The topic broaches on a unified understanding of the function of neuroinflammation in the pathophysiology, neuropathology, and molecular biology of neuropathy. We define the classical science of pathophysiology as the study of abnormal function, pathology as the study of mechanisms of dysfunction, and molecular biology as the study of the structure and function of macromolecules. Neuropathic pain, by definition, is a pain state caused by injury to components of the nervous system, and most typically manifests days or weeks after injury to peripheral nerves. It is often burning in nature with components of extreme sensitivity to mechanical and thermal stimuli. We present a perspective on neuroinflammation that links the pathology of the classical disciplines and helps in understanding the systemic consequences of focal nerve injury, which includes changes in the basic science of neurobiology and the clinical science of pain medicine. As the Peripheral Nerve Society meets this year for the first time without John Griffin, we want to emphasize the importance of cross-training and interaction between basic and clinical scientists, which was one of his most important gifts to the academic study of neuropathy, and which continues in his tradition to be the essence of our success as a scientific society.
The pathogenic role of neuroinflammation in neuropathy has gained stature as it became clear that proinflammatory cytokines were key modulators of the pathophysiology of and cross-talk between immune cells, neurons, and glia (peripheral, Schwann cells and central, microglia and astrocytes), and their environment during the processes of degeneration, regeneration, and pain (Myers et al., 2006). It has been argued that the cytokine-driven process of Wallerian degeneration is the key pathologic mechanism most closely associated with the development of significant neuropathic pain states, a supposition that has an approximately 18-year history of experimental support. Initially, it was shown that the magnitude of the Wallerian degeneration response associated with several forms of nerve injury, including the chronic constriction injury (CCI) of Bennett and Xie (1988), is related directly to the magnitude and duration of the hyperalgesic pain behavior that characterizes the neuropathic pain state (Myers et al., 1993; Sommer et al., 1993). Since proinflammatory cytokines, particularly tumor necrosis factor-alpha (TNF-α), were becoming first recognized as key regulators of macrophage activity in Wallerian degeneration, it was shown that TNF-α by itself produced a similar form of hyperalgesia when injected into nerve (Wagner and Myers, 1996a). We later established that activated Schwann cells upregulate TNF-α and that the initial action of TNF-α at the site of nerve injury begins the cascade of chemotropic and macrophage-mediated pathologic events associated with Wallerian degeneration (Myers et al., 1996; Wagner and Myers 1996b). This scenario was supported by alternative studies in which inhibition of TNF-α and other cytokines with several different drugs (as they became available) resulted in temporal modifications of Wallerian degeneration and reduction in pain (Sommer et al., 1998; 2001; Wagner et al., 1998; Üçeyler et al., 2007; 2009). Details of these effects are given in the following sections on pathophysiology, pathology, and molecular biology of cytokines in neuropathy. It should be noted that although the discussion is focused on the effects of TNF-α, other proinflammatory cytokines such as IL-1, 6, and 15 are involved. We limit discussion to TNF-α because it is considered to be the prototypical proinflammatory cytokine and because its upregulation influences and often precedes the upregulation of other cytokines.
The question remains as to how degenerative, regenerative, and pro-nociceptive events might be linked. Following nerve injury there are certainly electrophysiologic and systemic endocrine changes that are important in communicating injury and pain. Acute nerve injury produces an electrophysiologic barrage that can result in the release of neurotransmitters (e.g., histamine, serotonin, bradykinin) onto neurons and the activation of kinases that phosphorylate constitutive transcription factors, and local release of cytokines can stimulate autonomic dysfunction (Hermann and Rogers, 2008) and systemic changes in circulating IL-1 that have been shown to mimic the complex behavior known as sickness-induced hyperalgesia (Watkins and Maier, 1999). But are these forms of communication sufficient to selectively activate dorsal root ganglia (DRG) cells and central neurons whose altered function has characterized the pathophysiology and pharmacology of the neuropathic pain state, a complex pathophysiological state that typically develops days or weeks after the primary injury? We have argued, and continue to argue with new data in this report, that axonal transport of TNF-α is a critical mechanism in activating neural structures in the pain pathway. Timing is important. Following the early-phase electrophysiological response to nerve injury, intermediate-phase injury signals occurring over the next few days include target-derived factors retrogradely transported to sensory neurons (Ambron et al., 1995). Significant pathophysiologic changes in dorsal root ganglion and dorsal horn sensory neurons are instigated at this time. The signals of the intermediate-phase response are contained within the injured axoplasm and directly affect sensory neurons; injection of axoplasm from injured Aplysia nerves into uninjured sensory neurons produced the same increase in excitability as seen with axonal injury, while axoplasm from normal nerves did not have this effect (Ambron et al., 1995). We suggest that the TNF-α-controlled neuroinflammation initiated at the site of nerve injury by activated Schwann cells is a key factor in this response in that it sequentially alters neuronal function in a centripetal fashion producing both acute pain and the neuronal sensitization that is key to the development of neuropathic pain. These actions are linked by local effects of TNF-α and by retrograde axonal transport of TNF-α to sequential neural and glial structures in the pain pathway. The consequences of these actions can be modified by inhibition of TNF-α protein and/or its signaling and sequestering molecules, and, ideally, are counteracted by late-phase signals originating within the nerve during regeneration that are communicated centrally to down regulate the proinflammatory state. That is, the cytokine milieu of degenerating and regenerating axons is dynamic and carefully balanced by signaling pathways regulating the production of both pro- and anti-inflammatory cytokines, resulting in changes such as upregulation of anti-inflammatory IL-10 and suppressors of cytokine signaling (SOCS), the imbalance of which controls the local environment and perhaps an ineffective transition from degeneration to regeneration, leading to aberrant repair and/or generation of pain. This imbalance may contribute to the mechanism by which non-nociceptive, large-diameter myelinating fibers enter nociceptive circuits and contribute to the development of neuropathic pain.
The discussion that follows is not restricted to the events of Wallerian degeneration, but may be more broadly considered in terms of new findings on the role of inflammation in the pathogenesis of other painful forms of neuropathy such as diabetes (Doupis et al., 2009), and to the imbalance of pro- and anti-inflammatory cytokines seen in other painful human diseases (Üçeyler and Sommer, 2008). Perhaps the many problems of neuropathic pain can be understood in terms of a unification paradigm linking the action of proinflammatory cytokines to the pathophysiology, neuropathology, and molecular biology of neuropathy, and to interactions with the factors that modulate proinflammatory cytokine actions.
Pathophysiology
The pathophysiology of neuroinflammation is extensive, as recently reviewed in the central nervous system (CNS) by Clark et al. (2010) and in the peripheral nervous system (PNS) by Üçeyler et al. (2007). We emphasize the pathophysiologic effects of TNF-α in peripheral nerve and related sensory pathways, particularly with respect to activation of neurons and glia, changes in the vascular and endoneurial environment, recruitment of macrophages, and generation of spontaneous electrophysiologic activity in pain fibers. Table 1 outlines the range of these changes, beginning with the remarkable ability of TNF-α to activate cells, especially neurons, glia (including Schwann cells), mast cells, endothelial cells, perineurial cells, and both resident and hematogenous macrophages. It is difficult to know what may be the absolute initial event that begins the self-reinforced upregulation of TNF-α, but the release of calcium and chloride by damaged axons may cause glial and other cell activation via ion channels such as the chloride intracellular channel 1, the Ca2+/calmodulin-activated K+ channel, or the ATP-gated P2X7 purinergic receptor cation channel (Skaper, 2011). However, within minutes of nerve injury, TNF-α is upregulated in Schwann cells, endothelial cells, mast cells, and resident macrophages with immediate effects on electrophysiology and the permeability of the blood–nerve barrier. TNF-α causes aberrant spontaneous electrophysiologic activity in normally quiescent polymodal C-nociceptor fibers, and this is thought to be an important mechanism in chronic pain states (Sorkin et al., 1997) by providing constant low-grade pain signals to dorsal horn neurons processing sensory input. TNF-α also contributes to upregulation of Nav1.3 and Nav1.8 voltage-gated sodium channels in DRG neurons (shown following ventral root transection), in correspondence with upregulation of TNF receptor 1 (TNFR1) in ipsilateral DRG and spinal cord dorsal horn neurons (Xu et al., 2006); Nav1.3 has been shown to be upregulated in injured DRG neurons following sensory nerve transection (Waxman et al., 1994). Thus, these ion channel and electrophysiologic changes alone can facilitate a neuronal tendency toward sensitization in which low-grade signals generated by changes in the axonal environment are recognized and amplified in the ascending pain pathway. This effect is potentiated by a direct action of TNF-α on neurons, as shown by Schäfers et al. (2003) who reported that exogenously applied TNF-α sensitized both injured and adjacent uninjured primary sensory neurons following spinal nerve ligation (Schäfers et al., 2003).
Table 1.
Pathophysiologic effects of proinflammatory cytokines
|
TNF-α plays a significant role in vascular dysfunction (Zhang et al., 2009) by increasing the expression of matrix metalloproteinases (MMPs), particularly MMP-2 and MMP-9 in nerve (Shubayev and Myers, 2000; 2002). Since these gelatinases are the only two basal lamina-degrading proteases, they are instrumental in altering blood-nerve barrier permeability, causing edema and macrophage traversal through blood vessels and the Schwann cell basal lamina (Shubayev et al., 2006). Associated changes include the TNF-α-induced upregulation of intercellular adhesion molecule-1 (ICAM-1), vascular adhesion molecule-1 (VCAM-1), and chemoattractant signals, particularly chemokine monocyte chemoattractant protein (MCP)-1, that facilitate hematogenous macrophage migration into the endoneurial space (Yadav et al., 2010). Thus begins a significant increase in the soluble 17-kDa form of TNF-α activated, presumably, by MMP-2 from the 26-kDa transmembrane precursor (Gearing et al., 1994; Shubayev and Myers, 2000). These pathophysiological changes in the vasculature and the endoneurial environment facilitate Wallerian degeneration.
Pathology
Wallerian degeneration is the term used for the pathological process originally described by Augustus Waller in 1850 relating to his observations on the disintegration of frog nerves after axotomy (Stoll et al., 2002). It is a macrophage-driven process (Perry et al., 1987) that proceeds from the site of nerve injury distally, culminating in phagocytosis of the injured axons and associated Schwann cells, the nerve fiber (Brück, 1997). Mice with a genetic defect that limits macrophage recruitment to the injured nerves (WLDS) (Perry et al., 1990) have delayed Wallerian degeneration and reduced pain (Myers et al., 1996), a finding consistent with the observation that the expression of TNF-α is delayed in these animals (Sommer and Schäfers, 1998) and with related studies showing that macrophage content in injured nerves is partially dependent on MMP-9 (Shubayev et al., 2006). TNF-α−/− mice also have a decreased number of macrophages invading transected nerves (Liefner et al., 2000).
The pathology is striking and begins with edema associated with interruption of the perineurial layer, hypertrophy of endothelial cells, and infiltration of macrophages. As reviewed by Chaudhry et al. (1992), early axonal changes include granular disintegration of the cytoskeleton. The axolemma degenerates at the same time followed by myelin sheath interruption with formation of ovoids or myelin blebs that are cleared by macrophages along with the axonal debris. In rodents this process is usually completed within 1 month, a time course corresponding to the associated hyperalgesic pain state.
The relationship between degeneration and regeneration is incompletely understood, but it appears that a certain level of degeneration is necessary for regeneration to occur, at least in vertebrate nerves (Hilliard, 2010). Generally, the processes of degeneration and regeneration occur simultaneously, although one process can predominate depending on intrinsic or extrinsic factors. Lack of TNF-α in TNF-α−/− mice delays myelin removal but does not interfere with macrophage phagocytosis in transected nerves (Liefner et al., 2000). Immediate or delayed anti-TNF-α therapy with etanercept reduces pain in chronic constriction injured nerves, but it does not affect the extent of axonal degeneration or macrophage content (Sommer et al., 2001). However, immediate therapy with etanercept following crush injury enhances axonal regeneration (Kato et al., 2009; 2010), a finding that can also be seen by reducing TNF-α levels via inhibition of p38 mitogen-activated protein kinase (MAPK) signaling (Myers et al., 2003).
Thus, inhibition of TNF-α activity is most closely associated with the reduction of pain during Wallerian degeneration, but it can also influence the dynamics of macrophage activity and the relationship between degeneration and regeneration. Molecular therapeutic insights into cytokine mechanisms of degeneration can hopefully be exploited to help control the balance between degeneration and regeneration and mitigate the consequences of painful neuropathies.
Molecular Neurobiology
Cytokines are pervasive modulators of intercellular communication that orchestrate the delicate balance of our physiological state. The cytokine currency theory suggests that pro- and anti-inflammatory cytokines are constantly available locally and neuronally to modulate gene expression in the injured nerve, and therefore to help balance cellular signals for proliferation, differentiation, or apoptosis, and to control responses of cells during nerve degeneration and regeneration.
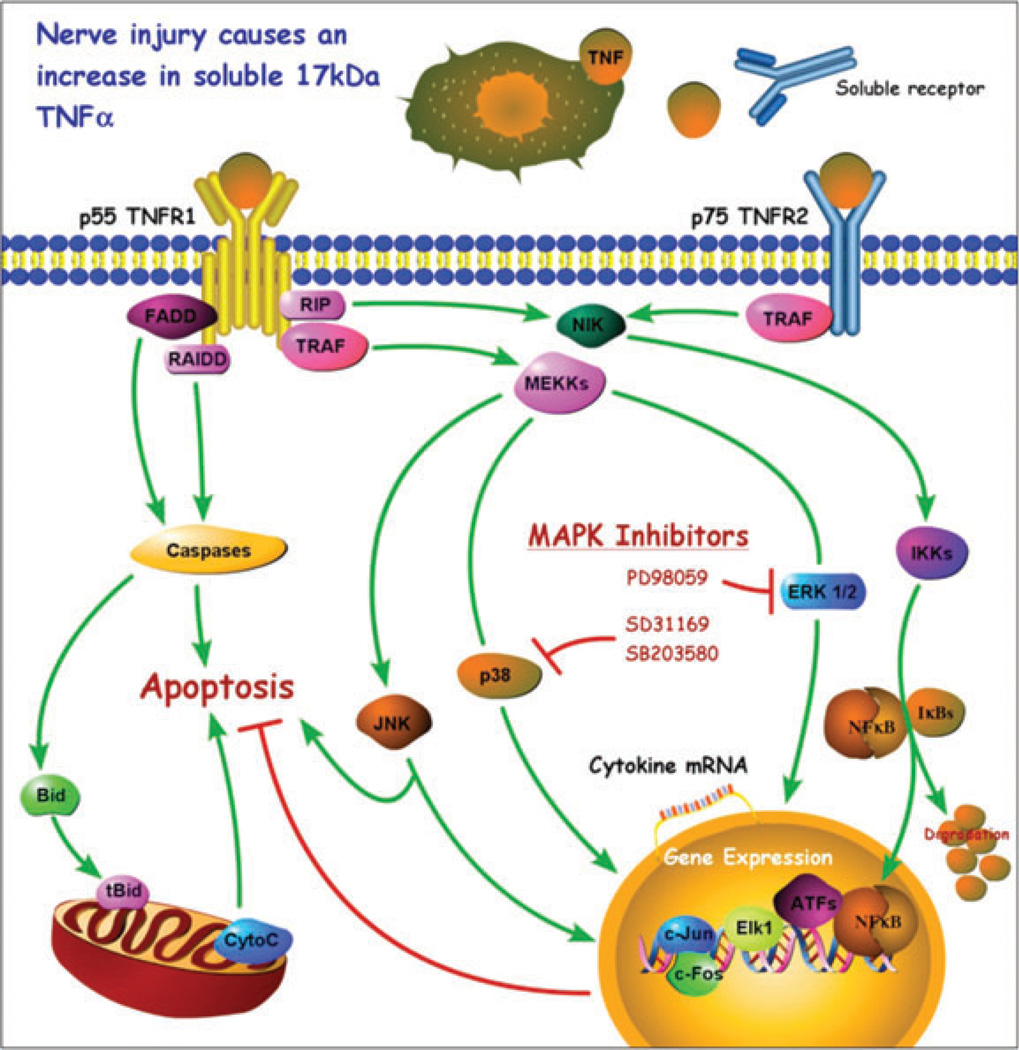
The upregulation and interaction of TNF-α with its two specific receptors (p55TNFR1 and p75TNFR2) control the pathogenesis of tissue destruction and pain. Activation of TNF-α receptors leads to phosphorylation of p38 MAPK, extracellular signal-regulated kinases (ERKs), and Jun N-terminal kinase (JNK), potentially activating NF-kB transcription pathways. TNFR1 modulates the majority of pro-inflammatory TNF-α cellular responses and is related to signal transduction pathways in which TNF-α has been characterized (Smith et al., 1994). TNFR2 has a somewhat opposite role in that it preferentially binds to a membrane-bound TNF-α form and mediates neuroprotection (Fontaine et al., 2002). Numerous studies with receptor knockout mice further refine the overlapping yet differential roles of these receptors in responding to TNF-α occupation: TNF-α can cause inflammatory demyelination and cell death on one hand and the promotion of remyelination, expression of growth factors, and neuroprotection on the other hand, depending on the balance of competing signaling factors. Figure 1 summarizes several TNF-α signaling pathways and their consequences in injured nerve. With respect to pain, it has been shown that thermal hyperalgesia requires TNFR1 while mechanical and cold allodynia depend on TNFR1 or TNFR2 (George et al., 2005; Vogel et al., 2006).
Figure 1.
A model diagram of tumor necrosis factor-alpha (TNF-α) signaling. TNF-α is enzymatically cleaved from a 26-kDa cell-surface-bound monomer by TNF-α-converting enzyme to form a 17-kDa soluble cytokine that is a target for therapeutic sequestration by soluble receptors. Both forms of TNF-α can interact with either of two distinct receptors, p55TNFR1 and p75TNFR2. Receptor-mediated effects can trigger cell activation, cytokine suppression, cytokine upregulation, or apoptosis, depending on the complex interactions between the pathways illustrated. Interactions between the p38 mitogen-activated protein kinase (MAPK) pathway and the JNK pathway are especially important. p38 inhibitors are of particular interest because of their relationship to gene expression which can result in reduction of TNF-α protein expression.
The signaling pathway p38 MAPK became of particular interest when it was shown that inflammation inhibitors affected its activity (Lee et al., 1994). p38 MAPK activation is now known to regulate cytokine expression. The p38 pathway phosphorylates and enhances the activity of many transcription factors, such as ELK-1, NF-kB, heat shock transcription factor-1, and SAP-1. p38 inhibition reduces Schwann cell TNF-α protein and increases the rate of regeneration in both crushed and transected nerve fibers (Myers et al., 2003). In spinal cord, p38 inhibition reduces TNF-α synthesis and attenuates mechanical allodynia 3 days after CCI (Xu et al., 2007). The ERK pathway is also of interest. Through this pathway, TNF-α can directly stimulate neuropeptide gene expression and further modulate its own production (Song et al., 2003). Noxious stimuli also induce ERK phosphorylation in primary sensory neurons (Dai et al., 2002), as does application of TNF-α to nerve roots (Takahashi et al., 2006). In this second study we found that phosphorylated ERK was preferentially expressed in small- and medium-sized neurons, and this may be related to the selective increase in TNF-α seen in these same sized neurons after peripheral nerve injury. A recent review of MAPK and pain provides additional insight into the complexity and interactions of cytokine signaling, particularly with respect to activation of glia and neurons in spinal cord (Ji et al., 2009). The pathologic roles of MAPK signaling pathways in human diseases is further elaborated by Kim and Choi (2010).
The attempt to interfere with TNF-α activity and MAPK pathways has highlighted the complexity of cytokine signaling, as indicated in part by our data (unpublished) demonstrating that there is proinflammatory cytokine compensation in the form of elevated IL-1 protein expression in nerve-injured animals unable to express TNF-α. That is, IL-1 protein expression was significantly upregulated 24 h following nerve crush injury in TNF-α−/− nerves relative to control animals receiving the same nerve lesion. It is not clear, however, if genetic or developmental compensation in cytokine activity results in the upregulation of IL-1 in TNF-α knockout animals, or if acutely TNF-α-inhibited animals would have the same response.
Nevertheless, experimental therapeutic interventions with cytokine signaling and particularly with TNF-α activity and availability (Tracey et al., 2008) have led directly to human use of anti-TNF-α agents for control of sciatica and low back pain. Sciatica is an important clinical problem normally treated by orthopedic/spine surgeons, while other radiculopathies not directly related to suspected vertebral disc herniation may be seen initially by orthopedists but referred to neurologists. The prevalence of sciatica is high, and in its most severe form has a rapid onset secondary to L4-5 nerve root compression by a herniated lumbar disc. The treatment consensus has been for emergent operation to relieve the compression, but recent epidemiological studies recommend a less aggressive approach if there is no strong radiographic evidence of compression, resulting in watchful waiting for months while the pain may be quite severe. It is now recognized that much of the sciatica problem is inflammatory in nature, caused not only by degeneration of compressed axons, but also by inflammatory mediators liberated from the herniated nucleus pulposus material within discs. This material contains significant concentrations of proinflammatory cytokines, especially TNF-α (Shamji et al., 2010). We showed that exogenous TNF-α applied to nerve roots mimics the pathological changes and pain caused by nucleus pulposus application (Igarashi et al., 2000), and that the pain caused by nucleus pulposus herniation and nerve root compression was additive (Omarker and Myers, 1998).
Thus, there can be a range in pain severity related to the degree of inflammation and compression of nerve roots. It may be difficult for the clinician to decide if surgery will bring rapid relief because surgery alone often does not provide relief, even combined with traditional pain medications. Fortunately, sciatica tends to resolve over a 6-month period secondary to natural resorptive processes, but pain may be persistent during this time. In this context, it may be advisable to use the interventional technique of epidural steroid injection if oral pain medications do not provide sufficient relief. Although there is persistent controversy about the effectiveness of paravertebral steroid injections, the American Pain Society has found that caudal epidural injection of corticosteroids can provide some relief for radiculopathy (Manchikanti et al., 2010). Steroid injections are often combined with local anesthetics, which have their own anti-inflammatory properties (Deruddre et al., 2010), and this can also be useful diagnostically in locating the pain generators. We have supported the thinking that if steroids are useful in reducing local inflammation and pain in radiculopathy, then targeted anti-cytokine therapies would be better. To this end, randomized, double-blind, placebo-controlled human studies using transforaminal epidural etanercept for the treatment of sciatica (Cohen et al., 2009) have been initiated, and there are promising early results.
The search for successful anti-cytokine biologics including p38 MAPK inhibitors is intense (Karcher and Laufer, 2009), and there is hope that insight into the molecular biology of neuroinflammation will provide new targets for therapeutic intervention (Myers et al., 2006).
Axonal Transport in Neuro-Immune Communication
We are left to consider how the initial local increase of TNF-α at the site of nerve injury by activated Schwann cells might give rise to, or link, the sequence of inflammatory events that can culminate in neuropathic pain states. We have previously reported that CCI induces the release of a soluble, active 17 kDa form of TNF-α at the injury site that can be detected in mid-axonal nerve segments as a migrating signal, and in corresponding DRG (Shubayev and Myers, 2001). A 14-kDa TNF-α form is also present exclusively in lesioned axonal preparations, suggesting that this form may be specific for injury-induced axonal transport. Other molecular forms of TNF-α seen in injured and control nerves may function as a supply for the active 17-kDa form. We also observed that exogenous biotinylated TNF-α tracer colocalized with TNFR1 and TNFR2 along the nerve trunk and in DRG.
In DRG neurons, TNF-α, TNFR1, and TNFR2 are greatly increased between days 3 and 5 post-CCI. Using a TNF-α tracer injected into nerve, we found that it was retrogradely transported in axoplasm to DRG neurons within 6 h and to nuclei of small dorsal horn and motor neurons and adjacent cells within 96 h (Shubayev and Myers, 2002). We now report ipsilateral activation of spinal cord dorsal horn astrocytes and localization of biotinylated TNF-α tracer in those astrocytes 96 h after injection of tracer into rat sciatic nerve (Figs. 2 and 3), based on the methodology reported in our other axonal transport studies (Shubayev and Myers, 2001; 2002). Activation is taken to mean expression of glial fibrillary acidic protein (GFAP), a useful marker because of its correlation with astroglial hypertrophy and release of inflammatory cytokines and chemokines. Others have reported activated spinal glia following nerve injury, and it is widely recognized that activated glia contribute to neuropathic pain processing by releasing a number of cytokine and chemokine molecules (Milligan and Watkins, 2009; Gao and Ji, 2010). Gao et al. (2010) suggest that MCP-1 may be the factor expressed by astrocytes that is responsible for producing a persistent pain state. They injected astrocytes briefly activated by TNF-α in culture into the lumbar intrathecal space of mice. This produced a substantial mechanical allodynia. The allodynia could be prevented by pretreating the astrocytes with an inhibitor of JNK, or by an MCP-1-neutralizing antibody after they were injected. Thorough washing of the astrocytes after activation to reduce surface TNF-α did not affect their ability to release MCP-1 or cause pain after being injected into the intrathecal space.
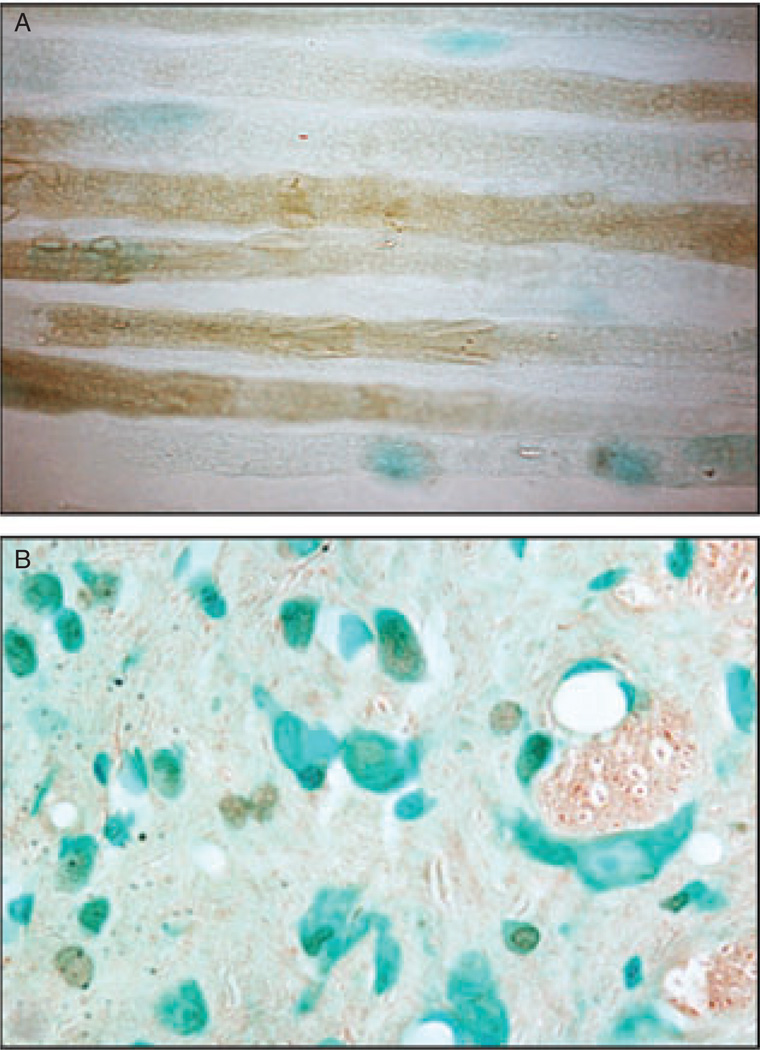
Figure 2.
Biotinylated tumor necrosis factor-alpha (TNF-α) was injected at the CCI injury site and detected 1 h later in axons midway between the CCI injury and DRG in rat sciatic nerve using avidin-biotin-peroxidase (brown) (A) and in the spinal cord dorsal horn ipsilateral to CCI injury 96 h after intra-sciatic injection (B). Methylene blue (blue) is used as counter-stain. Magnification 1200×.
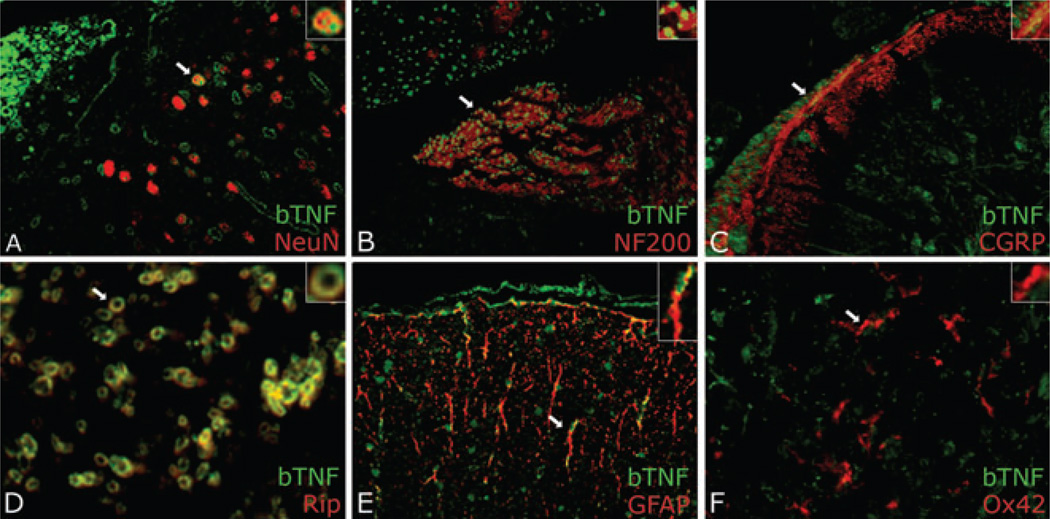
Figure 3.
Biotinylated tumor necrosis factor-alpha (TNF-α) detected using neutravidin-fluorescein isothiocyanate (FITC) (green) in the spinal cord dorsal horn ipsilateral to sciatic CCI nerve injury 96 h after intra-sciatic injection. Sections were dual-labeled with neuronal marker NeuN (A) for large neurons, NF200 (B) for small neurons, calcitonin gene-related peptide (CGRP) (C) for CGRP+ fibers, Rip (D) for oligodendrocytes, glial fibrillary acidic protein (GFAP) (E) for astrocytes, or Ox42 (F) for microglia. TNF-α colocalized (yellow) with all markers except Ox42. Magnification 1200× (A, C, D) or 600× (B, E, F).
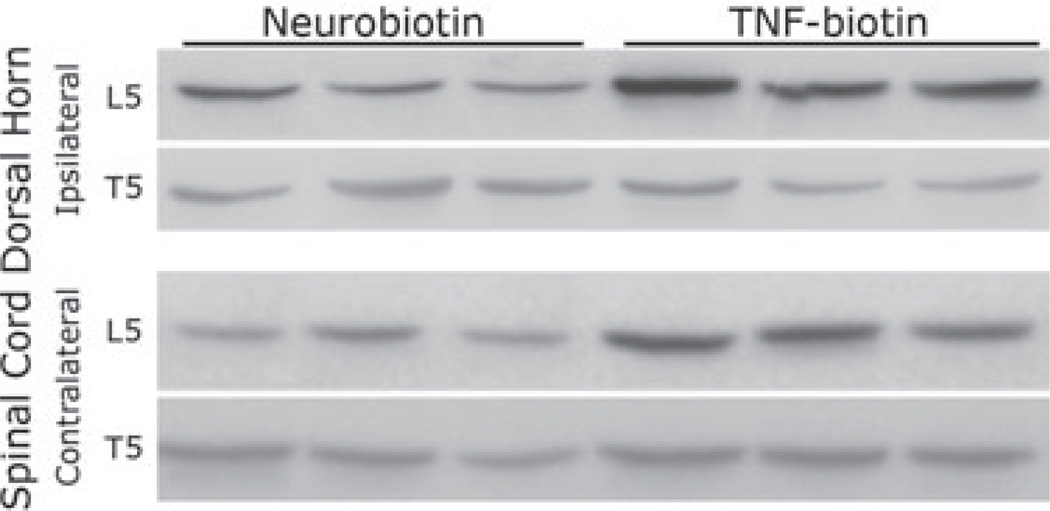
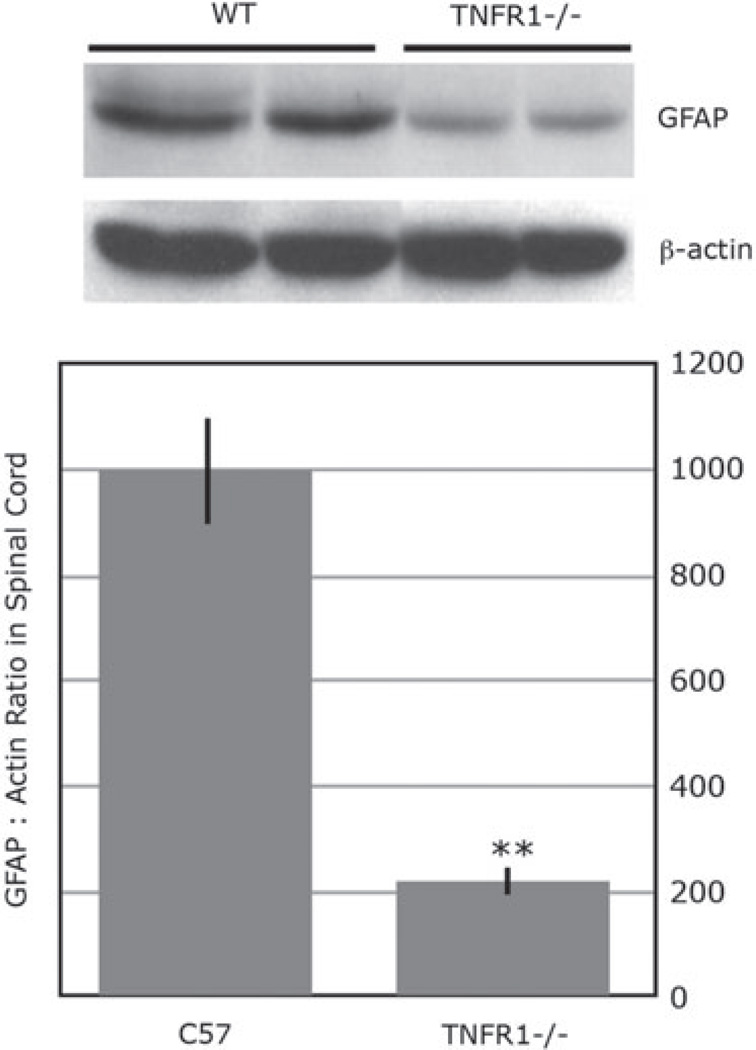
We suggest that axoplasmic-transported TNF-α causes glial activation. Our studies (Fig. 3) show axonally transported biotinylated TNF-α from CCI injury site, colocalizing with astrocytes, second-order sensory dorsal horn neurons, and oligodendrocytes, but not microglia, 96 h after injection of the tracer into rat sciatic nerve. Using Western blotting of GFAP (Fig. 4), we observed a strong positive signal in the ipsilateral L5 dorsal horn after TNF-α-biotin but not control neurobiotin injection, and not contralateral L5 or control T5 dorsal horn. Of specific interest is that GFAP activation observed in L5 dorsal horn 5 days after sciatic nerve crush is significantly diminished in TNFR1-deficient mice (Fig. 5).
Figure 4.
Western blotting for glial fibrillary acidic protein (GFAP) (40 kDa) in ipsilateral and contralateral spinal cord dorsal horn segmental level lumbar L5 and thoracic T5 (for control) tissue 96 h after biotinylated tumor necrosis factor-alpha (TNF-α) or control Neurobiotin™ injection into the sciatic nerve CCI injury site. N of 3/group is presented.
Figure 5.
Western blotting for glial fibrillary acidic protein (GFAP) (40 kDa) in ipsilateral L5 dorsal horn spinal cord 5 days after sciatic nerve crush in C57BL/6 wild-type (WT) and TNFR1 knockout (−/−) mice. The graph represents the mean GFAP to −actin ratios ± SE of n = 4/group (**, p ≤ 0.01).
Conclusion
Peripheral nerve injury can result in neuropathic pain, an incompletely understood pain state involving the entire sensory neuroaxis. Neuropathic pain is typically refractory to standard therapeutic interventions and may be at times unbearable. The condition is more likely to arise following axonal injury and Wallerian degeneration, but any neuropathologic process involving significant neuroinflammation is suspect.
We have reviewed the role of neuroinflammation in Wallerian degeneration, focusing particularly on TNF-α liberated by Schwann cells at the site of nerve injury and its effect on the pathophysiology, neuropathology, molecular biology, and pain of the neuropathy. We conclude that axonal transport of TNF-α from the site of injury to ascending neurons and glia in the sensory pathway is a key factor in sensitizing the nervous system to afferent input, changing the pharmacology of sensory neurons and facilitating neuropathic pain. TNF-α by itself is painful, and strategies to interfere with its transport, production, and availability to occupy its receptors may be effective as new therapy.
Our discussion is purposefully focused to make the point that integrative mechanisms linking the detailed observations within the many scientific disciplines operative in neuropathy are of value in promoting interactions and discussions between basic and clinical scientists.
References
- Ambron RT, Dulin MF, Zhang XP, Schmied R, Walters ET. Axoplasm enriched in a protein mobilized by nerve injury induces memory-like alterations in Aplysia neurons. J Neurosci. 1995;15:3440–3446. doi: 10.1523/JNEUROSCI.15-05-03440.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- Brück W. The role of macrophages in Wallerian degeneration. Brain Pathol. 1997;7:741–752. doi: 10.1111/j.1750-3639.1997.tb01060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry V, Glass JD, Griffin JW. Wallerian degeneration in peripheral nerve disease. Neurol Clin. 1992;10:613–627. [PubMed] [Google Scholar]
- Clark IA, Alleva LM, Vissel B. The roles of TNF in brain dysfunction and disease. Pharmacol Ther. 2010;128:519–548. doi: 10.1016/j.pharmthera.2010.08.007. [DOI] [PubMed] [Google Scholar]
- Cohen SP, Bogduk N, Dragovich A, Buckenmaier CC, III, Griffith S, Kuihara C, Raymond J, Richter PJ, Williams N, Yaksh TL. Randomized, double-blind, placebo-controlled, dose-response, and preclinical safety study of transforaminal epidural etanercept for the treatment of sciatica. Anesthesiology. 2009;110:1116–1126. doi: 10.1097/ALN.0b013e3181a05aa0. [DOI] [PubMed] [Google Scholar]
- Dai Y, Iwata K, Fukuoka T, Kondo E, Tokunaga A, Yamanaka H, Tachibana T, Liu Y, Noguchi K. Phosphorylation of extracellular signal-regulated kinase in primary afferent neurons by noxious stimuli and its involvement in peripheral sensitization. J Neurosci. 2002;22:7737–7745. doi: 10.1523/JNEUROSCI.22-17-07737.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deruddre S, Combettes E, Estebe JP, Duranteau J, Benhamou D, Beloeil H, Mazoit JX. Effects of a bupivacaine nerve block on the axonal transport of tumor necrosis factor-alpha (TNF-alpha) in a ratmodel of carrageenan-induced inflammation. Brain Behav Immun. 2010;24:652–659. doi: 10.1016/j.bbi.2010.01.013. [DOI] [PubMed] [Google Scholar]
- Doupis J, Lyons TE, Wu S, Gnardellis C, Dinh T, Veves A. Microvascular reactivity and inflammatory cytokines in painful and painless peripheral diabetic neuropathy. J Clin Endocrinol Metab. 2009;94:2157–2163. doi: 10.1210/jc.2008-2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontaine V, Mohand-Said S, Hanoteau N, Fuchs C, Pfizenmaier K, Eisel U. Neurodegenerative and neuroprotective effects of tumor necrosis factor (TNF) in retinal ischemia: opposite roles of TNF receptor 1 and TNF receptor 2. J Neurosci. 2002;22:RC216. doi: 10.1523/JNEUROSCI.22-07-j0001.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao YJ, Ji RR. Targeting astrocyte signaling for chronic pain. Neurotherapeutics. 2010;7:482–493. doi: 10.1016/j.nurt.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao YJ, Zhang L, Ji RR. Spinal injection of TNF-alpha-activated astrocytes produces persistent pain symptom mechanical allodynia by releasing monocyte chemoattractant protein-1. Glia. 2010;58:1871–1880. doi: 10.1002/glia.21056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gearing AJ, Beckett P, Christodoulou M, Churchill M, Clements J, Davidson AH, Drummond AH, Galloway WA, Giulbert R, Gordon JL, Leber TM, Mangan M, Miller K, Nayee P, Owen K, Patel S, Thomas W, Wells G, Wood LM, Wolley K. Processing of tumour necrosis factor-alpha precursor by metalloproteinases. Nature. 1994;370:555–557. doi: 10.1038/370555a0. [DOI] [PubMed] [Google Scholar]
- George A, Buehl A, Sommer C. Tumor necrosis factor receptor 1 and 2 proteins are differentially regulated during Wallerian degeneration of mouse sciatic nerve. Exp Neurol. 2005;192:163–166. doi: 10.1016/j.expneurol.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Girolami HI, Bouhy D, Haber M, Johnson H, David S. Differential expression and potential role of SOCS1 and SOCS3 in Wallerian degeneration in injured peripheral nerve. Exp Neurol. 2010;223:173–182. doi: 10.1016/j.expneurol.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann GE, Rogers RC. TNFalpha: a trigger of autonomic dysfunction. Neuroscientist. 2008;14:53–67. doi: 10.1177/1073858407305725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilliard MA. Axonal degeneration and regeneration: a mechanistic tug-of-war. J Neurochem. 2009;108:23–32. doi: 10.1111/j.1471-4159.2008.05754.x. [DOI] [PubMed] [Google Scholar]
- Igarashi T, Kikuchi S, Shubayev V, Myers RR. 2000 Volvo Award winner in basic science studies: exogenous tumor necrosis factor-alpha mimics nucleus pulposus-induced neuropathology. Molecular, histologic, and behavioral comparisons in rats. Spine. 2000;25:2975–2980. doi: 10.1097/00007632-200012010-00003. [DOI] [PubMed] [Google Scholar]
- Ji RR, Gereau RW, IV, Malcangio M, Strichartz GR. MAP kinase and pain. Brain Res Rev. 2009;60:135–148. doi: 10.1016/j.brainresrev.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karcher SC, Laufer SA. Successful structure-based design of recent p38 MAP kinase inhibitors. Curr Top Med Chem. 2009;9:655–676. doi: 10.2174/156802609789007363. [DOI] [PubMed] [Google Scholar]
- Kato K, Kikuchi S, Shubayev VI, Myers RR. Distribution and tumor necrosis factor-alpha isoform binding specificity of locally administered etanercept into injured and uninjured rat sciatic nerve. Neuroscience. 2009;160:492–500. doi: 10.1016/j.neuroscience.2009.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K, Liu H, Kikuchi S, Myers RR, Shubayev VI. Immediate anti-tumor necrosis factor-alpha (etanercept) therapy enhances axonal regeneration after sciatic nerve crush. J Neurosci Res. 2010;88:360–368. doi: 10.1002/jnr.22202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EK, Choi EJ. Pathological roles of MAPK signaling pathways in human diseases. Biochim Biophys Acta. 2010;1802:396–405. doi: 10.1016/j.bbadis.2009.12.009. [DOI] [PubMed] [Google Scholar]
- Lee JC, Laydon JT, McDonnell PC, Gallagher TF, Kumar S, Green D, McNulty D, Blumenthal MJ, Keys JR, Vatter SWL, Strickler JE, McLaughlin MM, Siemens IR, Fisher SM, Livi GP, White JR, Adams JL, Young PR. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature. 1994;372:739–746. doi: 10.1038/372739a0. [DOI] [PubMed] [Google Scholar]
- Liefner M, Siebert H, Sachse T, Michel U, Kollias G, Brück W. The role of TNF-alpha during Wallerian degeneration. J Neuroimmunol. 2000;108:147–152. doi: 10.1016/s0165-5728(00)00262-9. [DOI] [PubMed] [Google Scholar]
- Manchikanti L, Datta S, Gupta S, Munglani R, Bryce DA, Ward SP, Benyamin RM, Sharma ML, Helm S, II, Fellows B, Hirsch JA. A critical review of the American Pain Society clinical practice guidelines for interventional techniques: part 2. Therapeutic interventions. Pain Physician. 2010;13:E215–E264. [PubMed] [Google Scholar]
- Milligan ED, Watkins LR. Pathological and protective roles of glia in chronic pain. Nat Rev Neurosci. 2009;10:23–36. doi: 10.1038/nrn2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers RR, Yamamoto T, Yaksh TL, Powell HC. The role of focal nerve ischemia andWallerian degeneration in peripheral nerve injury producing hyperesthesia. Anesthesiology. 1993;78:308–316. doi: 10.1097/00000542-199302000-00015. [DOI] [PubMed] [Google Scholar]
- Myers RR, Heckman HM, Rodriguez M. Reduced hyperalgesia in nerve-injured WLDmice: relationship to nerve fiber phagocytosis, axonal degeneration and regeneration in normal mice. Exp Neurol. 1996;141:94–101. doi: 10.1006/exnr.1996.0142. [DOI] [PubMed] [Google Scholar]
- Myers RR, Sekiguchi Y, Kikuchi S, Scott B, Medicherla S, Protter A, Campana WM. Inhibition of p38 MAP kinase activity enhances axonal regeneration. Exp Neurol. 2003;184:606–614. doi: 10.1016/S0014-4886(03)00297-8. [DOI] [PubMed] [Google Scholar]
- Myers RR, Campana WM, Shubayev VI. The role of neuroinflammation in neuropathic pain: mechanisms and therapeutic targets. Drug Discov Today. 2006;11:8–20. doi: 10.1016/S1359-6446(05)03637-8. [DOI] [PubMed] [Google Scholar]
- Omarker K, Myers RR. Pathogenesis of sciatic pain: role of herniated nucleus pulposus and deformation of spinal nerve root and dorsal root ganglion. Pain. 1998;78:99–105. doi: 10.1016/S0304-3959(98)00119-5. [DOI] [PubMed] [Google Scholar]
- Perry VH, Brown MC, Gordon S. The macrophage response to central and peripheral nerve injury. A possible role for macrophages in regeneration. J Exp Med. 1987;165:1218–1223. doi: 10.1084/jem.165.4.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry VH, Brown MC, Lunn ER, Tree P, Gordon S. Evidence that very slow Wallerian degeneration in C57BL/Ola mice is an intrinsic property of the peripheral nerve. Eur J Neurosci. 1990;2:802–808. doi: 10.1111/j.1460-9568.1990.tb00472.x. [DOI] [PubMed] [Google Scholar]
- Schäfers M, Lee DH, Brors D, Yaksh TL, Sorkin LS. Increased sensitivity of injured and adjacent uninjured rat primary sensory neurons to exogenous tumor necrosis factor-alpha after spinal nerve ligation. J Neurosci. 2003;23:3028–3038. doi: 10.1523/JNEUROSCI.23-07-03028.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamji MF, Setton LA, Jarvis W, So S, Chen J, Jing L, Bullock R, Isaacs RE, Brown C, Richardson WJ. Proinflammatory cytokine expression profile in degenerated and herniated human intervertebral disc tissues. Arthritis Rheum. 2010;62:1974–1982. doi: 10.1002/art.27444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shubayev VI, Myers RR. Upregulation and interaction of TNFalpha and gelatinases A and B in painful peripheral nerve injury. Brain Res. 2000;855:83–89. doi: 10.1016/s0006-8993(99)02321-5. [DOI] [PubMed] [Google Scholar]
- Shubayev VI, Myers RR. Axonal transport of TNF-alpha in painful neuropathy: distribution of ligand tracer and TNF receptors. J Neuroimmunol. 2001;114:48–56. doi: 10.1016/s0165-5728(00)00453-7. [DOI] [PubMed] [Google Scholar]
- Shubayev VI, Myers RR. Anterograde TNF alpha transport from rat dorsal root ganglion to spinal cord and injured sciatic nerve. Neurosci Lett. 2002;320:99–101. doi: 10.1016/s0304-3940(02)00010-1. [DOI] [PubMed] [Google Scholar]
- Shubayev VI, Angert M, Dolkas J, Campana WM, Palenscar K, Myers RR. TNFalpha-induced MMP-9 promotes macrophage recruitment into injured peripheral nerve. Mol Cell Neurosci. 2006;31:407–415. doi: 10.1016/j.mcn.2005.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaper SD. Ion channels on microglia: therapeutic targets for neuroprortection. CNS Neurol Disord Drug Targets. 2011;10:44–56. doi: 10.2174/187152711794488638. [DOI] [PubMed] [Google Scholar]
- Smith CA, Farrah T, Goodwin RG. The TNF receptor superfamily of cellular and viral proteins: activation, costimulation and death. Cell. 1994;76:959–962. doi: 10.1016/0092-8674(94)90372-7. [DOI] [PubMed] [Google Scholar]
- Sommer C, Schäfers M. Painful mononeuropathy in C57BL/Wld mice with delayed Wallerian degeneration: differential effects of cytokine production and nerve regeneration on thermal and mechanical hypersensitivity. Brain Res. 1998;784:154–162. doi: 10.1016/s0006-8993(97)01327-9. [DOI] [PubMed] [Google Scholar]
- Sommer C, Galbraith JA, Heckman HM, Myers RR. Pathology of experimental compression neuropathy producing hyperesthesia. J Neuropathol Exp Neurol. 1993;52:223–233. doi: 10.1097/00005072-199305000-00006. [DOI] [PubMed] [Google Scholar]
- Sommer C, Marziniak M, Myers RR. The effect of thalidomide treatment on vascular pathology and hyperalgesia caused by chronic constriction injury of rat nerve. Pain. 1998;74:83–92. doi: 10.1016/S0304-3959(97)00154-1. [DOI] [PubMed] [Google Scholar]
- Sommer C, Schäfers M, Marziniak M, Toyka KV. Etanercept reduces hyperalgesia in experimental painful neuropathy. J Peripher Nerv Syst. 2001;6:67–72. doi: 10.1046/j.1529-8027.2001.01010.x. [DOI] [PubMed] [Google Scholar]
- Song CH, Lee JS, Lee SH, Lim K, Kim HJ, Park JK, Palk TH, Jo EK. Role of mitogen-activated protein kinase pathways in the production of tumor necrosis factor-alpha, interleukin-10, and monocyte chrmotactic protein-1 by Mycobacterium tuberculosis H37Rv-infected human monocytes. J Clin Immunol. 2003;23:194–201. doi: 10.1023/a:1023309928879. [DOI] [PubMed] [Google Scholar]
- Sorkin LS, Xiao WH, Wagner R, Myers RR. Tumor necrosis factor-alpha induces ectopic activity in nociceptive primary afferent fibers. Neuroscience. 1997;81:255–262. doi: 10.1016/s0306-4522(97)00147-4. [DOI] [PubMed] [Google Scholar]
- Stoll G, Jander S, Myers RR. Degeneration and regeneration of the peripheral nervous system: from Augustus Waller’s observations to neuroinflammation. J Peripher Nerv Syst. 2002;7:13–27. doi: 10.1046/j.1529-8027.2002.02002.x. [DOI] [PubMed] [Google Scholar]
- Takahashi N, Kikuchi S, Shubayev VI, Campana WM, Myers RR. TNF-alpha and phosphorylation of ERK in DRG and spinal cord: insights into mechanisms of sciatica. Spine. 2006;31:523–529. doi: 10.1097/01.brs.0000201305.01522.17. [DOI] [PubMed] [Google Scholar]
- Tracey D, Klareskog L, Sasso EH, Salfeld JG, Tak PP. Tumor necrosis factor antagonist mechanisms of action: a comprehensive review. Pharmacol Ther. 2008;117:244–279. doi: 10.1016/j.pharmthera.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Üçeyler N, Sommer C. Cytokine regulation in animal models of neuropathic pain and in human diseases. Neurosci Lett. 2008;437:194–198. doi: 10.1016/j.neulet.2008.03.050. [DOI] [PubMed] [Google Scholar]
- Üçeyler N, Tscharke A, Sommer C. Early cytokine expression in mouse sciatic nerve after chronic constriction nerve injury depends on calpain. Brain Behav Immun. 2007;21:553–560. doi: 10.1016/j.bbi.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Üçeyler N, Schäfers M, Sommer C. Mode of action of cytokines on nociceptive neurons. Exp Brain Res. 2009;196:67–78. doi: 10.1007/s00221-009-1755-z. [DOI] [PubMed] [Google Scholar]
- Vogel C, Stallforth S, Sommer C. Altered pain behavior and regeneration after nerve injury in TNF receptor deficient mice. J Peripher Nerv Syst. 2006;11:294–303. doi: 10.1111/j.1529-8027.2006.00101.x. [DOI] [PubMed] [Google Scholar]
- Wagner R, Myers RR. Endoneurial injection of TNF-alpha produces neuropathic pain behaviors. Neuroreport. 1996a;7:2897–2901. doi: 10.1097/00001756-199611250-00018. [DOI] [PubMed] [Google Scholar]
- Wagner R, Myers RR. Schwann cells produce tumor necrosis factor alpha: expression in injured and non-injured nerves. Neuroscience. 1996b;73:625–629. doi: 10.1016/0306-4522(96)00127-3. [DOI] [PubMed] [Google Scholar]
- Wagner R, Janjigian M, Myers RR. Anti-inflammatory interleukin-10 therapy in CCI neuropathy decreases thermal hyperalgesia, macrophage recruitment, and endoneurial TNF-alpha expression. Pain. 1998;74:35–42. doi: 10.1016/S0304-3959(97)00148-6. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Maier SF. Implications of immune-to-brain communication for sickness and pain. Proc Natl Acad Sci U S A. 1999;96:7710–7713. doi: 10.1073/pnas.96.14.7710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman SG, Kocsis JD, Black JA. Type III sodium channel mRNA is expressed in embryonic but not adult spinal sensory neurons, and is reexpressed following axotomy. J Neurophysiol. 1994;72:466–470. doi: 10.1152/jn.1994.72.1.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu JT, Xin WJ, Zang Y, Wu CY, Liu XG. The role of tumor necrosis factor-alpha in the neuropathic pain induced by lumbar 5 ventral root transection in rat. Pain. 2006;123:306–321. doi: 10.1016/j.pain.2006.03.011. [DOI] [PubMed] [Google Scholar]
- Xu L, Huang Y, Yu X, Yue J, Yang N, Zuo P. The influence of p38 mitogen-activated protein kinase inhibitor on synthesis of inflammatory cytokine tumor necrosis factor alpha in spinal cord or rats with chronic constriction injury. Anesth Analg. 2007;105:1838–1844. doi: 10.1213/01.ane.0000287660.29297.7b. [DOI] [PubMed] [Google Scholar]
- Yadav A, Saini V, Arora S. MCP-1: chemoattractant with a role beyond immunity: a review. Clin Chim Acta. 2010;411:1570–1579. doi: 10.1016/j.cca.2010.07.006. [DOI] [PubMed] [Google Scholar]
- Zhang H, Park Y, Wu J, Chen X, Lee S, Yang J, Dellsperger KC, Zhang C. Role of TNF-alpha in vascular dysfunction. Clin Sci (Lond) 2009;116:219–230. doi: 10.1042/CS20080196. [DOI] [PMC free article] [PubMed] [Google Scholar]