Abstract
Age at epilepsy onset has a broad impact on brain plasticity and epilepsy pathomechanisms. Prolonged febrile seizures in early childhood (FS) constitute an initial precipitating insult (IPI) commonly associated with mesial temporal lobe epilepsy (MTLE). FS-MTLE patients may have early disease onset, i.e. just after the IPI, in early childhood, or late-onset, ranging from mid-adolescence to early adult life. The mechanisms governing early (E) or late (L) disease onset are largely unknown. In order to unveil the molecular pathways underlying E and L subtypes of FS-MTLE we investigated global gene expression in hippocampal CA3 explants of FS-MTLE patients submitted to hippocampectomy. Gene coexpression networks (GCNs) were obtained for the E and L patient groups. A network-based approach for GCN analysis was employed allowing: i) the visualization and analysis of differentially expressed (DE) and complete (CO) - all valid GO annotated transcripts - GCNs for the E and L groups; ii) the study of interactions between all the system’s constituents based on community detection and coarse-grained community structure methods. We found that the E-DE communities with strongest connection weights harbor highly connected genes mainly related to neural excitability and febrile seizures, whereas in L-DE communities these genes are not only involved in network excitability but also playing roles in other epilepsy-related processes. Inversely, in E-CO the strongly connected communities are related to compensatory pathways (seizure inhibition, neuronal survival and responses to stress conditions) while in L-CO these communities harbor several genes related to pro-epileptic effects, seizure-related mechanisms and vulnerability to epilepsy. These results fit the concept, based on fMRI and behavioral studies, that early onset epilepsies, although impacting more severely the hippocampus, are associated to compensatory mechanisms, while in late MTLE development the brain is less able to generate adaptive mechanisms, what has implications for epilepsy management and drug discovery.
Introduction
Mesial temporal lobe epilepsy with childhood febrile seizures (FS-MTLE) is a distinctive entity that can be delineated from afebrile MTLE as demonstrated by epidemiological [1], radiological [2], and genomic [3, 4] studies. The age at onset in FS-MTLE is trimodal, with peaks at early childhood, adolescence, and early adult-life [5]. The age of seizure onset exerts a relevant impact on brain activity and connectivity because epilepsy-associated processes interfere with normal brain developmental changes, as evidenced by fMRI and network (graph theory) computational studies of brain connectivity [6, 7, 8]. These studies show that late-onset MTLE causes more pronounced neuronal network alterations (whole-brain properties), since the mature brain has a diminished capacity to generate adaptive responses to epilepsy effects, whereas in early-onset MTLE several compensatory mechanisms are activated in the more plastic younger brain. On the other hand, early MTLE onset is associated to a more severe functional abnormality in the ictal hippocampus (local alteration) [8], what is in agreement with the inverse correlation between age of seizure onset and severity of mesial temporal sclerosis [9]. About 40% of the patients with FS-MTLE develop refractory epilepsy [10] and early onset of seizures is a predictive factor for pharmacoresistancy [11, 12].
Prolonged febrile seizures (FS) and febrile status epilepticus (FSE) in early childhood have long been associated to a higher risk of temporal lobe epilepsy and mesial temporal sclerosis, but a causal relationship was just recently established, based on epidemiological and imaging investigations, as well as on studies with animal models (reviewed in [13]). FS and FSE can cause hippocampal injury due to the interplay between inflammation and fever: fever increases neuronal firing and causes the overexpression of inflammatory molecules (IL-1β, TNF-α, HMGB1), leading to neuronal injury, neuronal excitability and epileptogenesis [14, 15, 16]. Studies in animal models showed that a single episode of neonatal seizure permanently alters glutamatergic synapses [17]. In fact, initial precipitating injuries, such as complex febrile seizures, are potent inducers of epigenetic alterations that modify brain functioning [18]. It was shown that DNA methylation is an early event triggered by FSE that may persists late in the epileptic hippocampus, leading to permanent changes in gene expression [19].
The predisposition to developing temporal lobe epilepsy and hippocampal sclerosis has been investigated in animal models of FS induced by hyperthermia and in prospective clinical studies of children with FSE. Altogether, these studies revealed that FS development and subsequent epilepsy results from a combination of environmental and genetic factors that vary in each individual [13, 20, 21]. In rodent models of FS-like seizures induced by hyperthermia, a quite regular latency period between the initial insult and the development of recurrent seizures is always observed [22], but in human FS-MTLE the age at onset varies from early childhood to adult life [5, 1]. The mechanisms governing early (E) or late (L) disease onset in FS-MTLE are largely unknown but their unraveling is crucial, since the latent period could be a therapeutic window for developing antiepileptogenic drugs [23].
In order to look into the molecular mechanisms leading to early or late FS-MTLE onset, we have decided to investigate comparatively the hippocampal CA3 transcriptional profile of a group of FS-MTLE patients where all individuals had their IPI before 4 years of age but who developed MTLE in early childhood or in mid-adolescence and adult life. Our rationale was based on the evidences that FS-induced epigenetic changes produce lasting effects on gene expression in human hippocampus, and in the tenets of network medicine: i) genes do not operate in isolation but as components of complex networks [24, 25, 26]; ii) genomic interaction data is basically composed of pairwise relationships among transcriptional modules (network communities [27]); iii) complex diseases rarely derive from alterations in a single gene but, on the contrary, reflect perturbations in cell’s genomic and protein-protein interaction networks, often caused by environmental factors [28, 29, 30]. Therefore, we sought to find out how FS, an initial precipitating insult of environmental origin, differentially impacted hippocampal CA3 gene coexpression networks of FS-MTLE patients with early or late disease onset. Conceivably, different precipitating insult effects on disease onset could arise from pre-existent CA3 gene network differences between E and L patients, due to inherited genetic differences, or stem from different network adaptations to the insult, also influenced by allelic differences among individuals. In order to investigate this issue, gene expression data was analyzed using network science parameters, i.e. with emphasis in complex network visualization, gene hierarchy categorization, community detection and coarse-grained community structure [4, 26, 31, 32].
Material and Methods
Patients
Ethics Statement
The patients with refractory MTLE and febrile IPI included in this study were selected through the CInAPCe-FAPESP Program (www.fapesp.br/en/; www.cinapce.org.br). This research has been approved by the research ethics committees of Hospital das Clínicas da FMUSP and of Hospital Albert Einstein under numbers 251/05 and CAEE 0122.0.028.174.05 respectively. A written informed consent was obtained from all patients.
Refractory epilepsy cases were defined as those who have not gained seizure control after treatment with three or more anticonvulsant drugs. In the last 3–4 years before surgery, seizure control was attempted with carbamazepine, oxicarbazepine, phenobarbital, clobazam, topiramate, and lamotrigine, in different drug combinations. The patients were submitted to clinical, electrophysiological, neuropsychological and neuroimaging evaluations before surgery. All patients included in this study (Table 1) had prolonged febrile seizures as the IPI at or before the age of 4 years. Early onset patients were those who developed the disease soon after the IPI, whereas the late onset patients developed the disease after ≥13 years old. In the present investigation we compared global gene expression profiles of CA3 explants obtained at surgery room from seven early-onset (group E) and seven late-onset RMTLE patients (group L) submitted to corticoamigdalohippocampectomy. E and L groups had the same gender composition: three males and four females. Hippocampal hypersignal was observed in T2-weighted MRimages in all cases, what is a hallmark of hippocampal sclerosis [33]. MRI evidence indicated that six of the E patients had MTLE on the right side and one on the left side, whereas five of the L patients had MTLE on the left side and two on the right side. Patients with bilateral hippocampal sclerosis, lesions other than hippocampal sclerosis (tumors, dysplasias, etc), and psychiatric disorders were not included in this study. No significant group differences were found for epilepsy duration or age at surgery between E and L groups (S1 Fig). All surgical specimens were classified as ILAE type 1 hippocampal sclerosis [34], that is, severe neuronal cell loss and gliosis predominantly in CA1 and CA4 regions.
Table 1. Patients' clinical and demographic data.
| Epilepsy | |||||||
|---|---|---|---|---|---|---|---|
| Patient ID | Gender | FR | IPI (yr/mo) | Onset (yr/mo) | Duration (yr/mo) | Age at surgery (yr) | Side |
| E1 | M | No | 4yr | 4yr | 9yr | 13 | R |
| E2 | M | No | 4yr | 4yr | 35yr | 39 | L |
| E3 | M | 2nd | 2yr | 2yr | 31yr | 33 | R |
| E4 | F | No | 6mo | 6mo | 55yr6mo | 56 | R |
| E5 | F | 1st/3rd | 8mo | 9mo | 28yr3mo | 29 | R |
| E6 | F | No | 2yr | 2yr | 18yr | 20 | R |
| E7 | F | 1st/3rd | 3yr | 5yr | 42yr | 47 | R |
| L1 | M | 2nd | 3yr | 14yr | 9yr | 23 | L |
| L2 | F | 2nd | 2yr | 29yr | 13yr | 42 | L |
| L3 | M | 2nd | 6mo | 15yr | 14yr | 29 | L |
| L4 | F | No | 2yr | 14yr | 14yr | 28 | R |
| L5 | M | 2nd | 2yr | 19yr | 31yr | 50 | L |
| L6 | F | No | 9mo | 13yr | 11yr | 24 | R |
| L7 | F | 1st | 6mo | 16yr | 38yr | 54 | L |
E-Early onset; L-Late onset; FR-Familial recurrence; IPI-Initial precipitant insult; 1st/2nd/3rd- first, second or third degree relative with epilepsy.
Brain tissue specimens for gene expression and neuropathological studies
Fresh ex-vivo explants from hippocampal CA3 of our patients were obtained at the surgery room and immediately preserved with RNAlater (Qiagen cat. no. 76106, Valencia, CA). MRI and histological studies were performed in all removed hippocampi for neuropathology analysis and for confirming that the explants for genomic studies were obtained at the proper site [2, 3].
RNA extraction
Brain tissue explants from CA3 (3–4 mm3) were homogenized with TissueRupter (Qiagen, cat. no. 9001272 Valencia, CA) and total RNA was extracted from the homogenates using the RNeasy Lipid Tissue Kit (Qiagen cat. no. 74804, Valencia, CA) according to the manufacturer’s instructions. RNA quality was assessed on the Agilent BioAnalyzer 2100 (Agilent, Santa Clara, CA). All samples were stored at -80°C until used in hybridization experiments.
Microarray hybridization and gene expression analysis
In order to determine gene expression profiles, 4x44K DNA microarrrays (Whole Human Genome Microarray Kit, Agilent Technologies, cat no. G4112F, Santa Clara, CA) were used. The procedures for hybridization followed the protocols provided by the manufacturer´s instructions (One-Color Microarray-Based Gene Expression Analysis—Quick Amp Labeling). The images were captured by the reader Agilent Bundle according to the parameters recommended for bioarrays and extracted by Agilent Feature Extraction software version 9.5.3 and considering spots present none or only one flag (i.e. low intensity, saturation, controls, etc.). The selected transcripts were used for analysis using the R software version 2.11.1 (R Development Core Team, 2010). We identified 13,427 valid GO annotated genes for the CA3 samples (early- and late-onset patients). By means of the TMEV software version 4.6.1 we obtained the differentially expressed (DE) Gene Ontology (GO) annotated genes using the t-test (P<0.05). All microarray raw data has been deposited in GEO public database (http://www.ncbi.nlm.nih.gov/geo), a MIAME compliant database, under accession number GSE57585. Differential gene expression data were validated through quantitative real-time polymerase chain reaction [3].
Gene coexpression networks (GCNs): visualization, analysis and community detection
Gene coexpression networks for differentially expressed GO annotated genes (DE) and for all valid GO annotated genes (CO) were constructed for E and L groups based on Pearson’s correlation, as we previously described [4]. Pearson’s correlation identifies sets of genes which covaries (positively or negatively), thus allowing us to construct networks by considering nodes as genes, with edges inferred if a pair presents high absolute value of correlation. Specifically, we define a correlation threshold that determines if edges are present or absent in the resulting network. This is done in a way that all nodes are connected to the major component and the network is stable in the sense that slight changes in the threshold value do not significantly affect its topological structure [4]. Networks were tested for scale free status by Kolmogorov-Smirnov (K-S) statistics, i.e. power law distributions in empirical data [35].
As these networks may grow larger in the number of components (e.g. tens of thousands genes) or present very intricate connections between them (such as hierarchical or modular structure), it becomes mandatory the use of tools and methodologies borrowed from network science to better characterize such systems.
We developed a network methodology for GCN visualization (3D) and analysis [4] that allows the categorization of network nodes according to node-centered connectivity taken along distinct hierarchical levels of gene-gene neighborhoods [36, 37]: hubs are highly connected nodes, VIPs—standing for “Very Important Person”, an acronym initially coined for the study of social networks [38] and equivalent to the term “date-hubs” in biological network papers [39]—have low node degree but connect only with hubs, and high-hubs have VIP status and high overall number of connections. We classified network nodes as VIPs, hubs or high-hubs by obtaining the node degree, k 0, and the first level concentric node degree, k 1, which takes into account all node connections leaving from its immediate neighborhood, then projecting all node values in a k 0 vs k 1 graphic. All calculations were done by using Python program and the conceptual framework is described at http://cyvision.if.sc.usp.br/~bant/hierarchical/.
Connectivity
The network connectivity k for non-directed networks was calculated by k = 2L/N, where L stands for the number of edges and N for the number of nodes [40].
Community detection
Community detection in complex networks is usually accomplished by discovering the network modular structure that optimizes the modularity measurement. Modularity takes into account the relationship between the number of links inside a community and between nodes in distinct communities compared to the random model [27; 40]. A diverse range of optimization techniques exist to optimize the modularity. Here we applied the method proposed by Blondel et al. [41] which attains good modularity values and presents excellent performance.
Coarse-grained community structure
As a complementary analysis for the community detection, each GCN was rearranged in a new network accounting only for the relationships between each community, also known as coarse-grained community structure (CGCS) [42]. Here the CGCS was generated by contracting all nodes inside each community into a single community node, likewise, edges are added up as connection weights between such communities. This structure can also be obtained directly by considering the mixing matrix [27] as an adjacency matrix of the new network.
Interactome analysis
The interactome networks were constructed using an in house free web tool developed by Leandro de A. Lima and Renato D. Puga—Centro Internacional de Pesquisa e Ensino (CIPE)—Hospital A. C. Camargo (http://bioinfo.lbhc.hcancer.org.br/cgi-bin/interactomegraph/index.cgi). Only genes categorized as hubs, VIPs or high hubs were considered in this analysis. MINT and IntAct databases (experimentally verified protein-protein interactions) were selected for comparison and data generation. Data analysis and visualization were accomplished through Cytoscape (version 3.1.0, www.cytoscape.org).
Results
GCN analyses
In the E versus L comparison, 761 DE genes were found to be upregulated and three were downregulated in the E group. Gene coexpression networks (GCNs) were inferred for E and L groups using DE or CO (encompassing all 13,427 valid transcripts) subsets of genes through Pearson’s correlation method. A 0.965 link-strength cut-off was adopted for DE networks. The resulting DE networks had 621 genes and 1367 links for the E-DE group, or 703 genes and 3,206 links for the L-DE group. We adopted a higher link-strength cut-off (0.998) to finalize the CO networks, which had 9,578 genes and 32,807 links for the E-CO group, or 11,321 genes and 76,711 links for CT group. All networks were validated as scale-free networks as seen in a normalized degree distribution log-log plot: DE and CO networks are shown in Figs 1 and 2, respectively. In these figures the nodes (genes) are depicted in different colors corresponding to their hierarchical level: blue for hubs, red for VIPs, and green for high-hubs. Gene categorization as hubs, VIPs, and high hubs, with their corresponding k 0 and k 1 values and biological function appear in Table 2 (DE networks) and Table 3 (CO networks).
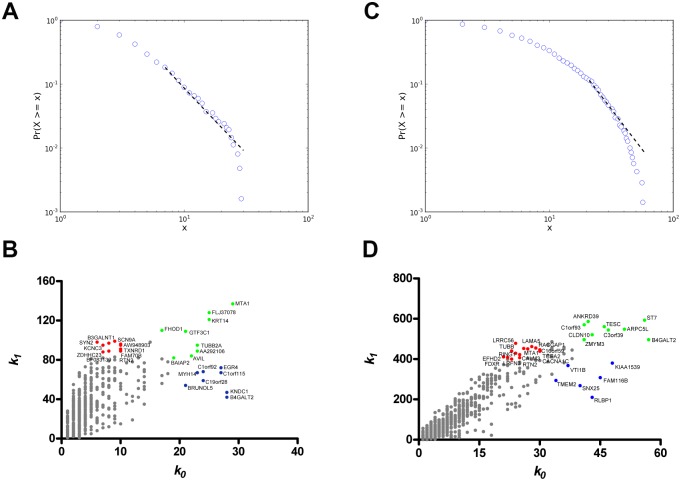
Fig 1. Node distribution and categorization for DE networks.
Kolmogorov-Smirnov test for scale free status for E-DE (A) and L-DE (B) gene coexpression networks (GCNs). Scatter plots of node degree (k0) vs concentric node degree (k1) measures of GO annotated genes in E-DE (C) and L-DE networks (D). Hubs (blue), VIPs (red) and high-hubs (green), identified by their gene symbols.
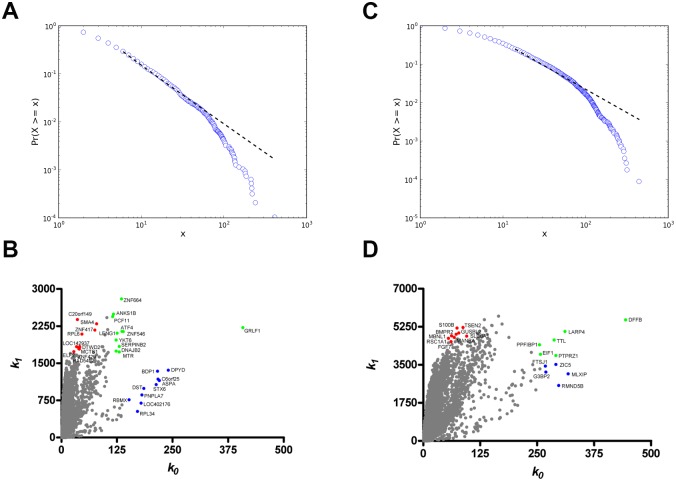
Fig 2. Node distribution and categorization for CO networks.
Kolmogorov-Smirnov test for scale free status for E-CO (A) and L-CO (B) networks. Scatter plots of node degree (k0) vs concentric node degree (k1) measures of GO annotated genes in E-CO (C) and L-CO networks (D). Hubs (blue), VIPs (red) and high-hubs (green), identified by their gene symbols.
Table 2. High-hubs, hubs, VIPs and communities in Early and/or Late DE networks.
| Early | Late | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Gene | Comm 1 | Cat 2 | K0 | K1 | Comm | Cat | K0 | K1 | Gene function/product [reference] |
| C1orf115 | A | Hub | 27 | 67 | Integral component of membrane (Gene ID: 79762) | ||||
| SYN2 | A | VIP | 6 | 98 | Regulation of epileptic and synaptic activity on hippocampus [67] | ||||
| AW948903 | A | VIP | 10 | 96 | Expressed sequence tag (EST) AW948903 | ||||
| FAM70B | A | VIP | 10 | 91 | Official symbol: TMEM255B. Integral component of membrane (Gene ID: 348013) | ||||
| FHOD1 | A | High-hub | 17 | 110 | Coordinates actin filament and microtubule alignment [67] | ||||
| TUBB2A | A | High-hub | 23 | 95 | Beta tubulin, a major component of hippocampal microtubules [71, 72] | ||||
| C19orf28 | B | Hub | 24 | 59 | Aliase: MFSD12. Mediates sodium butyrate (HDAC inhibitor) inhibition of Sirtuin-2 (HDAC III)-mediated hippocampal synaptic plasticity [73, 74] | ||||
| ZDHHC23 | B | VIP | 7 | 88 | Aliase: NIDD. Controls surface expression of calcium-activated potassium channels (BK) [76] | ||||
| KRT14 | B | High-hub | 25 | 121 | Codes for keratin 14 and modulates Notch signaling [80] | ||||
| BRUNOL5 | C | Hub | 21 | 54 | Member of the CELF-Bruno Like family of RNA-binding proteins [52] | ||||
| SCN9A | C | VIP | 9 | 99 | Febrile seizure associated gene coding for Nav 1.7 sodium channel; Allelic variants may cause FS epilepsy [46, 47] | ||||
| RTN2 | C | VIP | 10 | 89 | C | VIP | 25 | 407 | Regulates the trafficking and function of glutamate transporter EAAC1 [48] |
| FLJ37078 | C | High-hub | 25 | 128 | Official symbol: SRRM3. Serine/arginine repetitive matrix 3 (Gene ID: 222183) | ||||
| AVIL | C | High-hub | 22 | 84 | Codes for advillin, an actin binding protein, and regulates neurite outgrowth [54, 55] | ||||
| BAIAP2 | C | High-hub | 19 | 82 | Adaptor protein IRSp53; involved in the regulation of NMDA receptor-mediated excitatory synaptic transmission [50] | ||||
| MYH14 | D | Hub | 23 | 67 | Neurite stabilization at adhesion sites [194] | ||||
| TXNRD1 | D | VIP | 10 | 95 | Regulator of cellular redox balance (protection against oxidative stress) [195] | ||||
| AA292106 | D | High-hub | 23 | 89 | Expressed sequence tag (EST) AA292106 | ||||
| C1orf92 | E | Hub | 24 | 68 | Aliase: LRRC71. Protein harboring a leucine-rich repeat motif (Gene ID: 149499) | ||||
| BF083139 | E | VIP | 8 | 89 | Expressed sequence tag (EST) BF083139 | ||||
| KNDC1 | F | Hub | 28 | 47 | Codes for a v-KIND domain containing protein involved in the control of dendrite arborization patterns [196] | ||||
| B4GALT2 | G | Hub | 28 | 42 | D | High-hub | 57 | 496 | Major regulator of glycan synthesis involved in neuronal development and neuron outgrowth [197, 198] |
| GTF3C1 | H | High-hub | 21 | 109 | Aliase: TFIIIC. Regulates the rearrangement of nuclear architecture allowing the coordinated expression of activity-dependent neuronal genes [82] | ||||
| EGR4 | I | Hub | 27 | 72 | Mediates BNDF induction of neuronal KCC2 transcription [56] | ||||
| B3GALNT1 | I | VIP | 8 | 97 | Glycosylation of HCN1 channels upon seizure activity in hippocampus [61] | ||||
| KCNC3 | I | VIP | 7 | 95 | Potassium channel related to febrile seizures and synaptic excitability [58, 59, 60] | ||||
| MTA1 | I | High-hub | 29 | 137 | H | VIP | 27 | 451 | Codes for a protein which is an integral part of the nucleosome remodeling and histone deacetylation complex [62] |
| KIAA1539 | A | Hub | 48 | 380 | Official symbol: FAM214B. Family with sequence similarity 214, member B (Gene ID: 80256) | ||||
| FAM116B | A | Hub | 45 | 308 | Aliase: DENND6B. DENN/MADD domain proteins regulate Rab-mediated trafficking role in neurite formation [199, 200] | ||||
| SNX25 | A | Hub | 40 | 268 | Codes for Sorting Nexin 25; modulates TGF-beta signaling pathway and is involved in epileptogenesis and TLE [106] | ||||
| VTI1B | A | Hub | 37 | 368 | Non-canonical SNARE molecule involved in synaptic vesicle recycling and spontaneous neurotransmitter release [201] | ||||
| TMEM2 | A | Hub | 34 | 294 | Transmembrane protein 2 (Gene ID: 23670) | ||||
| ARPC5L | A | High-hub | 51 | 548 | Arp2/3 complex protein involved in actin polymerization and control of neurite outgrowth of hippocampal neurons [107, 108] | ||||
| RLBP1 | B | Hub | 43 | 210 | Retinaldehyde binding protein 1, a molecule controlling PAX6 (candidate genefor epilepsy) expression [110, 111] | ||||
| LRRC56 | C | VIP | 24 | 478 | Leucine rich repeat containing 5 (Gene ID: 115399) | ||||
| RACGAP1 | C | VIP | 28 | 462 | Constituent of the IQGAP1–filamin-A—RacGAP1 pathway that coordinates directional cell migration [99] | ||||
| C16orf59 | C | VIP | 29 | 455 | Chromosome 16 open reading frame 59 (Gene ID: 80178) | ||||
| TCEA2 | C | VIP | 30 | 446 | Aliase: TFIIS. SII class transcription elongation factor; prevents cellular death due to oxidative DNA damage [88] | ||||
| TUBB | C | VIP | 23 | 440 | Tubulin beta I; cytoskeleton protein aberrantly expressed in the hippocampus of TLE patients [75] | ||||
| CACNA1C | C | VIP | 30 | 439 | L-type voltage-gated calcium channel Cav1.2. Involved in synaptic activity-dependent gene expression [83]; neurotransmitter release in hippocampal interneurons [80]; control of neurite extension [85] | ||||
| LAMA5 | C | VIP | 26 | 453 | Hippocampal laminin matrix essential for its dynamic structure and for neuronal survival under stress conditions [92] | ||||
| RING1 | C | VIP | 24 | 429 | Promotes transcriptional activation/silencing via Polycomb [87] | ||||
| CALM3 | C | VIP | 25 | 422 | Calcium signal transducer involved in the NFKB activation pathway [94] | ||||
| EFHD2 | C | VIP | 21 | 411 | Conserved calcium binding protein that regulates F-actin access to cofilin [96] | ||||
| FDXR | C | VIP | 22 | 408 | Maintenance of cytosolic and mitochondrial iron homeostasis [89] | ||||
| LRFN3 | C | VIP | 23 | 400 | Aliase: SALM4. Leucine-rich repeat and synaptic adhesion-like molecule; promotes neurite outgrowth and branching [202] | ||||
| ST7 | C | High-hub | 56 | 593 | Cytoplasmic protein involved in remodeling extracellular matrix structure [203] | ||||
| ANKRD39 | C | High-hub | 42 | 586 | Ankyrin repeat domain 39 (Gene ID: 51239) | ||||
| C1orf93 | C | High-hub | 41 | 570 | Official symbol: FAM213. Family with sequence similarity 213, member B (Gene ID: 127281) | ||||
| TESC | C | High-hub | 46 | 561 | Calcineurin B-like protein involved in rapid hippocampal network adaptation to recurring synchronous activity [93] | ||||
| C3orf39 | C | High-hub | 47 | 544 | POMGNT2; aliase: GTDC2. Glycosyltransferase catalyzing GlcNAcylation o fO-mannosylated α-DG in the ER [204] | ||||
| CLDN10 | C | High-hub | 43 | 521 | Tight junction protein which mediates cell adhesion [205] | ||||
| ZMYM3 | C | High-hub | 41 | 496 | Transcriptional repressor and component of HDAC complexes; abundantly expressed in the brain [206] | ||||
1Community
2 Node category; Bold indicates genes present in both E and L networks, but belonging to different communities.
Table 3. High-hubs, hubs, VIPs and community in Early and/or Late CO networks.
| Early | Late | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Gene | Comm 1 | Cat 2 | K0 | K1 | Comm | Cat | K0 | K1 | Gene function/product [reference] |
| DPYD | B | Hub | 240 | 1361 | Dihydropyrimidine dehydrogenase involved in pyrimidine catabolism and modulation of beta-alanine production [146] | ||||
| STX6 | B | Hub | 213 | 1072 | t-SNARE family member that regulates intracellular membrane trafficking [149] | ||||
| DST | B | Hub | 185 | 994 | Cytoskeletal-associated protein essential for maintaining neuronal cytoskeleton organization [150] | ||||
| BDP1 | C | Hub | 216 | 1342 | Aliase: TFIIIB. Subunit of the TFIIIB transcription initiation complex; essential component of human TFIIIC activity [195,110] | ||||
| ZNF417 | C | VIP | 75 | 2171 | Zinc finger protein (Gene ID: 147687) | ||||
| ZNF429 | C | VIP | 41 | 1821 | DNA-binding protein associated with analgesic onset in humans [207] | ||||
| MCTS1 | C | VIP | 40 | 1788 | Aliase: MCT1.Carboxylate transporter; its deficiency was observed in human epileptogenic hippocampus [115] | ||||
| GRLF1 | C | High-hub | 408 | 2221 | Aliase: ARHGAP35. Rho-GTPase involved in the promotion of neurite outgrowth [119] | ||||
| ANKS1B | C | High-hub | 117 | 2494 | Aliase: AIDA1.Postsynaptic scaffolding protein linking signal events occurring at neuronal synapse with global changes in gene expression [118] | ||||
| PCF11 | C | High-hub | 115 | 2443 | 3’-end processing factor enhancing RNA polymerase II nascent RNA degradation and transcriptional termination [120] | ||||
| C6orf25 | D | Hub | 217 | 1182 | Aliase: G6bB. Inhibitory platelet receptor bearing ITAM and ITIM motifs; modulates microglia-neuron interaction[126, 127] | ||||
| PNPLA7 | D | Hub | 181 | 862 | Patatin-like 7hospholipase involved in lipid/energy homeostasis and axonal and synaptic integrity [129, 130,127] | ||||
| C20orf149 | D | VIP | 36 | 2384 | Aliase: PPDPF. Pancreatic progenitor cell differentiation and proliferation factor (gene ID 79144) | ||||
| RPL6 | D | VIP | 46 | 2092 | Ribosomal protein L6 regulates HDM2—p53 pathway in response to ribosomal stress [124] | ||||
| LOC142937 | D | VIP | 34 | 1837 | Uncharacterized gene products BC008131 (Gene ID: 142937) | ||||
| DTWD2 | D | VIP | 41 | 1837 | Prothymosin alpha involved in response to oxidative stress and neuronal survival [125] | ||||
| ZNF664 | D | High-hub | 135 | 2800 | Zinc finger protein 664 (Gene ID: 144348) | ||||
| ZNF546 | D | High-hub | 136 | 2144 | Zinc finger protein 546 (Gene ID: 339327) | ||||
| DNAJB2 | D | High-hub | 123 | 1747 | Aliase: HSP70. heat-shock protein expressed in hippocampal neurons; stress marker of TLE [121] | ||||
| MTR | D | High-hub | 130 | 1728 | Regulation of homocysteine, an excitatory amino acid, protecting hippocampal neurons against oxidative stress [122, 123] | ||||
| LOC402176 | E | Hub | 179 | 697 | Aliase: RPL21. Ribosomal protein L21 (Gene ID: 6144) | ||||
| ASPA | H | Hub | 220 | 1150 | Aspartoacylase, catabolizes NAA (N-acetyil-L-aspartic acid) in oligodendrocytes; responsive to glutamatergic activity [144] | ||||
| SMA4 | H | VIP | 79 | 2299 | Aliase: SMN1. SMN protein that modulates neuronal survival [132] | ||||
| RAD54L2 | H | VIP | 39 | 1793 | Aliase: ARIP4. Chromatin remodeling factor; modulates excitation/inhibition balance in hippocampal neurons towards seizure inhibition [133] | ||||
| ELF2 | H | VIP | 28 | 1737 | E1f transcription factor;promotes cell survival under cytokine stress [134] | ||||
| ATF4 | H | High-hub | 139 | 2142 | Transcription factor mediating neuronal resistance against oxidative stress [136, 137] | ||||
| LENG1 | H | High-hub | 125 | 2109 | Leukocyte receptor cluster (LRC) member 1 [140] | ||||
| YKT6 | H | High-hub | 123 | 1970 | Conserved SNARE essential for ER-Gogi transport, highly expressed in neurons and enriched in hippocampus [141] | ||||
| SERPINB2 | H | High-hub | 130 | 1840 | Synaptic activity-induced neuroprotection against seizure-induced brain-damage [143] | ||||
| RPL34 | J | Hub | 171 | 529 | Ribosomal protein L34, a Cdk5 inhibitor [151] | ||||
| RBMX | S | Hub | 152 | 764 | RNA binding motif protein involved in the promotion of neurite growth [154] | ||||
| MLXIP | B | Hub | 318 | 3089 | Aliase: MondoA. glucose sensing transcription factor involved in glucose homeostasis [178] | ||||
| ZIC5 | C | Hub | 291 | 3523 | C2H2-type zinc finger protein involved in neuronal development and inhibition of neuronal differentiation via Notch [208] | ||||
| S100B | C | VIP | 74 | 5192 | Astrocyte-derived cytokine; promotes neurite outgrowth; increases intracellular calcium in hippocampal neurons [155] | ||||
| GUSBL2 | C | VIP | 78 | 4968 | Aliase: GUSBP4. Glucuronidase beta pseudogene 4 (Gene ID: 375513) | ||||
| BMPR2 | C | VIP | 72 | 4907 | Bone morphogenetic protein receptor 2; involved in astrocyte development and survival/differentiation of GABAergic and dopaminergic neurons [165] | ||||
| MBNL1 | C | VIP | 62 | 4837 | Regulatory splicing factor involved in the splicing of the microtubule-associated protein Tau [169] | ||||
| MANBA | C | VIP | 68 | 4764 | Glycosyl hydrolase 2 family (Gene ID: 4126) | ||||
| RSC1A1 | C | VIP | 55 | 4723 | Regulates neuronal expression of the Na+-D-glucose cotransporter SGLT1; increased in the hippocampus during epileptic seizures [172] | ||||
| FGF7 | C | VIP | 61 | 4568 | Fibroblast growth factor 7; essential for inhibitory synapse formation in the hippocampus [173] | ||||
| DFFB | C | High-hub | 444 | 5575 | Aliase: DFF40. Triggering of DNA fragmentation, an early event in apoptotic neuronal cell death after brain injury [175, 176] | ||||
| LARP4 | C | High-hub | 311 | 5039 | La-related protein 4; interacts with poly(A)-binding protein and promotes mRNA homeostasis [177] | ||||
| EIF1 | C | High-hub | 257 | 3987 | Eukaryotic translation initiation factor 1 [178] | ||||
| PTPRZ1 | C | High-hub | 291 | 3935 | Receptor-type protein tyrosine phosphatase; it's concentration is increased in the hippocampus of MTLE patients [174] | ||||
| G3BP2 | D | Hub | 269 | 3168 | Ras-GTPase activating protein; contributes to stress granule formation [180]; expression levels altered in TLE [181] | ||||
| FTSJ1 | D | Hub | 268 | 3447 | RNA methyltransferase expressed in the hippocampus; associated to seizures [182] | ||||
| RMND5B | F | Hub | 297 | 2551 | Aliase: GID2. E3 ubiquitin ligase involved in the catabolic-induced degradation of gluconeogenic enzymes [157] | ||||
| TSEN2 | F | VIP | 87 | 5225 | tRNA splicing endonuclease complex subunit; associated with seizures [159] | ||||
| SLC6A1 | F | VIP | 95 | 4821 | Aliase: GAT1. GABA transporter 1; a major GABA transporter in the brain [156]; hippocampal expression increased in TLE patients [153] | ||||
| TTL | G | High-hub | 287 | 4647 | Tubulin-tyrosine-ligase; involved in neuronal organization and control of neurite extensions [184] | ||||
| PPFIBP1 | G | High-hub | 255 | 4425 | Liprin-family scaffold protein regulating cell adhesion, cell migration and synapse development [185] | ||||
1Community
2 Node Category
Connectivity
E networks exhibited lower connectivity when compared to L networks. The k values for these four networks were: E-DE = 4.40; E-CO = 6.85; L-DE = 9.12; L-CO = 13.55.
Community detection
An overall picture of DE gene communities (modules) is depicted in Fig 3A for E-DE and in Fig 4A for L-DE networks. Different node colors identify the distinct gene communities in each network. Fig 3B, Fig 4B and 4C present, respectively, E-DE and L-DE hierarchy-categorized selected nodes identified by their corresponding GO gene symbols. Symbol letter colors indicate hubs (blue), VIPs (red) or high hubs (green). E-CO and L-CO networks, respective communities, and hierarchy-categorized selected nodes can be properly visualized only in 3D and are shown in S1 and S2 Videos. DE and CO networks presented good quality of community structure and modularity values were quite similar for all networks: E-DE = 0.657; L-DE = 0.530; E-CO = 0.514; L-CO = 0.506. It is interesting to note that the E-CO network has more gene communities (24) than the L-CO network (16) as depicted in Fig 5A and 5B respectively. Fig 5C shows the number of nodes per community in each of the CO networks. E networks (DE and CO) have lower connectivity and their communities are more sparsely connected, what may indicate a higher grade of dysregulation in cell’s functional organization [4, 43]. A set of simulations run with slightly different link-strength thresholds (from 0.930 up to 0.980 for DE networks, and from 0.990 up to 0.998 for CO networks) did not reveal alterations in community structure, thus indicating its robustness.
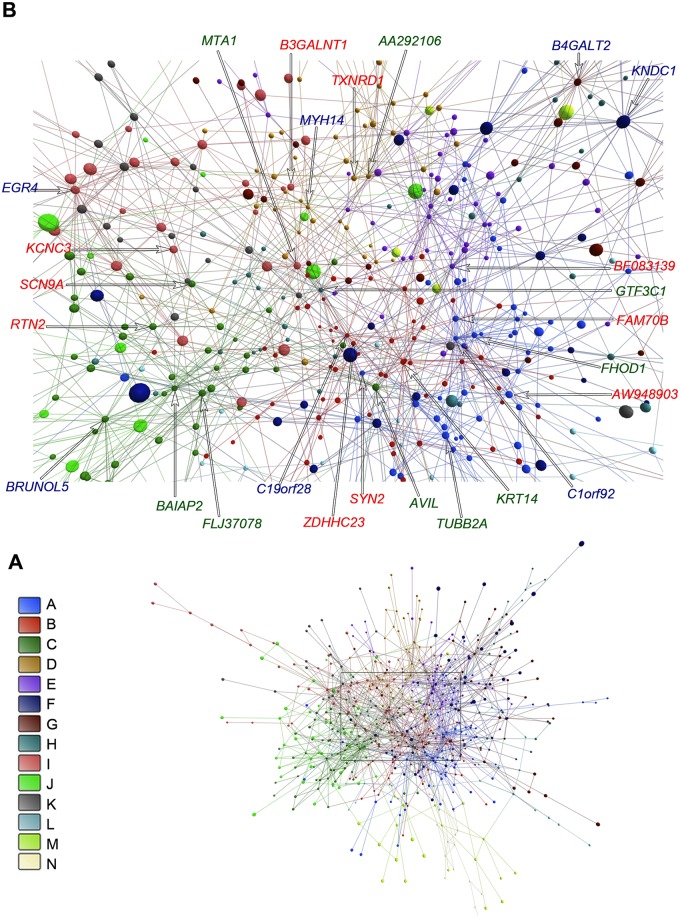
Fig 3. Community analysis for E-DE network.
The communities are indicated by different colors (A). Highly connected nodes (B) occupy central positions and their correspondent GO gene symbols are depicted in different colors corresponding to their hierarchical level: blue for hubs, red for VIPs, and green for high-hubs. In amplification B node size is not related to node degree.
Fig 4. Community analysis for L-DE network.
The communities are indicated by different colors (A). Highly connected nodes (B-C) occupy central positions and their correspondent GO gene symbols are depicted in different colors corresponding to their hierarchical level: blue for hubs, red for VIPs, and green for high-hubs. In the amplifications B-C node size is not related to node degree.
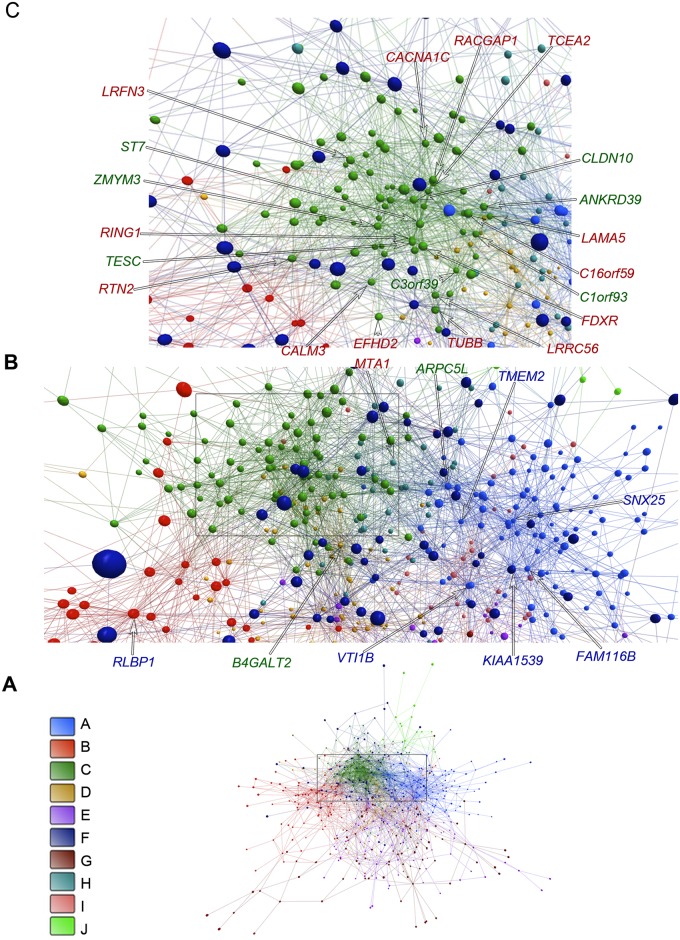
Fig 5. Community analysis for CO networks.
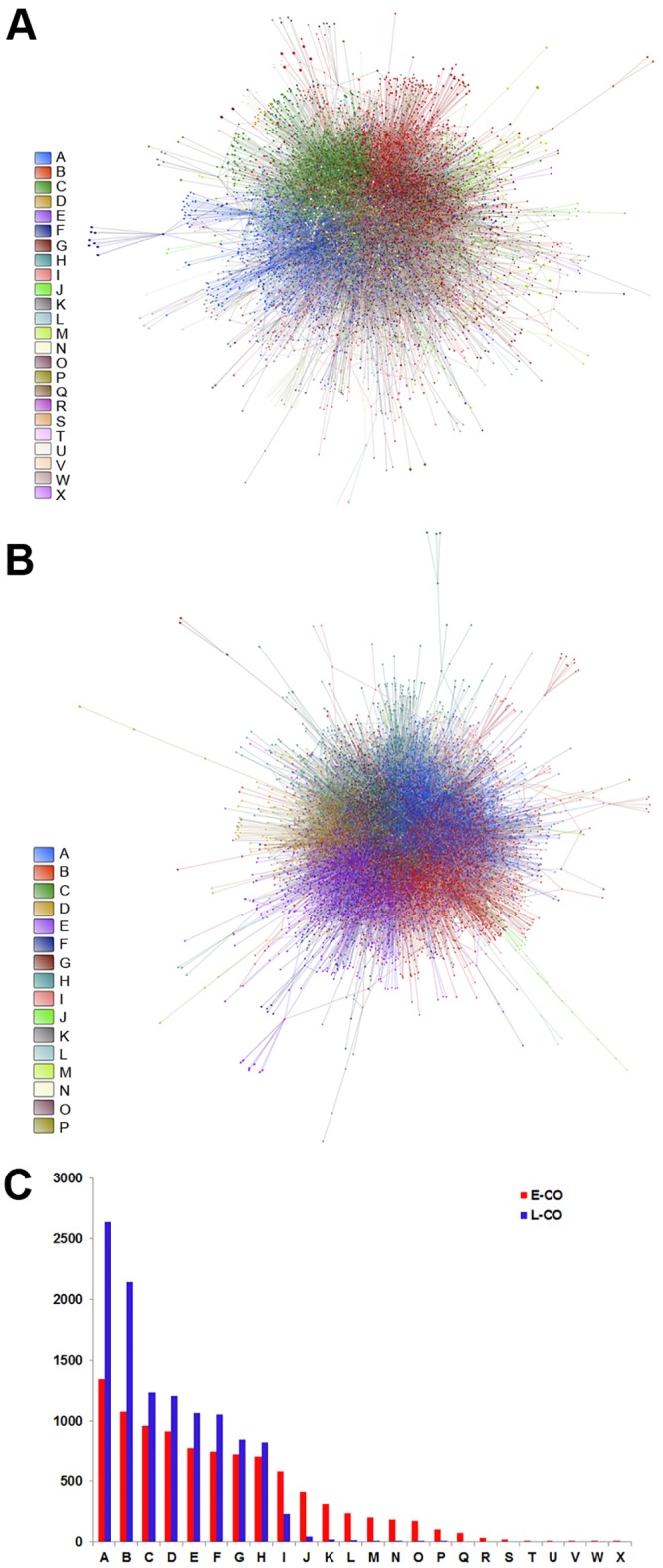
The communities are indicated by different colors for E-CO (A) and for L-CO (B). Fig 5C shows the number of nodes per community in each of the CO networks (indicated by red and blue bars for E-CO and L-CO, respectively).
Community structure analysis of transcriptional networks
This section portrays the biological functions of selected hubs, VIPs and high- hubs in the context of the distinct gene communities, i.e. transcriptional modules, found in DE and CO networks generated for E and L groups. Coarse-grained community structure (CGCS) was obtained for each network, yielding the relationships between each community in the network (Fig 6). Communities with the strongest connection weights (fraction of edges linking distinct communities) hold the most significant functional interactions in the network [30, 44, 45]. Therefore, the subsequent analysis of gene communities in DE and CO networks was performed considering not only the gene/node hierarchy but, and principally, the networks’ CGCS.
Fig 6. CGCS summarizing the relationships among the communities.
CGCS is depicted for E-DE (A), L-DE (B), E-CO (C) and L-CO (D) networks. The edge width is proportional to the fraction of edges linking distinct communities. The node size is proportional to the number of nodes in each community. In each of the four networks only the top nine communities in number of nodes (A to I) were considered for this analysis, except for communities J and S in the E- CO network (C), since these communities contain relevant hubs.
E-DE network
This network encompasses 14 gene communities, of which 10 contain high hierarchy nodes/genes—categorized as hubs, VIPs or high-hubs—with high network centrality (Fig 3A, 3B and Table 2). Most of these high hierarchy genes play relevant roles in epilepsy and brain functioning. These roles will be analyzed below in the setting of gene community relationships. In the E-DE network communities C and I have the strongest connection weights (Fig 6A), followed by communities A and B.
Community C harbors six E-DE high hierarchy genes and four of them are related to neuronal excitability. Two of these genes are VIPs: SCN9A, which codes for Nav1.7 sodium channel and whose allelic variants are implicated in genetic epilepsies with febrile seizures [46, 47] and RTN2, also a VIP in L-DE network module C, a regulator of the trafficking and function of glutamate transporter EAAC1 (excitatory amino acid carrier 1) [48]; dysregulation of EAAC1 was reported in experimental models of epilepsy and also in the hippocampus of temporal lobe epilepsy (TLE) patients [49]. The third gene, BAIAP2, is a high-hub. This gene codes for an adaptor protein (IRSp53) involved in the regulation of NMDA receptor-mediated excitatory synaptic transmission, long-term potentiation, and learning and memory [50]. Rapid surface accumulation of NMDA receptors in dentate gyrus and CA3 pyramidal cells increases glutamatergic excitation during status epilepticus [51]. The fourth gene in this functional group, BRUNOL5, aliase CELF5, codes for a member of the CELF-Bruno Like family of RNA-binding proteins. BRUNOL5 expression is restricted to brain [52]. CELF proteins share structural and functional redundancy. Human BRUNOL4, closely related to BRUNOL5 [48], is involved in fine-tuning synaptic transmission and its deficiency causes recurrent seizures in mice and man [53].
The remaining two genes in community C are high-hubs: AVIL codes for advillin, an actin binding protein, and regulates neurite outgrowth [54, 55]; and FLJ37078 encodes a hitherto uncharacterized protein.
In community I all four high hierarchy genes are clearly related to febrile seizures, synaptic activity and epilepsy. EGR4, a hub, mediates BNDF induction of neuronal KCC2 (potassium chloride cotransporter 2) transcription [56]. KCC2 variants determine susceptibility to febrile seizures [57]. KCN3, a VIP, codes for a potassium channel related to febrile seizures and synaptic excitability [58, 59, 60]. B3GALTN1, also a VIP, glycosylates and promotes heteromerization of HCN1-HCN2 channels in hippocampus upon seizure activity, thus enhancing network excitability [61]. MTA1, a high-hub, and a VIP in L-DE community H, codes for a core component of NuRD (Nucleosome Remodeling and histone Deacetylation) complex [62], where it enhances histone deacetylases (HDACs) 1 and 2 activity in order to repress gene expression [63]. HDAC2—the target of the antiepileptic drug Valproate—is overexpressed in the brain of TLE patients and its transcriptional repression activity plays an important role in the pathogenesis of epilepsy [64].
Communities A and B have the second strongest connection weights in E-DE. Noteworthy, community A harbors SYN2, a VIP that encodes Synapsin II, a phosphoprotein which desynchronizes neurotransmitter release at inhibitory synapses by interacting with presynaptic Ca channels, modulating synaptic transmission and plasticity; allelic variants of SYN2 contribute to epilepsy predisposition [65, 66]. In community A are also located two high-hubs—FOHD1 and TUBB2A—related to microtubule function and structure. FOHD1 coordinates actin filament and microtubule alignment to mediate cell elongation [67], being possibly involved in hippocampal neuronal polarization [68, 69]. There is a loss of normal neuronal polarization in temporal lobe epilepsy [70]. TUBB2A codes for beta tubulin, which is a major component of hippocampal microtubules [71, 72].
Community B includes three genes acting in several epilepsy pathways. C19orf28 (aliase MFSD12) is a hub and its gene product (protein pp3501) mediates sodium butyrate (HDAC inhibitor) inhibition of Sirtuin-2 (HDAC III)-mediated hippocampal synaptic plasticity [73, 74]. Sirtuin-2 expression is decreased in mesial temporal lobe epilepsy (MTLE) [75]. ZDHHC23 (aliase NIDD) is a VIP and codes for a DHHC-containing protein involved in: i) regulation of cell surface expression of calcium-activated potassium (BK) channels [76]; ii) regulation of NO signaling pathways at postsynaptic sites [77]; iii) control of neuronal hiperexcitability via BK channels [78]. Interestingly, a seizure—related down regulation of BK channel protein levels was described in the pilocarpine model of temporal lobe epilepsy [79], indicating a role for BK and NIDD in MTLE. The high hub KRT14, codes for keratin 14 and modulates Notch signaling [80]. Notch signaling is up-regulated in temporal lobe epilepsy (reviewed in [81]).
The remaining communities and respective high hierarchy genes are described in Table 2. Here is worth to mention here community H, which harbors GTF3C1 (aliase TFIIIC) a gene that regulates the rearrangement of nuclear architecture allowing the coordinated expression of activity-dependent neuronal genes [82]. This gene controls the expression of BAIAP2, which is high hub in E-DE community C.
In synthesis, it is possible to say that communities C and I, which share the strongest connection weights, include genes closely related to neural excitability and febrile seizures mechanisms, with relevance for SCN9A and KCCN3, implicated in febrile seizures and synaptic excitability, followed by the regulators of synaptic excitability (RNT2, BAIAP2 and B3GALTN1) and by MTA1, the enhancer of HDAC2 and a Valproate target. Several of the high hierarchy genes in A and B communities are also related to neuronal excitability, or to genomic mechanisms favoring this mechanism. The implications of these results for the development and onset age of MTLE in patients with febrile seizure history will be discussed later in this paper.
L-DE network
This network (Fig 4A–4C) has 10 gene communities, of which 5 (Table 3) contain high hierarchy nodes (hubs, VIPs or high-hubs). Here the strongest connection weights are centered in module C, which also contains most of the VIPs and high-hubs of the L-DE network, which are involved in relevant epilepsy mechanisms, as follows.
Two community C VIPs, RTN2 and CACN1C, are significantly linked to neuronal excitability. RTN2, also a VIP in E-DE network module C, is a regulator of the glutamate transporter EAAC1 [48]; the dysregulation of EAAC1 was reported in animal models of epilepsy and in the hippocampus of TLE patients [49]. CACNA1C encodes the alpha 1C subunit of the L-type voltage-gated calcium channel Cav1.2 which plays relevant roles in: i) synaptic activity-dependent gene expression [83] ii) regulation of neurotransmitter release in hippocampal interneurons [84]; iii) control of neurite extension [85]. Here is very important to note that Cav1.2 calcium channels are temperature- sensitive and support the intrinsic firing of pyramidal neurons during hyperthermia, thus providing a target for the treatment of febrile seizures [86].
The VIPs RING1, TCEA2, FDXR, LAMA5, and the high-hub TESC, are associated to mechanisms of stress response and brain homeostasis. RING1 promotes transcriptional activation/silencing via Polycomb [87]. TCEA2, codes for a SII class transcription elongation factor that plays an important role in preventing cell death due to oxidative DNA damage [88]. FDXR codes for the sole human ferredoxin reductase; it is involved in the maintenance of cytosolic and mitochondrial iron homeostasis [89], and in cell sensitization to oxidative stress and apoptosis in TLE [90, 91]. LAMA5 codes for a component of hippocampal laminin matrix essential for its dynamic structure and for neuronal survival under stress conditions, such as excitotoxicity [92]. TESC codes for a Ca2+ binding calcineurin B-like protein involved in rapid hippocampal network adaptation to recurring synchronous activity [93].
The other high hierarchy genes community C with functions associated to epilepsy-related mechanisms are: i) the VIP CALM3, that encodes a calcium signal transducer involved in the NFKB activation pathway [94], which is dysregulated in hippocampal tissues of TLE patients [95]; ii) the high-hub EFHD2; its gene product is a conserved calcium binding protein that regulates F-actin access to cofilin [96], influencing actin cytoskeleton remodeling and the excitability of epileptic hippocampus [97], as well as astrogial loss following status epilepticus [98]; iii) the VIP RACGAP1 a constituent of the IQGAP1—filamin-A—RacGAP1 pathway that coordinates directional cell migration [99], which is frequently aberrant in epileptic hippocampus [70, 100].
Although strongly connected with community C, community F (Fig 6B) does not harbor high hierarchy genes within the k 0 and k 1 cut-off values adopted for L-DE network (see Fig 1D). A scatter plot of node degrees for module F genes and the high hierarchy genes of L-DE appear in S2 Fig Among the module F highly connected genes are: i) CNTNAP1, which encodes contactin associated protein1, a member of the contactin-PTPRZ1 complex that regulates the traffic and synaptic content of AMPA glutamate receptor subunit GluA1 in hippocampal neurons [101]; ii) CCNE1, a Sirtuin-2 regulator [102] and iii) PKIG which encodes a protein kinase involved in endothelial barrier function [103]. Remarkably, GluA1 hippocampal expression is altered during status epilepticus [101, 104], MTLE patients show reduced hippocampal expression of Sirtuin-2 [75], and endothelial barrier function is altered in epilepsy [105].
The other significant connections of community C (Fig 6B) occur with community A—where are located SNX25 and ARPC5L, markers of intractable epilepsy—and with communities H and B which harbor, respectively, the genes MTA1 (a high-hub in E-DE community I, functionally described above) and RBPL1, regulators of two epilepsy-associated genes (HDAC2 and PAX6, targets of the antiepileptic drug Valproate). The hub SNX25 codes for Sorting Nexin 25, a PX domain protein which modulates TGF-beta signaling pathway and is involved in epileptogenesis and TLE development [106]. SNX25 is a biomarker of intractable epilepsy, being overexpressed in TLE patients [106]. ARPC5L encodes an Arp2/3 complex protein involved in actin polymerization [107] and in the control of neurite outgrowth of hippocampal neurons [108]. Arp2/3 expression is increased in the temporal lobe cortex of intractable epilepsy patients [109]. RLBP1 encodes the retinaldehyde binding protein 1, a retinoic acid (RA) signalling molecule. It is noteworthy that RA-signalling promotes the expression of PAX6 [110], which is a candidate gene for epilepsy [111] and whose expression on neuronal cells may be altered by Valproate [112].
The overall picture that emerged from the analysis of community relationships in L-DE is somewhat different from that obtained for E-DE. There is a quite dissimilar functional scenario and also different key players (excepting for RTN2, B4GALT2 and MTA1). Here community C—which centers the strongest connection weights,—harbors most of the VIPs and high-hubs, what indicates that this community is essential for keeping L-DE network stability and functionality. Contrarily to the C-I excitability axis in E-DE, only part of the genes in L-DE community C, such as CACNA1C and RNT2, is associated to neuronal excitability. In fact, a sizable number of genes are related to stress response and brain homeostasis, and others to different epilepsy-associated mechanisms, including those related to cytoskeleton and cell migration. The communities F and A, both strongly connected with community A, harbor genes involved in different biological process with relevancy for epilepsy, like CNTAP1, a regulator of GluA1, and the two markers of intractable epilepsy, SNX25 and ARPCL5. The community detection data for L-DE and E-DE networks will be considered comparatively in the Discussion section.
E-CO network
The complete gene coexpression network for the E group encompasses 24 gene communities of which only 7 contain high hierarchy nodes (Figs 2B and 5A) with high network centrality (S1 Video). In this network the strongest connection weights involve communities D and C, followed by communities H and B (Fig 6C). It is noteworthy that communities C, D and H concentrate all the VIPs and high-hubs of E-CO network. The summarized functional description of all high hierarchy genes contained in E-CO appears in Table 3.
Community C includes five high hierarchy genes related to brain homeostasis and regulation of neuronal gene expression: BDP1, MCTS1, ANKS1B, GRLF1 and PCF11. BDP1 (aliase TFIIIB) is a hub and codes for a subunit of the TFIIIB transcription initiation complex, being an essential component of human TFIIIC activity [113, 114]. TFIIIC regulates the coordinated expression of activity-dependent neuronal genes, such as BAIAP2 [82], a high-hub in E-DE community C. The VIP MCTS1 (aliase MCT1) codes for a carboxylate transporter and its deficiency was observed in human epileptogenic hippocampus [115]. MCTS1 is involved in TLE (and especially in MTLE) by influencing brain energy homeostasis, mitochondrial function GABAergic and glutamatergic transmission and flux of lactate through the brain [116, 117]. ANKS1B (aliase AIDA1), a high-hub, codes for a postsynaptic scaffolding protein that links persistent signal events occurring at neuronal synapse with global changes in gene expression [118]. The high-hubs GRLF1 (aliase ARHGAP35) and PCF11 codes, respectively, for a Rho-GTPase involved in the promotion of neurite outgrowth [119] and for a 3’-end processing factor that enhances RNA polymerase II nascent RNA degradation and transcriptional termination [120].
The high hierarchy genes of community D are mostly involved in responses to stress and neuronal survival. Two high-hubs, DNJB2 and MTR have relevant roles in this task. DNJB2 (aliase HSP70), codes for a heat-shock protein expressed in hippocampal neurons. HSP70 is a well-known stress marker of TLE; its expression before insult improves neuronal survival [121]. MTR codes for the enzyme 5-methyltetrahydrofolate-homocysteine methyltransferase. This enzyme regulates the brain levels of homocysteine, an excitatory amino acid, protecting hippocampal neurons against oxidative stress [122, 123]. Two VIPs are also involved in responses to stress: RPL6 whose gene product is ribosomal protein L6, a regulator of the HDM2 —p53 pathway in response to ribosomal stress [124]; and DTWD2, coding for prothymosin alpha, a highly acidic nuclear protein of the alpha-thymosin family involved in response to oxidative stress and neuronal survival [125]. Finally, two hubs, C6orf25 and PNPLA7, are also related to protective roles. C6orf 25 (aliase G6bB) encodes an inhibitory platelet receptor bearing ITAM and ITIM motifs and it is involved in the modulation of microglia-neuron interaction [126, 127, 128]. PNPLA7 codes for a patatin-like phospholipase, structurally similar to PNPLA6, involved in lipid and energy homeostasis [129] and possibly involved in axonal and synaptic integrity [130, 131].
Community H encompasses set of three VIPs, four high-hubs and one hub, all exerting important roles in neuroprotection, modulation of neuronal excitability, and seizure inhibition. These genes are in the following described according to their hierarchic category and biological functions
VIPs: SMA4 (aliase SMN1) codes for the survival motor neuron (SMN) protein and modulates neuronal survival cooperating with PP4R2 (a regulatory subunit of phosphatase 4) [132]. RAD54L2 (aliase ARIP4) encodes a chromatin remodeling factor that interacts with serine/threonine kinase DIRK1A (minibrain kinase) modulating excitation/inhibition balance in hippocampal neurons towards seizure inhibition [133]. ELF2 codes for an E1f transcription factor and promotes cell survival under cytokine stress (a condition present in MTLE) by increasing valosin-containing protein (VCP) expression [134]. Here is interesting to note that hippocampal valosin is vulnerable to oxidative stress in excitotoxin-induced neuronal injury [135].
High-hubs: ATF4 codes for a transcription factor mediating neuronal resistance against oxidative stress [136, 137]. Interestingly, ATF4 expression is regulated by ELF2 [138], a VIP in this community (see above) Moreover, ATF4 is involved in the differential control of hippocampal GABABR1a and GABABR1b Subunit Gene Expression through Alternative Promoters [139]. LENG1 codes for the leukocyte receptor cluster (LRC) member 1 with uncharacterized function in brain [140]. YKT6 codes for an evolutionary conserved SNARE essential for ER-Gogi transport, highly expressed in neurons and enriched in hippocampus [141]. YKT6 is co-upregulated with S100B (a VIP in L-CO network, module C) upon seizures [142]. SERPINB2 is one of the nine hippocampal core genes for synaptic activity-induced neuroprotection against seizure-induced brain-damage [143]. These genes are collectively termed Activity-regulated Inhibitor of Death (AID) genes. All AID genes are activated by calcium signaling [143].
Hub: ASPA codes for aspartoacylase, an enzyme that catabolizes NAA (N-acetyil-L-aspartic acid) in oligodendrocytes and is responsive to glutamatergic activity [144]. Decreased NAA levels in hippocampal CA3 are characteristic of MTLE [145].
Community B contains three hubs and their biological functions are detailed here. DPYD codes for dihydropyrimidine dehydrogenase, an enzyme involved in pyrimidine catabolism which also modulates the production of beta-alanine, a neuromodulator of inhibitory transmission in the brain [146]. Altered function of DYPD is associated with seizures [147] and intellectual disability [148]. STX6 codes for syntaxin 6, a t-SNARE family member that regulates intracellular membrane trafficking [149]; DST, encodes a large multidomain cytoskeletal-associated protein essential for maintaining neuronal cytoskeleton organization [150].
Finally, also depicted in Fig 6C are communities J and S, where two relevant hubs are located. Community J harbors the hub RPL34, which encodes ribosomal protein L34, a Cdk5 inhibitor [151]. Cdk5 is a mediator of neuronal death and survival [152] and is involved in cell degeneration in hippocampal neurons after an excitotoxic injury [153]. Community S has the hub RBMX, a gene coding for a RNA binding motif protein involved in the promotion of neurite growth [154]. Neurite growth is a hallmark of TLE [155].
Altogether, E-CO network most connected communities encompass genes predominantly related to compensatory pathways in epilepsy (seizure inhibition, neuronal survival and responses to stress conditions). These genes are concentrated in communities C, D and H, which also contain all VIPs and high-hubs in this network, pointing out for the role of those genes in supporting network stability. This issue will be further developed in the Discussion section.
L-CO network
The complete gene coexpression network for the L group has 16 gene communities) of which only 5 contain high hierarchy nodes (Fig 2D) with high network centrality (S2 Video). In this network the strongest weight connections are centered in community F and between the communities C and D (Fig 6D). Community F contains two VIPs and one hub whose altered expression is associated with pro-epileptic effects. The most relevant of these gene is SLC6A1 (aliase GAT1), which codes for GABA transporter 1 (GAT1), a major GABA transporter in the brain [156]. In the epileptogenic sclerotic hippocampus of MTLE patients the expression of GAT1 is decreased in CA3 but it is increased along granule cell dendrites [157]; inhibitors of GAT1 have been studied and developed for epilepsy control [158]. The other VIP is this community is TSEN2, whose gene product is a tRNA splicing endonuclease complex subunit whose mutations are associated with seizures and pontocerebellar hypoplasia [159]. The hub is RMND5B (aliase GID2), a gene that encodes an E3 ubiquitin ligase involved in the catabolic-induced degradation of gluconeogenic enzymes [160]. Gluconeogenesis occurs in astrocytes and is pro-epileptic [161].
Community C concentrates most of the VIPs and high-hubs in L-CO network. In this community all the VIPs with known biological functions are associated to seizure activity/severity, or with vulnerability to epilepsy. S100B is a well-known MTLE marker, which codes for an astrocyte-derived cytokine that promotes neurite outgrowth and increases the levels of intracellular calcium in hippocampal neurons [155, 162]. S100B coded protein is a marker of astroglial activation: its hippocampal levels were found to be higher at the side of seizure onset in patients with refractory MTLE [163] and its plasma concentration was reported to be elevated in MTLE patients [164]. BMPR2 encodes the bone morphogenetic protein (BMP) receptor 2, involved in astrocyte development and survival and differentiation of GABAergic and dopaminergic neurons [165]. BMPR2 was found to be strongly expressed in the hippocampal formation of human and rat adult brain [166, 167] and it is upregulated in rat adult hippocampus during neuroplasticity or repair upon brain injury [168]. MBNL1 codes for a regulatory splicing factor involved in the splicing of the microtubule-associated protein tau [169]. Elevated brain tau levels are associated with seizure severity [170]. MAMBA encodes a member of the glycosyl hydrolase 2 family. The encoded protein localizes to the lysosome where it is the final exoglycosidase in the pathway for N-linked glycoprotein oligosaccharide catabolism. Mutations in this gene cause beta-mannosidosis, including severe forms with neonatal onset epilepsy [171]. RSC1A1 gene product regulates the neuronal expression of the Na+-D-glucose cotransporter SGLT1, which is increased in the hippocampus during epileptic seizures [172]. FGF7 codes for the fibroblast growth factor 7 (FGF7), which is essential for inhibitory synapse formation in the hippocampus. Mice with deficiency in this gene display mossy fiber sprouting and increased neurogenesis, becoming vulnerable to epilepsy [173].
The high-hubs in community C are either involved in mechanisms related to epilepsy pathogenesis (DFF40 and PTPRZ1) or in the control of gene expression and translation initiation (LARP4, EIF1). PTPRZ1 encodes a member of the receptor protein tyrosine phosphatase family whose concentration is increased in the hippocampus of MTLE patients [174]. This receptor is involved damaged-induced gliosis and neuronal reorganization in the hippocampus of MTLE patients [174]. DFFB (aliase DFF40) codes for a caspase-activated deoxyribonuclease (CAD)/DNA fragmentation factor 40 (DFF40) involved in the triggering of DNA fragmentation, an early event in apoptotic neuronal cell death after brain injury [175, 176]. LARP4 gene product, the La-related protein 4, interacts with poly(A)-binding protein and promotes mRNA homeostasis [177]. EIF1 codes for the eukaryotic initiation factor 1, which integrates the scanning mechanism of eukaryotic translation initiation [178]
Community D has only two hubs: G3BP2, which codes for a Ras-GTPase activating protein that contributes to stress granule formation following cellular stress [179, 180] and has its expression levels altered in TLE [181] and FTSJ1, whose gene product is a RNA methyltransferase expressed in the hippocampus and associated to intellectual disabilities and seizures [182]
The remaining communities containing high hierarchy genes in L-CO network are: B, which has the second largest number of nodes but harbors just one hub, MLXIP (aliase MondoA), which codes for a glucose sensing transcription factor involved in glucose homeostasis [183]; and G, containing two high-hubs, TTL that encodes a tubulin-tyrosine-ligase with a vital role in neuronal organization and control of neurite extensions [184], and PPFIBP1 whose gene product is a liprin-family scaffold protein regulating cell adhesion, cell migration and synapse development [185].
The general picture of L-CO network is rather different of that depicted in E-CO, where the genes related to compensatory pathways predominated in the communities with strongest weight connections. Here the communities with the most relevant relationships harbor several genes related to pro-epileptic effects, seizure related mechanisms and vulnerability to epilepsy (SLC6A1, S100B, RSCA1 and PTPRZ1), and just a few ones acting on compensatory mechanisms (BMPR2) or homeostasis (MLXIP). These different scenarios for CO networks will be discussed latter, considering the late and early onset forms of MTLE with a history of febrile seizures.
In both E- and L-CO networks the community with the largest number of nodes, i.e. community A (Fig 6C and 6D), was devoid of high-hierarchy genes, with most of their nodes well below of the k 0 and k 1 cut-off values (Fig 2B and 2D) adopted for these networks.
Interactome network analysis
An interactome analysis was performed in order to validate GCN results. Only the high hierarchy genes (hubs, VIPs or high-hubs) were considered in this analysis. MINT and IntAct databases were selected for data generation, which resulted in interactomes with 106 nodes and 222 edges for the E-DE group and 187 nodes and 690 edges for L-DE (Fig 7A and 7B); 161 nodes and 318 edges for E-CO and 215 nodes and 454 edges for L-CO (Fig 8A and 8B). The nodes (proteins) of interactomes corresponding to genes present in gene coexpression networks—and previously categorized as hubs, VIPs or high-hubs—appear colored in blue, red or green, respectively. Node size is related to node degree (number of links). Links in red represent the first and second node connections, centered in all hubs, VIPs and high-hubs, except for TUBB, a VIP in L-DE, and FTSJ1, a hub in L-CO, two ubiquitously distributed proteins. A functional description of the interactome nodes for all DE and CO networks based on Gene Ontology (biological process) and PubMed databases is presented in S1, S2, S3 and S4 Tables.
Fig 7. Interactome for DE networks.
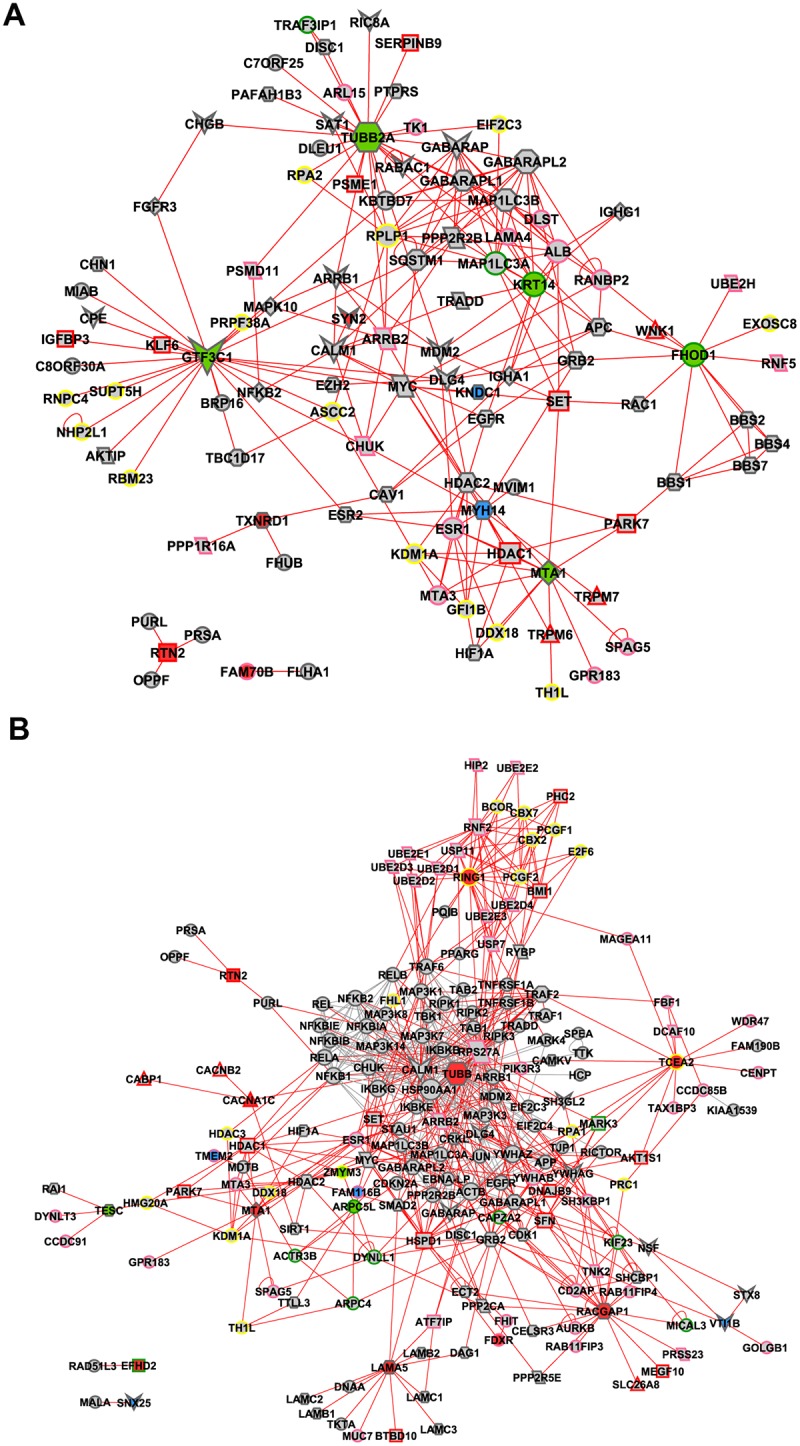
Interactome for E-DE (A) and L-DE (B) selected hubs (depicted in blue), VIPs (depicted in red) and high-hubs (depicted in green) using MINT and IntAct databases. Node size is related to node degree (number of links). Links in red represent the first and second node connections, centered in all hubs, VIPs and high-hubs for E-DE and in all hubs, VIPs and high-hubs, except TUBB for L-DE. Node shapes and border colors represent biological processes, as follows: parallelogram for apoptosis; parallelogram with pink border for ubiquitination; octagon for autophagy; circle with pink or green or yellow border stand for cell processes or cytoskeleton or transcriptional regulation respectively; diamond for inflammation; triangle with red border stand for ion channel; rectangle with green or red or pink stand for neurodegeneration or neuroprotection or response to oxidative stress respectively; hexagon for neuronal development; vee for synaptic transmission.
Fig 8. Interactome for CO networks.
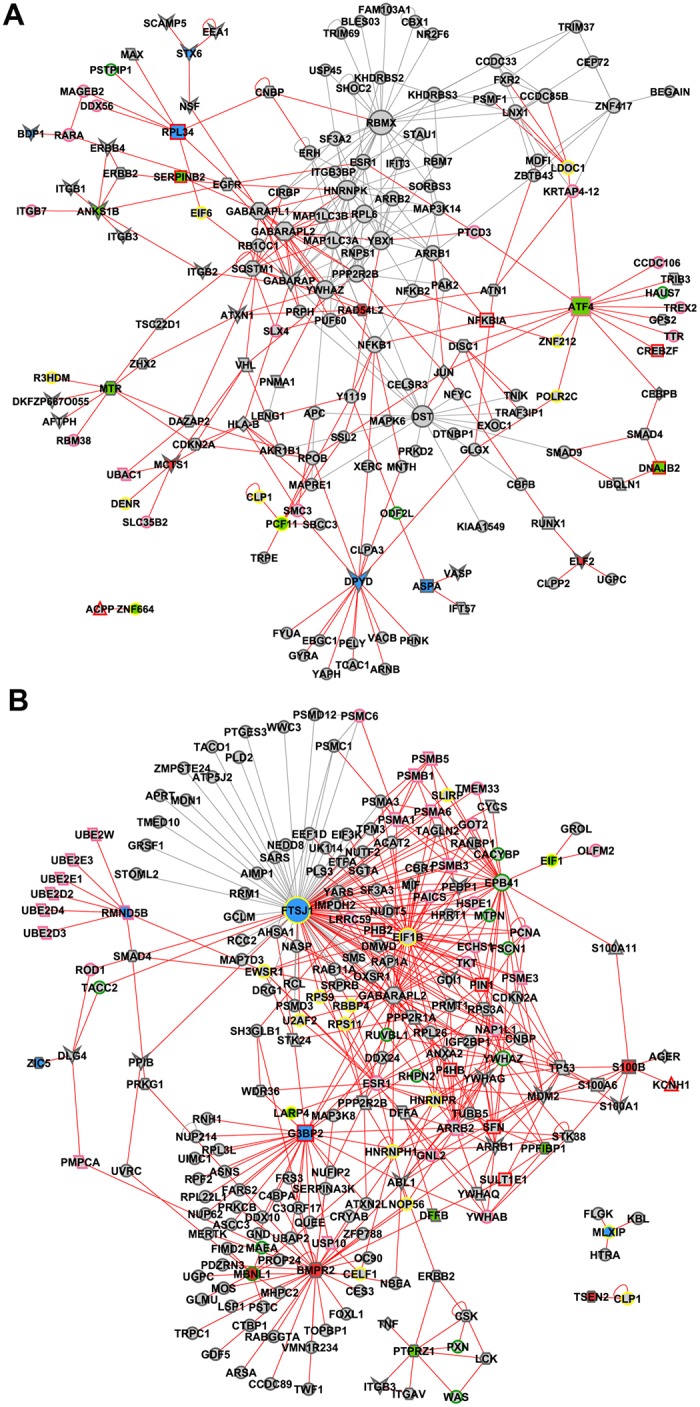
Interactome for E-CO (A) or L-CO (B) selected hubs (depicted in blue), VIPs (depicted in red) and high-hubs (depicted in green) using MINT and IntAct databases. Node size is related to node degree (number of links). Links in red represent the first and second node connections, for E-CO, or first, second and third node connections, for L-CO, centered in all hubs, VIPs and high-hubs, except FTSJ1 for L-CO. Node shapes and border colors represent biological processes, as follows: parallelogram for apoptosis; parallelogram with pink border for ubiquitination; octagon for autophagy; circle with pink or green or yellow border stand for cell processes or cytoskeleton or transcriptional regulation respectively; diamond for inflammation; triangle for ion binding; triangle with red border stand for ion channel; rectangle for neuroglia processes; rectangle with red or pink stand for neuroprotection or response to oxidative stress respectively; hexagon for neuronal development; vee for synaptic transmission.
Discussion
Community detection analysis of modular transcriptional repertoires for DE and CO gene coexpression networks, and the subsequent CGCS data analysis, revealed distinct molecular pathways for early- and late-onset forms of MTLE associated with childhood febrile seizures, as discussed below. Moreover, network connectivity is lower in both E networks when compared to the corresponding L networks, thus indicating that the hippocampal CA3 region of patients with early-onset FS-MTLE present a higher degree of dysregulation in cells’ functional organization [29, 32, 43]. Interactome analysis for experimentally verified protein-protein interactions confirmed the relevance of most high-hierarchy genes in E and L networks.
In the E-DE network coarse-grained community structure shows communities C and I as the ones with strongest connection weights. This C-I “axis”, described earlier in this paper, harbors high hierarchy genes with relevant roles in febrile-driven epilepsy and MTLE, such as SCN9A, RTN2 and BAIAP2, in community C, and B3GALTN1, KCN3 and MTA1 in community I. This synaptic excitability “axis” is seconded by the axis A-B, whose high hierarchy genes are also related to pro-epileptic roles, as SYN2 and NIDD (ZDHHC23). All this gene set is directly or indirectly involved with the molecular hallmarks of febrile seizures and epilepsy, namely: calcium and potassium channels, glutamate, NMDA receptors, HCN channels and HDACs [13]. Therefore on can conclude that E-DE network may well represent the “ground zero” of FS impact in hippocampal CA3.
The E-CO network, adopting the former analogy, would depict the derived “shock-waves” of FS impact on hippocampal CA3 cells. Indeed, the E-CO community with the strongest connection weights is community C, followed by communities D and H. The majority of the genes in the C-D-H interconnected communities are related to compensatory effects in epilepsy (seizure inhibition, neuronal survival and stress responses), what is in accordance with the concept that early onset epilepsies, although impacting more severely the ictal hippocampus, are associated to compensatory mechanisms [8]. Interestingly, this concept emerged from fMRI studies using network (graph theory) computational studies for assessing brain connectivity [6, 7, 8]. Hence, we can conclude that FS and FSE cause a perturbation in genomic and brain networks, determining “adaptive” rearrangements in both. The disease can be viewed as the breakdown of functional modules causing network reorganization. This issue will be further addressed below.
The L-DE network represents the “ground zero” of late-onset FS-MTLE, i.e., the GCN inferred from differentially expressed genes in the CA3 region of a hippocampus damaged by febrile IPI in early childhood but where temporal lobe epilepsy onset occurred after a long latent period (see Table 1). Here community C centers the strongest connection weights and also harbors most of the network’s VIPs and high hubs, what indicates its importance for network stability and functionality. The connection between C and F communities (Fig 6B) is the equivalent of the E-DE synaptic excitability C-I “axis”. Accordingly, the C-F “axis’ also harbors genetic and molecular hallmarks of epilepsy, such as: i) in community C the L-type calcium channel gene CACNA1, RTN2, a regulator of EAAC1 (also a VIP in E-DE network), the calcium binding/signaling genes TESC, CALM3, EFHD2; ii) in community F the gene CTNAP1, regulator of the glutamate receptor subunit GluA1. Moreover, as described before, other significant connections of community C—the communities A, H, and B—include several genes involved in epilepsy processes, like SXN25 and ARPC5L, markers of intractable epilepsy. Since L-DE high hierarchy genes, as a whole, are involved neuronal excitability or playing roles in other epilepsy-related processes, this network is functionally similar to E-DE. However, it reflects distinctive adaptive response to febrile seizures, probably related to late-onset MTLE development.
Contrarily to the observed for E-CO network, L-CO communities with strongest weighted connections harbor more high hierarchy genes related to pro-epileptic effects, or vulnerability to epilepsy, than to compensatory mechanisms. This finding, fully described in the Results section, is in accordance to the concept that early-onset epilepsies are associated with compensatory mechanisms, because the younger brain is more plastic, whereas in late-onset developed epilepsies the mature injured brain is less able to generate adaptive compensatory mechanisms [8, 186].
Imaging studies (structural and functional MRI) and graph theory models of brain connectivity led to significant progress in understanding the pathophysiology of temporal lobe epilepsy, [6, 8, 187]. Essentially, these studies showed that TLE is a network disease, i.e. a system disorder that alters local and distributed brain networks [6, 188]. Similarly to the application of complex network analysis to the study of gene-gene and protein-protein interactions, graph theory modeling of brain networks allows a quantitative description of the topological organization of brain connectivity based on network property measures, where nodes represent brain regions interconnected by edges. In this context, communities, or modules, are groups of highly connected nodes within the brain networks, modularity describes hierarchical organization, and network hubs are the nodes with greater degree centrality, i.e., those mediating most of the short path lengths between nodes. According to their community insertion and connectivity profile, hubs mediate intra- or inter module connectivity [6, 26, 188].
Complex network analyses of human brain connectivity revealed that the hippocampal formation has a concentration of densely linked nodes with very high degree centrality [189]. Increased clustering, an indicator of low connectivity, and alterations in the distribution of network hubs were detected in patients with refractory MTLE comparatively to normal controls [190]. Moreover, it was found, by comparing brain connectivity parameters of early- and late-onset MTLE patients who underwent resting-state fMRI scan, that late-onset patients had lower connectivity and higher modularity [8], thus indicating a certain degree of modular interaction disorganization in late-onset cases. These MRI data have a striking similarity with our findings on community structure analysis of transcriptional networks in early- and late-onset MTLE: i) comparative alterations in modularity/connectivity and hub and modular distributions; ii) evidences that in late MTLE development the brain is less able to generate adaptive mechanisms.
Altogether, gene coexpression and MRI networks studies on MTLE show that this disease, as other chronic non-communicable diseases, stems from environmentally-induced perturbations of complex intra and intercellular networks, probably modulated by the individual’s genomic makeup. Network reorganization following these perturbations may be kept stable for long periods due to epigenetic mechanisms acting just after the insult, and/or just after epilepsy onset [18, 19]. In fact, it was shown in kainic acid mouse model of epilepsy that genome-wide methylation status change after status epilepticus and in epileptic tolerance [191], thus contributing to regulate gene expression (and to reorganize gene-gene interaction networks) in the seizure-damaged hippocampus.
The complex network analyses performed in this study allowed a broad and more detailed view of genomic and molecular mechanisms involved in early and late-onset MTLE, in comparison to analyses centered solely on differentially expressed genes. Network centrality in DE and CO networks, is consistent with the network disease model, where a group of nodes whose perturbation (e.g. febrile IPI) leads to a disease phenotype occupies a central position in the network [4, 28, 192].
Most importantly, our data on hippocampal CA3 gene coexpression networks are in agreement with previous fMRI data showing that early onset epilepsies, although impacting more severely the ictal hippocampus, are associated to compensatory mechanisms, while in late MTLE development the brain is less able to generate adaptive mechanisms. This is very significant if one considers that the identification of windows of opportunity for antiepileptogenic interventions depends on a better understanding of the mechanisms occurring during the seizure-free interval, or latent period, in MTLE [23]. On the other hand, the probability of exerting therapeutic effects through the modulation of particular genes will be higher if these genes are highly interconnected in transcriptional networks [4, 2, 193]. In epilepsy—a disease affecting more than 50 million people around the world and where 30% of the patients do not respond to the available antiepileptic drugs—this systems biology approach seems to be mandatory for discovering new multi-target drugs, since hitting a single target does not treat complex diseases. In conclusion, a network-based approach to intractable epilepsy would be probably more effective than the “silver bullets” sought at the beginning of medical genomics.
Supporting Information
Scatter-plot of epilepsy duration (Figure A) and age at surgery (Figure B), in years, for early and late-onset MTLE patients and t-test p-value.
(TIF)
Scatter plots of node degree (k0) vs concentric node degree (k1) measures of GO annotated genes in L-DE. Hubs (blue), VIPs (red) and high-hubs (green), identified by their gene symbols. The nodes from community F are identified by orange dots/gene symbols.
(TIF)
Functional description of interactome nodes linked in first and second levels; centered in hubs, high-hubs and VIPs.
(PDF)
Functional description of interactome nodes linked in first and second levels; centered in hubs, high-hubs and VIPs.
(PDF)
Functional description of interactome nodes linked in first and second levels; centered in hubs, high-hubs and VIPs.
(PDF)
Functional description of interactome nodes linked in first and second levels; centered in hubs, high-hubs and VIPs.
(PDF)
High-hubs, Hubs and VIPs are identified by their gene symbols. Communities are indicated by different colors.
(MP4)
High-hubs, Hubs and VIPs are identified by their gene symbols. Communities are indicated by different colors.
(MP4)
Acknowledgments
We are indebted to Leandro Rodrigues Ferreira from the Laboratory of Pediatric Genomics, Department of Pediatrics, FMUSP, for valuable technical help.
Data Availability
All data are contained within the paper and Supporting Information Files.
Funding Statement
This work was funded by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) research grants 2009/53443-1 and 2005/56446-0 and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) grant 305635/2009-3 to CAM-F. LdFC was funded by FAPESP grants 2005/00587- 5 and 2011/50761-2 and CNPq grants 307333/2013-2. FNS and PI were funded by Coordenação de Aperfeiçoamento de Pessoal de Nivel Superior (CAPES). FNS, CAM-F and LdFC are funded by FAPESP-MCT/CNPq/PRONEX grant 2011/50761-2.
References
- 1. Heuser K, Cvancarova M, Gjerstad L, Taubøll E (2011) Is Temporal Lobe Epilepsy with childhood febrile seizures a distinctive entity? A comparative study. Seizure 20: 163–166. 10.1016/j.seizure.2010.11.015 [DOI] [PubMed] [Google Scholar]
- 2. Alegro MC, Silva AV, Bando SY, Lopes RD, Castro LH, Hungtsu W, et al. (2012) Texture analysis of high resolution MRI allows discrimination between febrile and afebrile initial precipitating injury in mesial temporal sclerosis. Magn Reson Med 68: 1647–1653. 10.1002/mrm.24174 [DOI] [PubMed] [Google Scholar]
- 3. Bando SY, Alegro MC, Amaro E Jr, Silva AV, Castro LHM, Wen H-T, et al. (2011) Hippocampal CA3 Transcriptome Signature Correlates with Initial Precipitating Injury in Refractory Mesial Temporal Lobe Epilepsy. PLoS ONE 6(10): e26268 10.1371/journal.pone.0026268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bando SY, Silva FN, Costa L da F, Silva AV, Pimentel-Silva LR, Castro LHM, et al. (2013) Complex Network Analysis of CA3 Transcriptome Reveals Pathogenic and Compensatory Pathways in Refractory Temporal Lobe Epilepsy. PLoS ONE 8(11): e79913 10.1371/journal.pone.0079913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Janszky J, Janszky I, Ebner A (2004) Age at onset in mesial temporal lobe epilepsy with a history of febrile seizures. Neurology 63: 1296–1298. [DOI] [PubMed] [Google Scholar]
- 6. Chiang S, Haneef Z (2014) Graph theory findings in the pathophysiology of temporal lobe epilepsy. Clin Neurophysiol 125: 1295–1305. 10.1016/j.clinph.2014.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Doucet G, Osipowicz K, Sharan A, Sperling MR, Tracy JI (2013) Extratemporal functional connectivity impairments at rest are related to memory performance in mesial temporal epilepsy. Hum Brain Mapp 34: 2202–2216. 10.1002/hbm.22059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Doucet GE, Sharan A, Pustina D, Skidmore C, Sperling MR, Tracy JI (2015) Early and late age of seizure onset have a differential impact on brain resting-state organization in temporal lobe epilepsy. Brain Topogr 28: 113–126. 10.1007/s10548-014-0366-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Davies KG, Hermann BP, Dohan FC Jr, Foley KT, Bush AJ, Wyler AR (1996) Relationship of hippocampal sclerosis to duration and age of onset of epilepsy, and childhood febrile seizures in temporal lobectomy patients. Epilepsy Res 24: 119–126. [DOI] [PubMed] [Google Scholar]
- 10. Chungath M, Shorvon S (2008) The mortality and morbidity of febrile seizures. Nat Clin Pract Neurol 4: 610–621. 10.1038/ncpneuro0922 [DOI] [PubMed] [Google Scholar]
- 11. Bilevicius E, Yasuda CL, Silva MS, Guerreiro CA, Lopes-Cendes I, Cendes F (2010) Antiepileptic drug response in temporal lobe epilepsy: a clinical and MRI morphometry study. Neurology 75: 1695–1701. 10.1212/WNL.0b013e3181fc29dd [DOI] [PubMed] [Google Scholar]
- 12. Sànchez J, Centanaro M, Solís J, Delgado F, Yépez L (2014) Factors predicting the outcome following medical treatment of mesial temporal epilepsy with hippocampal sclerosis. Seizure 23: 448–453. 10.1016/j.seizure.2014.03.003 [DOI] [PubMed] [Google Scholar]
- 13. Patterson KP, Baram TZ, Shinnar S (2014) Origins of temporal lobe epilepsy: febrile seizures and febrile status epilepticus. Neurotherapeutics 11: 242–250. 10.1007/s13311-014-0263-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Balosso S, Ravizza T, Aronica E, Vezzani A (2013) The dual role of TNF-α and its receptors in seizures. Exp Neurol 247: 267–271. 10.1016/j.expneurol.2013.05.010 [DOI] [PubMed] [Google Scholar]
- 15. Choy M, Dubé CM, Patterson K, Barnes SR, Maras P, Blood AB, et al. (2014) A novel, noninvasive, predictive epilepsy biomarker with clinical potential. J Neurosci 34: 8672–8684. 10.1523/JNEUROSCI.4806-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vezzani A (2014) Epilepsy and inflammation in the brain: overview and pathophysiology. Epilepsy Curr 14(1 Suppl): 3–7. 10.5698/1535-7511-14.s2.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cornejo BJ, Mesches MH, Coultrap S, Browning MD, Benke TA (2007) A single episode of neonatal seizures permanently alters glutamatergic synapses. Ann Neurol 61: 411–426. [DOI] [PubMed] [Google Scholar]
- 18. Kobow K, Blümcke I (2012). The emerging role of DNA methylation in epileptogenesis. Epilepsia 53(Suppl 9): 11–20. 10.1111/epi.12031 [DOI] [PubMed] [Google Scholar]
- 19. Ryley Parrish R, Albertson AJ, Buckingham SC, Hablitz JJ, Mascia KL, Davis Haselden W, et al. (2013) Status epilepticus triggers early and late alterations in brain-derived neurotrophic factor and NMDA glutamate receptor Grin2b DNA methylation levels in the hippocampus. Neuroscience 248: 602–619. 10.1016/j.neuroscience.2013.06.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ahmad S, Marsh ED (2010) Febrile status epilepticus: current state of clinical and basic research. Semin Pediatr Neurol 17: 150–154. 10.1016/j.spen.2010.06.004 [DOI] [PubMed] [Google Scholar]
- 21. McClelland S, Dubé CM, Yang J, Baram TZ (2011) Epileptogenesis after prolonged febrile seizures: mechanisms, biomarkers and therapeutic opportunities. Neurosci Lett 497: 155–162. 10.1016/j.neulet.2011.02.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dubé C, Richichi C, Bender RA, Chung G, Litt B, Baram TZ (2006) Temporal lobe epilepsy after experimental prolonged febrile seizures: prospective analysis. Brain 129: 911–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. White HS, Löscher W (2014) Searching for the ideal antiepileptogenic agent in experimental models: single treatment versus combinatorial treatment strategies. Neurotherapeutics 11: 373–384. 10.1007/s13311-013-0250-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Barabasi AL, Albert R (1999) Emergence of scaling in random networks. Science 286: 509–512. [DOI] [PubMed] [Google Scholar]
- 25. Newman MEJ (2003) The structure and function of complex networks. SIAM Rev 45: 167–256 [Google Scholar]
- 26. Costa L da F, Oliveira ON Jr, Travieso G, Rodrigues FA, Villas Boas PR, Antiqueira L, et al. (2011) Analyzing and modeling real-world phenomena with complex networks: a survey of applications. Adv Phys 60: 329–412. [Google Scholar]
- 27. Newman MEJ, Girvan M (2004) Finding and evaluating community structure in networks. Phys Rev E 69: 026113 [DOI] [PubMed] [Google Scholar]
- 28. Barabási AL, Gulbahce N, Loscalzo J (2011) Network Medicine: a network based approach to human disease. Nat Rev Genet 13: 56–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sahni N, Yi S, Zhong Q, Jailkhani N, Charloteaux B, Cusick ME, et al. (2013) Edgotype: a fundamental link between genotype and phenotype. Curr Opin Genet Dev 23: 649–657. 10.1016/j.gde.2013.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chaussabel D, Baldwin N (2014) Democratizing systems immunology with modular transcriptional repertoire analyses. Nat Rev Immunol 14: 271–280. 10.1038/nri3642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Moreira-Filho CA, Bando SY, Bertonha FB, Silva FN, Costa L da F (2014a) Methods for gene coexpression network visualization and analysis In: Passos GA, editor. Transcriptomics in Health and Disease. Cham, Switzerland: Springer International Publishing AG; pp. 79–94. [Google Scholar]
- 32. Moreira-Filho CA, Bando SY, Bertonha FB, Silva FN, Costa L da F, Carneiro-Sampaio M (2014b) Thymus gene coexpression networks: a comparative study in children with and without Down Syndrome In: Passos GA, editor. Transcriptomics in Health and Disease. Cham, Switzerland: Springer International Publishing AG; pp. 123–136. [Google Scholar]
- 33. Baulac M (2015) MTLE with hippocampal sclerosis in adult as a syndrome. Rev Neurol 26. pii: S0035-3787(15)00614-1. [DOI] [PubMed] [Google Scholar]
- 34. Blümcke I, Thom M, Aronica E, Armstrong DD, Bartolomei F, Bernasconi A, et al. (2013) International consensus classification of hippocampal sclerosis in temporal lobe epilepsy: a Task Force report from the ILAE Commission on Diagnostic Methods. Epilepsia 54:1315–29. 10.1111/epi.12220 [DOI] [PubMed] [Google Scholar]
- 35. Clauset A, Shallizi CR, Newman MEJ (2009) Power-law distributions in empirical data. SIAM Review 51: 661–703. [Google Scholar]
- 36. Costa L da F, Silva FN (2006) Hierarchical characterization of complex networks. J Stat Phys 125: 845–876. [Google Scholar]
- 37. Costa L da F, Tognetti MAR, Silva FN (2008) Concentric characterization and classification of complex network nodes: Application to an institutional collaboration network. Physica A 387: 6201–6214. [Google Scholar]
- 38. Brockmann D (2009) Human mobility and spatial disease dynamics In: Schuster HG, editor. Reviews of Nonlinear Dynamics and Complexity, vol 2 Weinheim (DE-BW), Germany: Wiley-VCH Verlag GmbH & Co. KGaA; pp. 1–24. [Google Scholar]
- 39. Zhu X, Gerstein M, Snyder M (2007) Getting connected: analysis and principles of biological networks. Genes Dev 21:1010–1024. [DOI] [PubMed] [Google Scholar]
- 40. Newman MEJ (2010) Networks: An Introduction. Oxford University Press, New York. [Google Scholar]
- 41. Blondel VD, Guillaume JL, Lambiotte R, Lefebvre E (2008) Fast unfolding of communities in large networks, J Stat Mech P10008. [Google Scholar]
- 42. Rosvall M, Bergstrom CT (2008) Maps of random walks on complex networks reveal community structure PNAS 105: 1118–1123. 10.1073/pnas.0706851105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Barabási AL, Oltvai ZN (2004) Network biology: understanding the cell's functional organization. Nat Rev Genet 5: 101–113. [DOI] [PubMed] [Google Scholar]
- 44. Hoang SA, Bekiranov S (2013) The network architecture of the Saccharomyces cerevisiae genome. PLoS One 8: e81972 10.1371/journal.pone.0081972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Narayanan T, Subramaniam S (2013) Community structure analysis of gene interaction networks in Duchenne muscular dystrophy. PLoS ONE 8: e67237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Singh NA, Pappas C, Dahle EJ, Claes LR, Pruess TH, De Jonqhe P, et al. (2009) A role of SCN9A in human epilepsies, as a cause of febrile seizures and as a potential modifier of Dravet syndrome. PLoS Genet 5: e1000649 10.1371/journal.pgen.1000649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mulley JC, Hodgson B, McMahon JM, Iona X, Bellows S, Mullen SA, et al. (2013) Role of the sodium channel SCN9A in genetic epilepsy with febrile seizures plus and Dravet syndrome. Epilepsia 54: e122–126. 10.1111/epi.12323 [DOI] [PubMed] [Google Scholar]
- 48. Liu Y, Vidensky S, Ruggiero AM, Maier S, Sitte HH, Rothstein JD (2008) Reticulon RTN2B regulates trafficking and function of neuronal glutamate transporter EAAC1. J Biol Chem 283: 6561–6571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bianchi MG, Bardelli D, Chiu M, Bussolati O (2014) Changes in the expression of the glutamate transporter EAAT3/EAAC1 in health and disease. Cell Mol Life Sci 71: 2001–2015. 10.1007/s00018-013-1484-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kim MH, Choi J, Yang J, Chung W, Kim JH, Paik SK, et al. (2009) Enhanced NMDA receptor-mediated synaptic transmission, enhanced long-term potentiation, and impaired learning and memory in mice lacking IRSp53. J Neurosci 29: 1586–1595. 10.1523/JNEUROSCI.4306-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Naylor DE, Liu H, Niquet J, Wasterlain CG (2013) Rapid surface accumulation of NMDA receptors increases glutamatergic excitation during status epilepticus. Neurobiol Dis 54: 225–238. 10.1016/j.nbd.2012.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Barreau C, Paillard L, Méreau A, Osborne HB (2006) Mammalian CELF/Bruno-like RNA-binding proteins: molecular characteristics and biological functions. Biochimie 88: 515–525. [DOI] [PubMed] [Google Scholar]
- 53. Wagnon JL, Briese M, Sun W, Mahaffey CL, Curk T, Rot G, et al. (2012) CELF4 regulates translation and local abundance of a vast set of mRNAs, including genes associated with regulation of synaptic function. PLoS Genet 8: e1003067 10.1371/journal.pgen.1003067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ravenall SJ, Gavazzi I, Wood JN, Akopian AN (2002) A peripheral nervous system actin-binding protein regulates neurite outgrowth. Eur J Neurosci 15: 281–290. [DOI] [PubMed] [Google Scholar]
- 55. Hasegawa H, Abbott S, Han BX, Qi Y, Wang F (2007) Analyzing somatosensory axon projections with the sensory neuron-specific Advillin gene. J Neurosci 27: 14404–14414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ludwig A, Uvarov P, Soni S, Thomas-Crusells J, Airaksinen MS, Rivera C (2011) Early growth response 4 mediates BDNF induction of potassium chloride cotransporter 2 transcription. J Neurosci 31: 644–649. 10.1523/JNEUROSCI.2006-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Puskarjov M, Seja P, Heron SE, Williams TC, Ahmad F, Iona X, et al. (2014) A variant of KCC2 from patients with febrile seizures impairs neuronal Cl- extrusion and dendritic spine formation. EMBO Rep 15: 723–729. 10.1002/embr.201438749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Dubé CM, Brewster AL, Baram TZ (2009) Febrile seizures: mechanisms and relationship to epilepsy. Brain Dev 31: 366–371. 10.1016/j.braindev.2008.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sasaki K, Matsuo M, Maeda T, Zaitsu M, Hamasaki Y (2009) Febrile seizures: characterization of double-stranded RNA-induced gene expression. Pediatr Neurol 41: 114–118. 10.1016/j.pediatrneurol.2009.03.003 [DOI] [PubMed] [Google Scholar]
- 60. Bürk K, Strzelczyk A, Reif PS, Figueroa KP, Pulst SM, Zühlke C, et al. (2013) Mesial temporal lobe epilepsy in a patient with spinocerebellar ataxia type 13 (SCA13). Int J Neurosci 123: 278–282. 10.3109/00207454.2012.755180 [DOI] [PubMed] [Google Scholar]
- 61. Zha Q, Brewster AL, Richichi C, Bender RA, Baram TZ (2008) Activity-dependent heteromerization of the hyperpolarization-activated, cyclic-nucleotide gated (HCN) channels: role of N-linked glycosylation. J Neurochem 105: 68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ghanta KS, Li DQ, Eswaran J, Kumar R (2011) Gene profiling of MTA1 identifies novel gene targets and functions. PLoS ONE 6: e17135 10.1371/journal.pone.0017135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Millard CJ, Fairall L, Schwabe JW (2014) Towards an understanding of the structure and function of MTA1. Cancer Metastasis Rev 33: 857–867. 10.1007/s10555-014-9513-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Huang Y, Zhao F, Wang L, Yin H, Zhou C, Wang X (2012) Increased expression of histone deacetylases 2 in temporal lobe epilepsy: a study of epileptic patients and rat models. Synapse 66: 151–159. 10.1002/syn.20995 [DOI] [PubMed] [Google Scholar]
- 65. Medrihan L, Cesca F, Raimondi A, Lignani G, Baldelli P, Benfenati F (2013) Synapsin II desynchronizes neurotransmitter release at inhibitory synapses by interacting with presynaptic calcium channels. Nat Commun 4: 1512 10.1038/ncomms2515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Feliciano P, Andrade R, Bykhovskaia M (2013) Synapsin II and Rab3a cooperate in the regulation of epileptic and synaptic activity in the CA1 region of the hippocampus. J Neurosci 33: 18319–18330. 10.1523/JNEUROSCI.5293-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Gasteier JE, Schroeder S, Muranyi W, Madrid R, Benichou S, Fackler OT. (2005) FHOD1 coordinates actin filament and microtubule alignment to mediate cell elongation. Exp Cell Res 306: 192–202. [DOI] [PubMed] [Google Scholar]
- 68. Witte H, Neukirchen D, Bradke F (2008) Microtubule stabilization specifies initial neuronal polarization. J Cell Biol 180: 619–632. 10.1083/jcb.200707042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Bartolini F, Gundersen GG (2010) Formins and microtubules. Biochim Biophys Acta 1803: 164–173. 10.1016/j.bbamcr.2009.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Lee MC, Kim GM, Woo YJ, Kim MK, Kim JH, Nam SC, et al. (2001) Pathogenic significance of neuronal migration disorders in temporal lobe epilepsy. Hum Pathol 32: 643–648. [DOI] [PubMed] [Google Scholar]
- 71. Pechenino AS, Frick KM (2009) The effects of acute 17beta-estradiol treatment on gene expression in the young female mouse hippocampus. Neurobiol Learn Mem 91: 315–322. 10.1016/j.nlm.2008.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Leandro-García LJ, Leskelä S, Landa I, Montero-Conde C, López-Jiménez E, Letón R, et al. (2010) Tumoral and tissue-specific expression of the major human beta-tubulin isotypes. Cytoskeleton (Hoboken) 67: 214–223. 10.1002/cm.20436 [DOI] [PubMed] [Google Scholar]
- 73. Wang Y, Ma C, Zhang H, Wu J (2012) Novel protein pp3501 mediates the inhibitory effect of sodium butyrate on SH-SY5Y cell proliferation. J Cell Biochem 113: 2696–2703. 10.1002/jcb.24145 [DOI] [PubMed] [Google Scholar]
- 74. Yoo DY, Kim DW, Kim MJ, Choi JH, Jung HY, Nam SM, et al. (2015) Sodium butyrate, a histone deacetylase Inhibitor, ameliorates SIRT2-induced memory impairment, reduction of cell proliferation, and neuroblast differentiation in the dentate gyrus. Neurol Res 37: 69–76. 10.1179/1743132814Y.0000000416 [DOI] [PubMed] [Google Scholar]
- 75. Yang JW, Czech T, Felizardo M, Baumgartner C, Lubec G (2006) Aberrant expression of cytoskeleton proteins in hippocampus from patients with mesial temporal lobe epilepsy. Amino Acids 30: 477–493. [DOI] [PubMed] [Google Scholar]
- 76. Tian L, McClafferty H, Knaus HG, Ruth P, Shipston MJ (2012) Distinct acyl protein transferases and thioesterases control surface expression of calcium-activated potassium channels. J Biol Chem 287: 14718–14725. 10.1074/jbc.M111.335547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Saitoh F, Tian QB, Okano A, Sakagami H, Kondo H, Suzuki T (2004) NIDD, a novel DHHC-containing protein, targets neuronal nitric-oxide synthase (nNOS) to the synaptic membrane through a PDZ-dependent interaction and regulates nNOS activity. J Biol Chem 279: 29461–29468. [DOI] [PubMed] [Google Scholar]
- 78. N'Gouemo P (2011) Targeting BK (big potassium) channels in epilepsy. Expert Opin Ther Targets 15: 1283–1295. 10.1517/14728222.2011.620607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Pacheco Otalora LF, Hernandez EF, Arshadmansab MF, Francisco S, Willis M, Ermolinsky B, et al. (2008) Down-regulation of BK channel expression in the pilocarpine model of temporal lobe epilepsy. Brain Res 1200: 116–131. 10.1016/j.brainres.2008.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Alam H, Sehgal L, Kundu ST, Dalal SN, Vaidya MM (2011) Novel function of keratins 5 and 14 in proliferation and differentiation of stratified epithelial cells. Mol Biol Cell 22: 4068–4078. 10.1091/mbc.E10-08-0703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sha L, Xu Q (2014) Complex Roles of Notch Signaling in the Development of Temporal Lobe Epilepsy: Evidence and Speculation. Molecular & Cellular Epilepsy 1:-Not available-, ahead of print. In press.
- 82. Crepaldi L, Policarpi C, Coatti A, Sherlock WT, Jongbloets BC, Down TA, et al. (2013) Binding of TFIIIC to SINE Elements Controls the Relocation of Activity-Dependent Neuronal Genes to Transcription Factories. PLoS Genet 9: e1003699 10.1371/journal.pgen.1003699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Simms BA, Zamponi GW (2014) Neuronal voltage-gated calcium channels: structure, function, and dysfunction. Neuron 82: 24–45. 10.1016/j.neuron.2014.03.016 [DOI] [PubMed] [Google Scholar]
- 84. Krug A, Witt SH, Backes H, Dietsche B, Nieratschker V, Shah NJ, et al. (2014) A genome-wide supported variant in CACNA1C influences hippocampal activation during episodic memory encoding and retrieval. Eur Arch Psychiatry Clin Neurosci 264: 103–110 10.1007/s00406-013-0428-x [DOI] [PubMed] [Google Scholar]
- 85. Gomez-Ospina N, Panagiotakos G, Portmann T, Pasca SP, Rabah D, Budzillo A, et al. (2013) A promoter in the coding region of the calcium channel gene CACNA1C generates the transcription factor CCAT. PLoS ONE 8: e60526 10.1371/journal.pone.0060526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Radzicki D, Yau HJ, Pollema-Mays SL, Mlsna L, Cho K, Koh S, et al. (2013) Temperature-sensitive Cav1.2 calcium channels support intrinsic firing of pyramidal neurons and provide a target for the treatment of febrile seizures. J Neurosci 33: 9920–9931. 10.1523/JNEUROSCI.5482-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Kondo T, Isono K, Kondo K, Endo TA, Itohara S, Vidal M, et al. (2014) Polycomb potentiates meis2 activation in midbrain by mediating interaction of the promoter with a tissue-specific enhancer. Dev Cell 28: 94–101. 10.1016/j.devcel.2013.11.021 [DOI] [PubMed] [Google Scholar]
- 88. Kuraoka I, Suzuki K, Ito S, Hayashida M, Kwei JS, Ikegami T, et al. (2007) RNA polymerase II bypasses 8-oxoguanine in the presence of transcription elongation factor TFIIS. DNA Repair (Amst) 6: 841–851. [DOI] [PubMed] [Google Scholar]
- 89. Shi Y, Ghosh M, Kovtunovych G, Crooks DR, Rouault TA (2012) Both human ferredoxins 1 and 2 and ferredoxin reductase are important for iron-sulfur cluster biogenesis. Biochim Biophys Acta 1823: 484–492. 10.1016/j.bbamcr.2011.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Liu G, Chen X (2002) The ferredoxin reductase gene is regulated by the p53 family and sensitizes cells to oxidative stress-induced apoptosis. Oncogene 21: 7195–7204. [DOI] [PubMed] [Google Scholar]
- 91. Rowley S, Patel M (2013) Mitochondrial involvement and oxidative stress in temporal lobe epilepsy. Free Radic Biol Med 62: 121–131. 10.1016/j.freeradbiomed.2013.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Chen ZL, Indyk JA, Strickland S (2003) The hippocampal laminin matrix is dynamic and critical for neuronal survival. Mol Biol Cell 14: 2665–2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Casanova JR, Nishimura M, Le J, Lam TT, Swann JW (2013) Rapid hippocampal network adaptation to recurring synchronous activity—a role for calcineurin. Eur J Neurosci 38: 3115–3127. 10.1111/ejn.12315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Choudhary S, Rosenblatt KP, Fang L, Tian B, Wu ZH, Brasier AR (2011) High throughput short interfering RNA (siRNA) screening of the human kinome identifies novel kinases controlling the canonical nuclear factor-κB (NF-κB) activation pathway. J Biol Chem 286: 37187–37195. 10.1074/jbc.M111.224923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Teocchi MA, Ferreira AÉ, da Luz de Oliveira EP, Tedeschi H, D'Souza-Li L (2013) Hippocampal gene expression dysregulation of Klotho, nuclear factor kappa B and tumor necrosis factor in temporal lobe epilepsy patients. J Neuroinflammation 10: 53 10.1186/1742-2094-10-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Huh YH, Kim SH, Chung KH, Oh S, Kwon MS, Choi HW, et al. (2013) Swiprosin-1 modulates actin dynamics by regulating the F-actin accessibility to cofilin. Cell Mol Life Sci 70: 4841–4854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Kim JE, Kim DW, Kwak SE, Kwon OS, Choi SY, Kang TC (2008) Potential role of pyridoxal-5'-phosphate phosphatase/chronopin in epilepsy. Exp Neurol 211: 128–140. 10.1016/j.expneurol.2008.01.029 [DOI] [PubMed] [Google Scholar]
- 98. Kim JE, Yeo SI, Ryu HJ, Kim MJ, Kim DS, Jo SM, et al. (2010) Astroglial loss and edema formation in the rat piriform cortex and hippocampus followingpilocarpine-induced status epilepticus. J Comp Neurol 518: 4612–4628. 10.1002/cne.22482 [DOI] [PubMed] [Google Scholar]
- 99. Jacquemet G, Morgan MR, Byron A, Humphries JD, Choi CK, Chen CS, et al. (2013) Rac1 is deactivated at integrin activation sites through an IQGAP1-filamin-A-RacGAP1 pathway. J Cell Sci 126: 4121–4135. 10.1242/jcs.121988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Yang F, Wang JC, Han JL, Zhao G, Jiang W (2008) Different effects of mild and severe seizures on hippocampal neurogenesis in adult rats. Hippocampus 18: 460–468. 10.1002/hipo.20409 [DOI] [PubMed] [Google Scholar]
- 101. Santos SD, Iuliano O, Ribeiro L, Veran J, Ferreira JS, Rio P, et al. (2012) Contactin-associated protein 1 (Caspr1) regulates the traffic and synaptic content of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)-type glutamate receptors. J Biol Chem 287: 6868–6877. 10.1074/jbc.M111.322909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Pandithage R, Lilischkis R, Harting K, Wolf A, Jedamzik B, Lüscher-Firzlaff J, et al. (2008) The regulation of SIRT2 function by cyclin-dependent kinases affects cell motility. J Cell Biol 180: 915–929. 10.1083/jcb.200707126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Lum H, Hao Z, Gayle D, Kumar P, Patterson CE, Uhler MD (2002) Vascular endothelial cells express isoforms of protein kinase A inhibitor. Am J Physiol Cell Physiol 282: C59–66. [DOI] [PubMed] [Google Scholar]
- 104. Russo I, Bonini D, Via LL, Barlati S, Barbon A (2013) AMPA receptor properties are modulated in the early stages following pilocarpine-induced status epilepticus. Neuromolecular Med 15: 324–338. 10.1007/s12017-013-8221-6 [DOI] [PubMed] [Google Scholar]
- 105. da Fonseca AC, Matias D, Garcia C, Amaral R, Geraldo LH, Freitas C, et al. (2014) The impact of microglial activation on blood-brain barrier in brain diseases. Front Cell Neurosci 8: 362 10.3389/fncel.2014.00362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Du Y, Zou Y, Yu W, Shi R, Zhang M, Yang W, et al. (2013) Expression pattern of sorting Nexin 25 in temporal lobe epilepsy: a study on patients and pilocarpine-induced rats. Brain Res 1509: 79–85. 10.1016/j.brainres.2013.03.005 [DOI] [PubMed] [Google Scholar]
- 107. Ma Y, Qi X, Du J, Song S, Feng D, Qi J, et al. (2009) Identification of candidate genes for human pituitary development by EST analysis. BMC Genomics 10: 109 10.1186/1471-2164-10-109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. You JJ, Lin-Chao S (2010) Gas7 functions with N-WASP to regulate the neurite outgrowth of hippocampal neurons. J Biol Chem 285: 11652–11666. 10.1074/jbc.M109.051094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Xiao F, Wang XF, Li JM, Xi ZQ, Lu Y, Wang Let et al. (2008) Overexpression of N-WASP in the brain of human epilepsy. Brain Res 1233: 168–175. 10.1016/j.brainres.2008.07.101 [DOI] [PubMed] [Google Scholar]
- 110. Holm PC, Mader MT, Haubst N, Wizenmann A, Sigvardsson M, Götz M (2007) Loss- and gain-of-function analyses reveal targets of Pax6 in the developing mouse telencephalon. Mol Cell Neurosci 34: 99–119. [DOI] [PubMed] [Google Scholar]
- 111. Woods JO, Singh-Blom UM, Laurent JM, McGary KL, Marcotte EM (2013) Prediction of gene-phenotype associations in humans, mice, and plants using phenologs. BMC Bioinformatics 14: 203 10.1186/1471-2105-14-203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Kim KC, Lee DK, Go HS, Kim P, Choi CS, Kim JW, et al. (2014) Pax6-dependent cortical glutamatergic neuronal differentiation regulates autism-like behavior in prenatally valproic acid-exposed rat offspring. Mol Neurobiol 49: 512–528. 10.1007/s12035-013-8535-2 [DOI] [PubMed] [Google Scholar]
- 113. Weser S, Gruber C, Hafner HM, Teichmann M, Roeder RG, Seifart KH, et al. (2004) Transcription factor (TF)-like nuclear regulator, the 250-kDa form of Homo sapiens TFIIIB", is an essential component of human TFIIIC1 activity. J BiolChem 279: 27022–27029. [DOI] [PubMed] [Google Scholar]
- 114. Wu CC, Lin YC, Chen HT (2011) The TFIIF-like Rpc37/53 dimer lies at the center of a protein network to connect TFIIIC, Bdp1, and the RNA polymerase III active center. Mol Cell Biol 31: 2715–2728. 10.1128/MCB.05151-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Lauritzen F, de Lanerolle NC, Lee TS, Spencer DD, Kim JH, Bergersen LH, et al. (2011) Monocarboxylate transporter 1 is deficient on microvessels in the human epileptogenic hippocampus. Neurobiol Dis 41: 577–584. 10.1016/j.nbd.2010.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Lauritzen F, Perez EL, Melillo ER, Roh JM, Zaveri HP, Lee TS, et al. (2012) Altered expression of brain monocarboxylate transporter 1 in models of temporal lobe epilepsy. Neurobiol Dis 45: 165–176. 10.1016/j.nbd.2011.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Lauritzen F, Eid T, Bergersen LH (2013) Monocarboxylate transporters in temporal lobe epilepsy: roles of lactate and ketogenic diet. Brain Struct Funct [Epub ahead of print]. In press. [DOI] [PubMed] [Google Scholar]
- 118. Smirnova E, Shanbhag R, Kurabi A, Mobli M, Kwan JJ, Donaldson LW (2013) Solution structure and peptide binding of the PTB domain from the AIDA1 postsynaptic signaling scaffolding protein. PLoS ONE 8: e65605 10.1371/journal.pone.0065605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Trollér U, Raghunath A, Larsson C (2004) A possible role for p190RhoGAP in PKCepsilon-induced morphological effects. Cell Signal 16: 245–252. [DOI] [PubMed] [Google Scholar]
- 120. West S, Proudfoot NJ (2008) Human Pcf11 enhances degradation of RNA polymerase II-associated nascent RNA and transcriptional termination. Nucleic Acids Res 36: 905–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Turturici G, Sconzo G, Geraci F (2011) Hsp70 and its molecular role in nervous system diseases. Biochem Res Int 2011: 618127 10.1155/2011/618127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Viggiano A, Viggiano E, Monda M, Ingrosso D, Perna AF, De Luca B (2012) Methionine-enriched diet decreases hippocampal antioxidant defences and impairs spontaneous behaviour and long-term potentiation in rats. Brain Res 1471: 66–74. 10.1016/j.brainres.2012.06.048 [DOI] [PubMed] [Google Scholar]
- 123. Bhargava S, Tyagi SC (2014) Nutriepigenetic regulation by folate-homocysteine-methionine axis: a review. Mol Cell Biochem 387: 55–61. 10.1007/s11010-013-1869-2 [DOI] [PubMed] [Google Scholar]
- 124. Bai D, Zhang J, Xiao W, Zheng X (2014) Regulation of the HDM2-p53 pathway by ribosomal protein L6 in response to ribosomal stress. Nucleic Acids Res 42:1799–1811. 10.1093/nar/gkt971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Halder SK, Ueda H (2012) Regional distribution and cell type-specific subcellular localization of Prothymosin alpha in brain. Cell Mol Neurobiol 32: 59–66. 10.1007/s10571-011-9734-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Coxon CH, Sadler AJ, Huo J, Campbell RD (2012) An investigation of hierachical protein recruitment to the inhibitory platelet receptor, G6B-b. PLoS ONE 11: e49543 10.1371/journal.pone.0049543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Linnartz B, Neumann H (2013) Microglial activatory (immunoreceptor tyrosine-based activation motif)- and inhibitory (immunoreceptor tyrosine-based inhibition motif)-signaling receptors for recognition of the neuronal glycocalyx. Glia 61: 37–46. 10.1002/glia.22359 [DOI] [PubMed] [Google Scholar]
- 128. Linnartz-Gerlach B, Mathews M, Neumann H (2014) Sensing the neuronal glycocalyx by glial sialic acid binding immunoglobulin-like lectins. Neuroscience 275: 113–124. 10.1016/j.neuroscience.2014.05.061 [DOI] [PubMed] [Google Scholar]
- 129. Kienesberger PC, Oberer M, Lass A, Zechner R (2009) Mammalian patatin domain containing proteins: a family with diverse lipolytic activities involved in multiple biological functions. J Lipid Res 50 Suppl: S63–68. 10.1194/jlr.R800082-JLR200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Richardson RJ, Hein ND, Wijeyesakere SJ, Fink JK, Makhaeva GF (2013) Neuropathy target esterase (NTE): overview and future. Chem Biol Interact 203: 238–244. 10.1016/j.cbi.2012.10.024 [DOI] [PubMed] [Google Scholar]
- 131. Glynn P (2013) Neuronal phospholipid deacylation is essential for axonal and synaptic integrity. Biochim Biophys Acta 1831: 633–641. 10.1016/j.bbalip.2012.07.023 [DOI] [PubMed] [Google Scholar]
- 132. Bosio Y, Berto G, Camera P, Bianchi F, Ambrogio C, Claus P, et al. (2012) PPP4R2 regulates neuronal cell differentiation and survival, functionallycooperating with SMN. Eur J Cell Biol 91: 662–674. 10.1016/j.ejcb.2012.03.002 [DOI] [PubMed] [Google Scholar]
- 133. Souchet B, Guedj F, Sahún I, Duchon A, Daubigney F, Badel A, et al. (2014) Excitation/inhibition balance and learning are modified by Dyrk1a gene dosage. Neurobiol Dis 69: 65–75. 10.1016/j.nbd.2014.04.016 [DOI] [PubMed] [Google Scholar]
- 134. Zhang B, Tomita Y, Qiu Y, He J, Morii E, Noguchi S, et al. (2007) E74-like factor 2 regulates valosin-containing protein expression. Biochem Biophys Res Commun 356: 536–541. [DOI] [PubMed] [Google Scholar]
- 135. Furukawa A, Kawamoto Y, Chiba Y, Takei S, Hasegawa-Ishii S, Kawamura N, et al. (2011) Proteomic identification of hippocampal proteins vulnerable to oxidative stress in excitotoxin-induced acute neuronal injury. Neurobiol Dis 43: 706–714. 10.1016/j.nbd.2011.05.024 [DOI] [PubMed] [Google Scholar]
- 136. Lewerenz J, Sato H, Albrecht P, Henke N, Noack R, Methner A, et al. (2012) Mutation of ATF4 mediates resistance of neuronal cell lines against oxidative stress by inducing xCT expression. Cell Death Differ 19: 847–858. 10.1038/cdd.2011.165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Lewerenz J, Baxter P, Kassubek R, Albrecht P, Van Liefferinge J, Westhoff MA, et al. (2014) Phosphoinositide 3-Kinases Upregulate System x(c)(-) via Eukaryotic Initiation Factor 2α and Activating Transcription Factor 4—A Pathway Active in Glioblastomas and Epilepsy. Antioxid Redox Signal 20: 2907–2922. 10.1089/ars.2013.5455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Lewerenz J, Maher P (2009) Basal levels of eIF2alpha phosphorylation determine cellular antioxidant status by regulating ATF4 and xCT expression. J Biol Chem 284: 1106–1115. 10.1074/jbc.M807325200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Steiger JL, Bandyopadhyay S, Farb DH, Russek SJ (2004) cAMP response element-binding protein, activating transcription factor-4, and upstream stimulatory factor differentially control hippocampal GABABR1a and GABABR1b subunit gene expression through alternative promoters. J Neurosci 24: 6115–6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Wende H, Colonna M, Ziegler A, Volz A (1999) Organization of the leukocyte receptor cluster (LRC) on human chromosome 19q13.4. Mamm Genome 10: 154–160. [DOI] [PubMed] [Google Scholar]
- 141. Wang Y, Tang BL (2006) SNAREs in neurons—beyond synaptic vesicle exocytosis (Review). Mol Membr Biol 23: 377–384. [DOI] [PubMed] [Google Scholar]
- 142. Huang CT, Chen CH (2008) Identification of gene transcripts in rat frontal cortex that are regulated by repeated electroconvulsive seizure treatment. Neuropsychobiology 58: 171–177. 10.1159/000191123 [DOI] [PubMed] [Google Scholar]
- 143. Zhang SJ, Zou M, Lu L, Lau D, Ditzel DA, Delucinge-Vivier C, et al. (2009) Nuclear calcium signaling controls expression of a large gene pool: identification of a gene program for acquired neuroprotection induced by synaptic activity. PLoS Genet 5: e1000604 10.1371/journal.pgen.1000604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Francis JS, Strande L, Pu A, Leone P (2011) Endogenous aspartoacylase expression is responsive to glutamatergic activity in vitro and in vivo. Glia 59: 1435–1446. 10.1002/glia.21187 [DOI] [PubMed] [Google Scholar]
- 145. Vielhaber S, Niessen HG, Debska-Vielhaber G, Kudin AP, Wellmer J, Kaufmann J, et al. (2008) Subfield-specific loss of hippocampal N-acetyl aspartate in temporal lobe epilepsy. Epilepsia 49: 40–50. 10.1111/j.1528-1167.2008.01449.x [DOI] [PubMed] [Google Scholar]
- 146. Tiedje KE, Stevens K, Barnes S, Weaver DF (2010) Beta-alanine as a small molecule neurotransmitter. Neurochem Int 57: 177–188. 10.1016/j.neuint.2010.06.001 [DOI] [PubMed] [Google Scholar]
- 147. van Kuilenburg AB, Vreken P, Abeling NG, Bakker HD, Meinsma R, Van Lenthe H, et al. (1999) Genotype and phenotype in patients with dihydropyrimidine dehydrogenase deficiency. Hum Genet 104: 1–9. [DOI] [PubMed] [Google Scholar]
- 148. Willemsen MH, Vallès A, Kirkels LA, Mastebroek M, Olde Loohuis N, Kos A, et al. (2011) Chromosome 1p21.3 microdeletions comprising DPYD and MIR137 are associated with intellectual disability. J Med Genet 48: 810–818. 10.1136/jmedgenet-2011-100294 [DOI] [PubMed] [Google Scholar]
- 149. Jung JJ, Inamdar SM, Tiwari A, Choudhury A (2012) Regulation of intracellular membrane trafficking and cell dynamics by syntaxin-6. Biosci Rep 32: 383–391. 10.1042/BSR20120006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Bhanot K, Young KG, Kothary R (2011) MAP1B and clathrin are novel interacting partners of the giant cyto-linker dystonin. J Proteome Res 10: 5118–5127. 10.1021/pr200564g [DOI] [PubMed] [Google Scholar]
- 151. Moorthamer M, Chaudhuri B (1999) Identification of ribosomal protein L34 as a novel Cdk5 inhibitor. Biochem Biophys Res Commun 255: 631–638. [DOI] [PubMed] [Google Scholar]
- 152. Cheung ZH, Ip NY (2004) Cdk5: mediator of neuronal death and survival. Neurosci Lett 361: 47–51. [DOI] [PubMed] [Google Scholar]
- 153. Putkonen N, Kukkonen JP, Mudo G, Putula J, Belluardo N, Lindholm D, et al. (2011) Involvement of cyclin-dependent kinase-5 in the kainic acid-mediated degeneration of glutamatergic synapses in the rat hippocampus. Eur J Neurosci 34: 1212–1221. 10.1111/j.1460-9568.2011.07858.x [DOI] [PubMed] [Google Scholar]
- 154. Buchser WJ, Smith RP, Pardinas JR, Haddox CL, Hutson T, Moon L, et al. (2012) Peripheral nervous system genes expressed in central neurons induce growth on inhibitory substrates. PLoS ONE 7: e38101 10.1371/journal.pone.0038101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Griffin WS, Yeralan O, Sheng JG, Boop FA, Mrak RE, Rovnaghi CR, et al. (1995) Overexpression of the neurotrophic cytokine S100 beta in human temporal lobe epilepsy. J Neurochem 65: 228–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Zhou Y, Danbolt NC (2013) GABA and Glutamate Transporters in Brain. Front Endocrinol (Lausanne) 4: 165 10.3389/fendo.2013.00165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Lee TS, Bjørnsen LP, Paz C, Kim JH, Spencer SS, Spence DD, et al. (2006) GAT1 and GAT3 expression are differently localized in the human epileptogenic hippocampus. Acta Neuropathol 111: 351–363. [DOI] [PubMed] [Google Scholar]
- 158. Sałat K, Kulig K (2011) GABA transporters as targets for new drugs. Future Med Chem 3: 211–222. 10.4155/fmc.10.298 [DOI] [PubMed] [Google Scholar]
- 159. Bierhals T, Korenke GC, Uyanik G, Kutsche K (2013) Pontocerebellar hypoplasia type 2 and TSEN2: review of the literature and two novel mutations. Eur J Med Genet 56: 325–330. 10.1016/j.ejmg.2013.03.009 [DOI] [PubMed] [Google Scholar]
- 160. Menssen R, Schweiggert J, Schreiner J, Kusevic D, Reuther J, Braun B, et al. (2012) Exploring the topology of the Gid complex, the E3 ubiquitin ligase involved in catabolite-induced degradation of gluconeogenic enzymes. J Biol Chem 287: 25602–25614. 10.1074/jbc.M112.363762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Cloix JF, Hévor T (2009) Epilepsy, regulation of brain energy metabolism and neurotransmission. Curr Med Chem 16: 841–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Rothermundt M, Peters M, Prehn JH, Arolt V (2003) S100B in brain damage and neurodegeneration. Microsc Res Tech 60:614–32. [DOI] [PubMed] [Google Scholar]
- 163. Steinhoff BJ, Tumani H, Otto M, Mursch K, Wiltfang J, Herrendorf G, et al. (1999) Cisternal S100 protein and neuron-specific enolase are elevated and site-specific markers in intractable temporal lobe epilepsy. Epilepsy Res 36: 75–82. [DOI] [PubMed] [Google Scholar]
- 164. Lu C, Li J, Sun W, Feng L, Li L, Liu A, et al. (2010) Elevated plasma S100B concentration is associated with mesial temporal lobe epilepsy in Han Chinese: a case—control study. Neurosci Lett 484: 139–142. 10.1016/j.neulet.2010.08.036 [DOI] [PubMed] [Google Scholar]
- 165. Miyagi M, Mikawa S, Hasegawa T, Kobayashi S, Matsuyama Y, Sato K (2011) Bone morphogenetic protein receptor expressions in the adult rat brain. Neuroscience 176: 93–109. 10.1016/j.neuroscience.2010.12.027 [DOI] [PubMed] [Google Scholar]
- 166. Zhang D, Mehler MF, Song Q, Kessler JA (1998) Development of bone morphogenetic protein receptors in the nervous system and possible roles in regulating trkC expression. J Neurosci 18: 3314–3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Sato T, Mikawa S, Sato K (2010) BMP2 expression in the adult rat brain. J Comp Neurol 518: 4513–4530. 10.1002/cne.22469 [DOI] [PubMed] [Google Scholar]
- 168. Charytoniuk DA, Traiffort E, Pinard E, Issertial O, Seylaz J, Ruat M (2000) Distribution of bone morphogenetic protein and bone morphogenetic protein receptor transcripts in the rodent nervous system and up-regulation of bone morphogenetic protein receptor type II in hippocampal dentate gyrus in a rat model of global cerebral ischemia. Neuroscience 100: 33–43. [DOI] [PubMed] [Google Scholar]
- 169. Carpentier C, Ghanem D, Fernandez-Gomez FJ, Jumeau F, Philippe JV, Freyermuth F, et al. (2014) Tau exon 2 responsive elements deregulated in myotonic dystrophy type I are proximal to exon 2 and synergistically regulated by MBNL1 and MBNL2. Biochim Biophys Acta 1842: 654–664. 10.1016/j.bbadis.2014.01.004 [DOI] [PubMed] [Google Scholar]
- 170. DeVos SL, Goncharoff DK, Chen G, Kebodeaux CS, Yamada K, Stewart FR, et al. (2013) Antisense reduction of tau in adult mice protects against seizures. J Neurosci 33: 12887–12897. 10.1523/JNEUROSCI.2107-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Broomfield A, Gunny R, Ali I, Vellodi A, Prabhakar P (2013) A Clinically Severe Variant of β-Mannosidosis, Presenting with Neonatal Onset Epilepsy with Subsequent Evolution of Hydrocephalus. JIMD Rep 11: 93–97. 10.1007/8904_2013_227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Poppe R, Karbach U, Gambaryan S, Wiesinger H, Lutzenburg M, Kraemer M, et al. (1997) Expression of the Na+-D-glucose cotransporter SGLT1 in neurons. J Neurochem 69: 84–94. [DOI] [PubMed] [Google Scholar]
- 173. Lee CH, Javed D, Althaus AL, Parent JM, Umemori H (2012) Neurogenesis is enhanced and mossy fiber sprouting arises in FGF7-deficient mice during development. Mol Cell Neurosci 51: 61–67. 10.1016/j.mcn.2012.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Perosa SR, Porcionatto MA, Cukiert A, Martins JR, Passeroti CC, Amado D, et al. (2002) Glycosaminoglycan levels and proteoglycan expression are altered in the hippocampus of patients with mesial temporal lobe epilepsy. Brain Res Bull 58: 509–516. [DOI] [PubMed] [Google Scholar]
- 175. Zhang C, Raghupathi R, Saatman KE, LaPlaca MC, McIntosh TK (1999) Regional and temporal alterations in DNA fragmentation factor (DFF)-like proteins following experimental brain trauma in the rat. J Neurochem 73: 1650–1659. [DOI] [PubMed] [Google Scholar]
- 176. Cao G, Pei W, Lan J, Stetler RA, Luo Y, Nagayama T, et al. (2001) Caspase-activated DNase/DNA fragmentation factor 40 mediatesapoptotic DNA fragmentation in transient cerebral ischemia and in neuronal cultures. J Neurosci 21: 4678–4690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Yang R, Gaidamakov SA, Xie J, Lee J, Martino L, Kozlov G, et al. (2011) La-related protein 4 binds poly(A), interacts with the poly(A)-binding protein MLLE domain via a variant PAM2w motif, and can promote mRNA stability. Mol Cell Biol 31: 542–556. 10.1128/MCB.01162-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Hinnebusch AG (2014) The Scanning Mechanism of Eukaryotic Translation Initiation. Annu Rev Biochem [Epub ahead of print]. In press. [DOI] [PubMed] [Google Scholar]
- 179. Kobayashi T, Winslow S, Sunesson L, Hellman U, Larsson C (2012) PKCα binds G3BP2 and regulates stress granule formation following cellular stress. PLoS ONE 7: e35820 10.1371/journal.pone.0035820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Matsuki H, Takahashi M, Higuchi M, Makokha GN, Oie M, Fujii M (2013) Both G3BP1 and G3BP2 contribute to stress granule formation. Genes Cells 18: 135–146. 10.1111/gtc.12023 [DOI] [PubMed] [Google Scholar]
- 181. Arion D, Sabatini M, Unger T, Pastor J, Alonso-Nanclares L, Ballesteros-Yáñez I, et al. (2006) Correlation of transcriptome profile with electrical activity in temporal lobe epilepsy. Neurobiol Dis 22: 374–387. [DOI] [PubMed] [Google Scholar]
- 182. El-Hattab AW, Bournat J, Eng PA, Wu JB, Walker BA, Stankiewicz P, et al. (2011) Microduplication of Xp11.23p11.3 with effects on cognition, behavior, and craniofacial development. Clin Genet 79: 531–538. 10.1111/j.1399-0004.2010.01496.x [DOI] [PubMed] [Google Scholar]
- 183. Kanari Y, Sato Y, Aoyama S, Muta T (2013) Thioredoxin-interacting protein gene expression via MondoA is rapidly and transiently suppressed during inflammatory responses. PLoS ONE 8: e59026 10.1371/journal.pone.0059026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Erck C, Peris L, Andrieux A, Meissirel C, Gruber AD, Vernet M, et al. (2005) A vital role of tubulin-tyrosine-ligase for neuronal organization. Proc Natl Acad Sci USA 102: 7853–7858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Wei Z, Zheng S, Spangler SA, Yu C, Hoogenraad CC, Zhang M (2011) Liprin-mediated large signaling complex organization revealed by the liprin-α/CASK and liprin-α/liprin-β complex structures. Mol Cell 43: 586–598. 10.1016/j.molcel.2011.07.021 [DOI] [PubMed] [Google Scholar]
- 186. Helmstaedter C, Sonntag-Dillender M, Hoppe C, Elger CE (2004) Depressed mood and memory impairment in temporal lobe epilepsy as a function of focus lateralization and localization. Epilepsy Behav 5: 696–701. [DOI] [PubMed] [Google Scholar]
- 187. Sequeira KM, Tabesh A, Sainju RK, Desantis SM, Naselaris T, Joseph JE, et al. (2013) Perfusion network shift during seizures in medial temporal lobe epilepsy. PLoS ONE 8: e53204 10.1371/journal.pone.0053204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Bernhardt BC, Hong S, Bernasconi A, Bernasconi N (2013) Imaging structural and functional brain networks in temporal lobe epilepsy. Front Hum Neurosci 7: 624 10.3389/fnhum.2013.00624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. van den Heuvel MP, Sporns O (2011) Rich-club organization of the human connectome. J Neurosci 31: 15775–15786. 10.1523/JNEUROSCI.3539-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Bernhardt BC, Chen Z, He Y, Evans AC, Bernasconi N (2011). Graph-theoretical analysis reveals disrupted small-world organization of cortical thickness correlation networks in temporal lobe epilepsy. Cereb Cortex 21: 2147–2157. 10.1093/cercor/bhq291 [DOI] [PubMed] [Google Scholar]
- 191. Miller-Delaney SF, Das S, Sano T, Jimenez-Mateos EM, Bryan K, Buckley PG, et al. (2012) Differential DNA methylation patterns define status epilepticus and epileptic tolerance. J Neurosci 32: 1577–1588. 10.1523/JNEUROSCI.5180-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Cai JJ, Borenstein E, Petrov DA (2010) Broker genes in human disease. Genome Biol Evol 2: 815–825. 10.1093/gbe/evq064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Margineanu DG (2012) Systems biology impact on antiepileptic drug discovery. Epilepsy Research 98: 104–115. 10.1016/j.eplepsyres.2011.10.006 [DOI] [PubMed] [Google Scholar]
- 194. Saha S, Dey SK, Biswas A, Das P, Das MR, Jana SS (2013) The effect of including the C2 insert of nonmuscle myosin II-C on neuritogenesis. J Biol Chem 288: 7815–7828. 10.1074/jbc.M112.417196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Soerensen J, Jakupoglu C, Beck H, Förster H, Schmidt J, Schmahl W, et al. (2008) The role of thioredoxin reductases in brain development. PLoS ONE 3: e1813 10.1371/journal.pone.0001813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Huang J, Furuya A, Hayashi K, Furuichi T (2011) Interaction between very-KIND Ras guanine exchange factor and microtubule-associated protein 2, and its role in dendrite growth—structure and function of the second kinase noncatalytic C-lobe domain. FEBS J 278: 1651–1661. 10.1111/j.1742-4658.2011.08085.x [DOI] [PubMed] [Google Scholar]
- 197. Shen A, Wang H, Zhang Y, Yan J, Zhu D, Gu J (2002) Expression of beta-1,4-galactosyltransferase II and V in rat injured sciatic nerves. Neurosci Lett 327: 45–48. [DOI] [PubMed] [Google Scholar]
- 198. Sasaki N, Manya H, Okubo R, Kobayashi K, Ishida H, Toda T, et al. (2005) Beta4GalT-II is a key regulator of glycosylation of the proteins involved in neuronal development. Biochem Biophys Res Commun 333: 131–137. [DOI] [PubMed] [Google Scholar]
- 199. Marat AL, Dokainish H, McPherson PS (2011) DENN domain proteins: regulators of Rab GTPases. J Biol Chem 286: 13791–13800. 10.1074/jbc.R110.217067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Villarroel-Campos D, Gastaldi L, Conde C, Caceres A, Gonzalez-Billault C (2014) Rab-mediated trafficking role in neurite formation. J Neurochem 129: 240–248. 10.1111/jnc.12676 [DOI] [PubMed] [Google Scholar]
- 201. Ramirez DM, Khvotchev M, Trauterman B, Kavalali ET (2012) Vti1a identifies a vesicle pool that preferentially recycles at rest and maintains spontaneous neurotransmission. Neuron 73: 121–134. 10.1016/j.neuron.2011.10.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Nam J, Mah W, Kim E (2011) The SALM/Lrfn family of leucine-rich repeat-containing cell adhesion molecules. Semin Cell Dev Biol 22: 492–498. 10.1016/j.semcdb.2011.06.005 [DOI] [PubMed] [Google Scholar]
- 203. Charong N, Patmasiriwat P, Zenklusen JC (2011) Localization and characterization of ST7 in cancer. J Cancer Res Clin Oncol 137: 89–97. 10.1007/s00432-010-0863-2 [DOI] [PubMed] [Google Scholar]
- 204. Ogawa M, Nakamura N, Nakayama Y, Kurosaka A, Manya H, Kanagawa M, et al. (2013) GTDC2 modifies O-mannosylated α-dystroglycan in the endoplasmic reticulum to generate N-acetyl glucosamine epitopes reactive with CTD110.6 antibody. Biochem Biophys Res Commun 440: 88–93. 10.1016/j.bbrc.2013.09.022 [DOI] [PubMed] [Google Scholar]
- 205. Günzel D, Stuiver M, Kausalya PJ, Haisch L, Krug SM, Rosenthal R, et al. (2009) Claudin-10 exists in six alternatively spliced isoforms that exhibit distinct localization and function. J Cell Sci 122: 1507–1517. 10.1242/jcs.040113 [DOI] [PubMed] [Google Scholar]
- 206. Hakimi MA, Dong Y, Lane WS, Speicher DW, Shiekhattar R (2003) A candidate X-linked mental retardation gene is a component of a new family of histone deacetylase-containing complexes. J Biol Chem 278: 7234–7239. [DOI] [PubMed] [Google Scholar]
- 207. Kim H, Ramsay E, Lee H, Wahl S, Dionne RA (2009) Genome-wide association study of acute post-surgical pain in humans. Pharmacogenomics 10: 171–179. 10.2217/14622416.10.2.171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Aruga J (2004) The role of Zic genes in neural development. Mol Cell Neurosci 26: 205–221. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Scatter-plot of epilepsy duration (Figure A) and age at surgery (Figure B), in years, for early and late-onset MTLE patients and t-test p-value.
(TIF)
Scatter plots of node degree (k0) vs concentric node degree (k1) measures of GO annotated genes in L-DE. Hubs (blue), VIPs (red) and high-hubs (green), identified by their gene symbols. The nodes from community F are identified by orange dots/gene symbols.
(TIF)
Functional description of interactome nodes linked in first and second levels; centered in hubs, high-hubs and VIPs.
(PDF)
Functional description of interactome nodes linked in first and second levels; centered in hubs, high-hubs and VIPs.
(PDF)
Functional description of interactome nodes linked in first and second levels; centered in hubs, high-hubs and VIPs.
(PDF)
Functional description of interactome nodes linked in first and second levels; centered in hubs, high-hubs and VIPs.
(PDF)
High-hubs, Hubs and VIPs are identified by their gene symbols. Communities are indicated by different colors.
(MP4)
High-hubs, Hubs and VIPs are identified by their gene symbols. Communities are indicated by different colors.
(MP4)
Data Availability Statement
All data are contained within the paper and Supporting Information Files.