Abstract
Background
Several Western studies have revealed that among non-diabetics, glycosylated hemoglobin A1c (HbA1c) levels are higher in smokers than non-smokers. While studies conducted in Western populations consistently support this association, a recent meta-analysis reported that studies carried out in non-Western populations, including studies of Chinese, Egyptian, and Japanese-Americans, did not detect any significant differences in HbA1c levels between smokers and non-smokers.
Objectives
We assessed the association between smoking habits and HbA1c levels in the general Korean adult population using data from the Korean National Health and Nutrition Examination Survey (KNHANES) performed in 2011–2012.
Methods
A total of 10,241 participants (weighted n=33,946,561 including 16,769,320 men and 17,177,241 women) without diabetes were divided into four categories according to their smoking habits: never smokers (unweighted n/ weighted n= 6,349/19,105,564), ex-smokers (unweighted n/ weighted n= 1,912/6,207,144), current light smokers (<15 cigarettes per day, unweighted n/ weighted n=1,205/5,130,073), and current heavy smokers (≥15 cigarettes per day, unweighted n/ weighted n=775/3,503,781).
Results
In age- and gender-adjusted comparisons, the HbA1c levels of each group were 5.52 ± 0.01% in non-smokers, 5.49 ± 0.01% in ex-smokers, 5.53 ± 0.01% in light smokers, and 5.61 ± 0.02% in heavy smokers. HbA1c levels were significantly higher in light smokers than in ex-smokers (p = 0.033), and in heavy smokers compared with light smokers (p < 0.001). The significant differences remained after adjusting for age, gender, fasting plasma glucose, heavy alcohol drinking, hematocrit, college graduation, and waist circumference. Linear regression analyses for HbA1c using the above-mentioned variables as covariates revealed that a significant association between current smoking and HbA1c (coefficient 0.021, 95% CI 0.003–0.039, p = 0.019).
Conclusions
Current smoking was independently associated with higher HbA1c levels in a cigarette exposure-dependent manner in a representative population of Korean non-diabetic adults. In this study, we have observed an association between smoking status and HbA1c levels in non-diabetics drawn from a non-Western population, consistent with previous findings in Western populations.
Introduction
Glycosylated hemoglobin A1c (HbA1c) is a marker for long-term glucose control and provides an index of overall glycemic exposure and risk for long-term complications in patients with diabetes. It is used to guide management and adjust treatments for diabetic individuals. In addition to monitoring glycemic control, HbA1c is also used to diagnose diabetes and identify individuals at high risk of developing diabetes [1]. However, HbA1c levels can be influenced by various factors including age, ethnicity, conditions that alter red cell turnover, socioeconomic factors, and glucose homeostasis [1–6].
Cigarette smoking is a major public health problem and a well-known risk factor for cardiovascular disease and several malignancies. Many studies have shown that cigarette smoking is also associated with an increased risk of diabetes and insulin resistance [7–11]. Furthermore, several studies have reported that current smokers exhibit higher HbA1c levels than non-smokers, even in populations without diabetes [9,12–16].
Recently, Soraya et al. analyzed the difference in mean HbA1c levels between current smokers and never-smokers. Their meta-analysis included individuals from 14 countries and suggested that HbA1c levels are higher in smokers than in non-smokers without known diabetes.[17] Therein, higher HbA1c levels in current smokers were consitently reported in 10 out of 14 Western population studies, including those from Australia, France, the United States, the Netherlands, and Denmark. However, in Chinese, Egyptian, Kenyan and Japanese-American populations, differences in HbA1c levels were not detected; current smokers even showed lower HbA1c levels. Therefore, race/ethnicity, or western lifestyle may modify the association between smoking and HbA1c levels. Nevertheless, few studies have investigated the association between smoking status and HbA1c levels in large study samples, particularly in non-Western populations.
In the current study, we performed a cross-sectional analysis to investigate the association between HbA1c and smoking habits in a racially homogeneous Korean adult population.
Method
Study population and data collection
This study used data from the 2011–2012 Korea National Health and Nutrition Examination Survey (KNHANES), a cross-sectional and nationally representative survey conducted by the Korean Center for Disease Control for Health Statistics. The KNHANES has been performed periodically since 1998 to assess the health and nutritional status of the civilian, non-institutionalized Korean population. Participants were selected using proportional allocation systematic sampling with multistage stratification. A standardized interview was conducted in the homes of the participants to collect information regarding demographic variables, family history, medical history, medications used, and a variety of other health-related variables. The health interview included well-established questions to determine the demographic and socioeconomic characteristics of the subjects, including questions concerning age, education level, occupation, income, marital status, smoking habits, alcohol consumption, exercise, previous and current diseases, and family disease history. Smoking status was divided into four categories: never smoker, ex-smoker, current light smoker (<15 cigarettes per day), and current heavy smoker (≥15 cigarettes per day).
Subjects were asked whether they exercised with an intensity that caused slight breathing difficulty and sweating. Subjects who exercised regularly at moderate intensity were asked about the frequency at which they exercised per week and the length of time per exercise session. Regular exercise was defined as exercising five or more times per week. Alcohol consumption was assessed by questioning the subjects about their drinking behavior during the month prior to the interview. Heavy alcohol drinking was categorized as drinking four or more times per week. Hypertension was defined as a systolic blood pressure (BP) ≥140 mmHg, diastolic BP ≥90 mmHg, or the use of antihypertensive medications, irrespective of blood pressure. Diabetes was defined as a fasting plasma glucose ≥ 126 mg/dL (7.0 mmol/l), current use of anti-diabetes medications, or a previous diagnosis of diabetes by a physician. In this study, the definition of diabetes did not include HbA1c level criteria. Obesity was defined as a body mass index (BMI) ≥25 kg/m2 according to the Asia-Pacific obesity classification [18].
Height and weight were obtained using standardized techniques and equipment. Height was measured to the nearest 0.1 cm using a portable stadiometer (Seriter, Bismarck, ND, USA). Weight was measured to the nearest 0.1 kg using a Giant-150N calibrated balance-beam scale (Hana, Seoul, Korea). BMI was calculated by dividing weight by the square of the height (kg/m2). Systolic and diastolic BP were measured by standard methods using a sphygmomanometer and with the patient in the sitting position. Three measurements were made for all subjects at 5-min intervals, and the mean of the second and third measurements was used in the analysis.
Laboratory methods
Blood samples were collected in the morning after fasting for at least 8 h. Fasting plasma glucose (FPG), total cholesterol, triglycerides (TG), low-density lipoprotein cholesterol (LDL-C), and serum creatinine levels were measured using a Hitachi Automatic Analyzer 7600 (Hitachi, Tokyo, Japan). HbA1c was measured using high performance liquid chromatography (HLC-723G7, Tosoh, Tokyo, Japan). The detailed methods for comparing and verifying the validity and reliability of each survey were described previously [19].
Ethics statement
The institutional review board of Ilsan Paik Hospital, Republic of Korea (IB-2-1404-016) approved this study. After approval of the study proposal, the KNHANES dataset was made available at the request of the investigator. Since the dataset did not include any personal information and participant consent had already been given for the KNHANES, our study was exempt from participant consent requirements.
Statistical analyses
Participants in the Korean NHANES were selected for participation based upon a stratified, multi-stage probability-sampling design. Weights for each respondent, representing the inverse of their sampling probability, were provided by the Korean Center for Disease Control and have been used in most analyses presented in order to produce estimates representative of the non-institutionalized Korean civilian population. However, linear models used to identify statistically significant determinants of HbA1C level were not weighted. Statistical analyses were performed using SPSS software (ver. 21.0 for Windows; SPSS, Chicago, IL, USA) for all analyses. To consider relationships among the study variables, we have drawn a direct acyclic diagram (DAG) based on the previous research findings or known facts. (Fig 1) To compare demographic and clinical characteristics among groups according to smoking habits, we evaluated age by ANOVA (analysis of variance) and the percentage of males by the χ2-test. ANCOVA (analysis of covariance) with Bonferroni post-hoc test was used to adjust for age and gender (Table 1). General linear models were used to assess weighted HbA1c levels according to smoking habits before and after adjustment for confounders (Table 2). Age (year), sex (men/women), and FPG (mmol/l) were adjusted in Model1.
Fig 1. DAG summarizes the established and hypothesized causal relationships among variables under study.
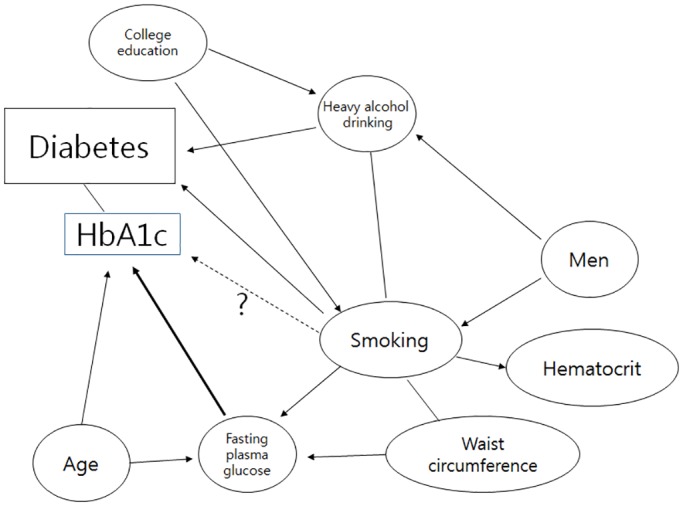
Table 1. Weighted, age- and sex-adjusted demographic and clinical characteristics by smoking status for Korean adults without diabetes, aged 19 years and older (2011–2012 KNHANES).
| Never smoker | Ex-smoker | Current smoker (<15 cigarettes/day) | Current smoker (≥ 15 cigarettes/day) | P | |
|---|---|---|---|---|---|
| Number (unweighted/weighted) | 6349/19105564 | 1912/6207144 | 1205/5130073 | 775/3503781 | |
| Age (years) | 44.5 ± 0.3 | 47.8 ± 0.5 | 38.9 ± 0.5 | 42.7 ± 0.5 | <0.001 |
| Women (%) | 78.0 ± 0.8 | 16.0 ± 0.9 | 22.0 ± 1.2 | 3.0 ± 0.6 | <0.001 |
| College graduation (%) | 32.9 ± 1.1 | 36.3 ± 1.6 | 30.6 ± 1.8 | 27.5 ± 2.1 | 0.022 |
| Heavy alcohol drinking (%) | 2.4 ± 0.3 | 10.6 ± 1.0 | 9.3 ± 1.0 | 19.5 ± 1.8 | <0.001 |
| Regular exercise (%) | 7.4 ± 0.5 | 8.2 ± 1.0 | 7.7 ± 1.0 | 6.5 ± 1.2 | 1.000 |
| Waist circumference (cm) | 80.1 ± 0.2 | 80.7 ± 0.3 | 80.6 ± 0.4 | 82.6 ± 0.5 | 0.006 |
| BMI (kg/m2) | 23.5 ± 0.1 | 23.6 ± 0.1 | 23.6 ± 0.2 | 24.2 ± 0.2 | 0.014 |
| Obesity (%) | 29.7 ± 0.9 | 30.9 ± 1.4 | 28.4 ± 1.8 | 41.2 ±2.4 | <0.001 |
| Systolic BP (mmHg) | 117.0 ± 0.3 | 115.9 ± 0.4 | 116.2 ± 0.5 | 116.6 ±0.6 | 0.162 |
| Diastolic BP (mmHg) | 76.0 ± 0.2 | 76.0 ± 0.3 | 75.3 ± 0.4 | 77.3 ±0.5 | 0.003 |
| Anti-hypertensive drug (%) | 10.7 ± 0.4 | 13.8 ± 0.9 | 11.9 ± 0.9 | 8.4 ± 1.0 | 0.005 |
| Hypertension (%) | 21.2 ± 0.7 | 25.2 ± 1.2 | 23.2 ± 1.3 | 24.4 ± 1.9 | 0.015 |
| FPG (mmol/l) | 5.12 ± 0.01 | 5.16 ± 0.02 | 5.09 ± 0.02 | 5.20 ± 0.03 | <0.001 |
| HbA1c (%) | 5.52 ± 0.01 | 5.49 ± 0.01 | 5.53 ± 0.01 | 5.61 ± 0.02 | <0.001 |
| HbA1c (mmol/mol) | 36.83 ± 0.11 | 36.50 ± 0.11 | 36.94 ± 0.11 | 37.81 ± 0.22 | <0.001 |
| Impaired fasting glucose (%) | 19.0 ± 0.8 | 21.0 ± 1.2 | 17.0 ± 1.3 | 26.0 ± 2.0 | 0.001 |
| Serum LDL-C (mg/dl) | 115.2 ± 1.3 | 115.6 ± 1.8 | 114.4 ± 2.1 | 119.2 ± 2.7 | 0.462 |
| Serum TG (mg/dl) | 118.0 ± 1.9 | 126.7 ± 2.7 | 138.6 ± 4.1 | 181.0 ± 7.1 | <0.001 |
| Anti-lipid drug (%) | 2.8 ± 0.2 | 3.8 ± 0.5 | 3.2 ± 0.4 | 3.3 ± 0.6 | 0.135 |
| Serum Creatinine (mg/dl) | 0.85 ± 0.01 | 0.84 ± 0.01 | 0.84 ± 0.01 | 0.84 ± 0.01 | 0.806 |
| Hematocrit (%) | 42.1 ± 0.1 | 41.9 ± 0.1 | 42.5 ± 0.1 | 43.3 ± 0.1 | <0.001 |
Data are expressed as mean with SEM. The estimates for age and sex are not adjusted. BMI, body mass index; BP, blood pressure; FPG, fasting plasma glucose; LDL-C, low density lipoprotein-cholesterol; TG, triglyceride; Heavy alcohol drinking, ≥ ×4 alcoholic drinks/week. Regular exercise, ≥ ×5 exercise/week; Obesity, BMI ≥ 25 kg/m2 or more; Hypertension, systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥90 mmHg or use of antihypertensive medications irrespective of BP;
Table 2. Weighted, multivariable-adjusted mean HbA1c (%) levels in Korean adults without diabetes by smoking status.
| Never smoker | Ex-smoker | Current smoker (<15 cigarettes/day) | Current smoker (≥ 15 cigarettes/day) | P | |
|---|---|---|---|---|---|
| Number (unweighted/weighted) | 6349/19105564 | 1912/6207144 | 1205/5130073 | 775/3503781 | |
| Model 1 | |||||
| NFG | 5.44 ± 0.01 | 5.41 ± 0.01 a | 5.46 ± 0.01 b | 5.53 ± 0.02 C | 0.001 |
| IFG | 5.82 ± 0.02 | 5.79 ± 0.02 | 5.87 ± 0.03 | 5.87 ± 0.03 | 0.096 |
| NFG+IFG | 5.52 ± 0.01 | 5.49 ± 0.01 a | 5.54 ± 0.01 b | 5.59 ± 0.02 C | 0.001 |
| Model 2 | |||||
| NFG | 5.44 ± 0.01 | 5.41 ± 0.01 a | 5.46 ± 0.01 b | 5.54 ± 0.02 C | <0.001 |
| IFG | 5.81 ± 0.02 | 5.80 ± 0.02 | 5.87 ± 0.03 b | 5.89 ± 0.03 | 0.116 |
| NFG+IFG | 5.52 ± 0.01 | 5.49 ± 0.01 a | 5.54 ± 0.01 b | 5.60 ± 0.02 C | <0.001 |
a, p< 0.05 vs. never-smoker;
b, p<0.01 vs. ex-smoker;
c, P<0.01 vs. current smoker (<15 cigarettes/day)
NFG, normal fasting glucose; IFG, impaired fasting glucose
Model 1, adjusted for age, sex, and fasting plasma glucose
Model 2, adjusted for heavy alcohol drinking, college graduation, hematocrit, waist circumference, and variables in Model 1
In Model 2, heavy alcohol drinking (≥ 4 alcoholic drinks/week/ <4 alcoholic drinks/week), college graduation (yes/no), hematocrit (%), and waist circumference (cm), as well as age, sex, and FPG, were adjusted for in the analysis.
Linear regression analysis for HbA1c was performed using age (years), gender (men/women), current smoking (yes/no), heavy alcohol drinking (≥ 4 alcoholic drinks/week/ <4 alcoholic drinks/week), college graduation (yes/no), waist circumference (cm), FPG (mmol/l), and hematocrit (%) levels as confounding variables (Table 3). There was no evidence that ANOVA and linear regression assumptions were violated. Logistic regression analysis was used to evaluate the odds ratios and 95% confidence intervals of ≥ 5.7% (the criteria of abnormal glucose regulation) and 6.1% HbA1c levels using age (by 10year increase), gender (men/ women), smoking status (never smoking/ ex-smoking/ current light smoking/current heavy smoking), heavy alcohol drinking (≥ 4 alcoholic drinks/week/ <4 alcoholic drinks/week), college graduation (yes/no), waist circumference (by 5cm increase), FPG (by 1 mmol/l), and hematocrit (by 2% increase) levels as confounding variables (Tables 4 and 5). The HbA1c cutoff value of 6.1% was selected, because a recent Korean study showed an HbA1c cutoff level of 6.1% to be the optimal corresponding value for diagnosing diabetes, along with the criteria of FPG ≥ 7.0 mmol/l and/or 2 hour plasma glucose ≥ 11.1 mmol/l upon a 75 gram oral glucose tolerance test (63.8% sensitivity and 88.1% specificity). An HbA1c threshold of 5.7% had reasonable sensitivity (48.6%) and specificity (65.7%) for identification of prediabetes.[20] All tests were two-sided, and P < 0.05 was considered statistically significant.
Table 3. Regression parameters from unweighted linear regression models to predict HbA1c levels in Korean adults without diabetes (n = 10,241).
| Coefficient (95% CI) | P | |
|---|---|---|
| Age (year) | 0.006 (0.006–0.0067) | <0.001 |
| Women | 0.060 (0.039–0.081) | <0.001 |
| Current smoking (10cm increase) | 0.021 (0.003–0.039) | 0.019 |
| Heavy alcohol drinking | -0.111 (-0.138- -0.084) | <0.001 |
| College graduation | -0.018 (-0.032- -0.003) | 0.019 |
| Waist circumference | 0.005 (0.004–0.005) | <0.001 |
| Fasting plasma glucose (mmol/l) | 0.283 (0.270–0296) | <0.001 |
| Hematocrit (%) | -0.001 (-0.004–0.001) | 0.176 |
| R2 | 0.2 | |
Table 4. Estimates of association from weighted logistic regression models to predict the risks of HbA1c ≥ 5.7% or ≥ 6.1% in Korean adults without diabetes (n = 10,241).
| For HbA1c ≥ 5.7% | For HbA1c ≥ 6.1% | |||
|---|---|---|---|---|
| Odds ratio (95% CI) | p | Odds ratio (95% CI) | p | |
| Age (10 year increase) | 1.52 (1.45–1.59) | <0.001 | 1.65 (1.53–1.77) | <0.001 |
| Men | 0.88 (0.71–1.08) | 0.203 | 0.68 (0.50–0.91) | 0.009 |
| Heavy alcohol drinking | 0.48 (0.37–0.64) | <0.001 | 0.42 (0.28–0.63) | <0.001 |
| Waist circumference (5cm increase) | 1.15 (1.10–1.19) | <0.001 | 1.20 (1.14–1.26) | <0.001 |
| College graduation | 0.95 (0.82–1.10) | 0.482 | 1.18 (0.91–1.53) | 0.211 |
| Smoking | <0.001 | <0.001 | ||
| Never smoking | reference | reference | ||
| Ex-smoking | 0.96 (0.78–1.17) | 0.675 | 0.83 (0.62–1.12) | 0.228 |
| Current smoking | ||||
| <15 cigarettes/day | 1.29 (1.04–1.60) | 0.002 | 1.21 (0.82–1.78) | 0.334 |
| ≥ 15 cigarettes /day | 1.84 (1.41–2.41) | <0.001 | 2.36 (1.58–3.52) | <0.001 |
| Hematocrit (2% increase) | 0.94 (0.90–0.99) | 0.011 | 0.93 (0.87–1.00) | 0.041 |
| Fasting plasma glucose (1mmol/l increase) | 3.72 (3.29–4.21) | <0.001 | 8.66 (7.24–10.35) | <0.001 |
All odds ratios were from weighted analyses.
Table 5. Estimates of association from weighted logistic regression models to predict the risks of HbA1c ≥ 5.7% or ≥ 6.1% in Korean adults without diabetes after exclusion of subjects with HbA1c ≥6.5% (n = 10,026).
| For HbA1c ≥ 5.7% | For HbA1c ≥ 6.1% | |||
|---|---|---|---|---|
| Odds ratio (95% CI) | p | Odds ratio (95% CI) | p | |
| Age (10 year increase) | 1.51 (1.44–1.58) | <0.001 | 1.62 (1.50–1.75) | <0.001 |
| Men | 0.88 (0.72–1.09) | 0.242 | 0.66 (0.48–0.91) | 0.011 |
| Heavy alcohol drinking | 0.49 (0.37–0.65) | <0.001 | 0.43 (0.27–0.68) | <0.001 |
| Waist circumference (5cm increase) | 1.14 (1.10–1.18) | <0.001 | 1.15 (1.09–1.22) | <0.001 |
| College graduation | 0.94 (0.82–1.09) | 0.423 | 1.14 (0.87–1.50) | 0.334 |
| Smoking | <0.001 | <0.001 | ||
| Never smoking | reference | reference | ||
| Ex-smoking | 0.97 (0.79–1.19) | 0.766 | 0.93 (0.67–1.28) | 0.644 |
| Current smoking | ||||
| <15 cigarettes/day | 1.29 (1.04–1.61) | 0.023 | 1.25 (0.82–1.90) | 0.297 |
| ≥ 15 cigarettes /day | 1.83 (1.39–2.41) | <0.001 | 2.53 (1.61–4.00) | <0.001 |
| Hematocrit (2% increase) | 0.94 (0.90–0.99) | 0.014 | 0.94 (0.87–1.01) | 0.082 |
| Fasting plasma glucose (1mmol/l increase) | 3.44 (3.04–3.89) | <0.001 | 6.82 (5.60–8.30) | <0.001 |
All odds ratios were from weighted analyses.
Results
Demographics and clinical characteristics of the study population
The demographics and clinical characteristics of the study population are shown in Table 1. Among the 11,473 adult participants (≥19 years of age) who participated in health interviews and laboratory examinations in the 2011–2012 KNHANES, 1,232 diabetic subjects were excluded. The remaining 10,241 participants (weighted n = 33,946,561 including 16,769,320 men and 17,177,241 women) were divided into four categories according to their smoking habits: never smokers (unweighted n/ weighted n = 6,349/19,105,564), ex-smokers (unweighted n/ weighted n = 1,912/6,207,144), current light smokers (<15 cigarettes per day, unweighted n/ weighted n = 1,205/5,130,073), and current heavy smokers (≥15 cigarettes per day, unweighted n/ weighted n = 775/3,503,781).
The mean ages of the never smokers, ex-smokers, current light smokers, and current heavy smokers were 44.5 ± 0.3, 47.8 ± 0.5, 38.9 ± 0.5, and 42.7 ± 0.5 years, respectively. The percentages of female participants in each smoking group were 78.0, 16.0, 22.0, and 3.0%, respectively (p < 0.001). In age- and gender-adjusted comparisons, the current heavy smokers had a higher BMI, waist circumference, serum TG levels, and fasting plasma glucose levels than current light smokers, never smokers, and ex-smokers. Heavy alcohol drinking and obesity were more common among current heavy smokers than current light smokers, never smokers, and ex-smokers. There was no difference in waist circumference or BMI among never smokers, ex-smokers, and current light smokers. Heavy alcohol drinking was more common in ex-smokers and current light smokers than never smokers.
Mean HbA1c levels according to smoking habits of the subjects with and without impaired FPG
After age- and gender-adjusted comparisons, mean HbA1c levels were higher among current smokers than never smokers and ex-smokers (5.56 ± 0.01, 5.52 ± 0.01, and 5.49 ± 0.01%, respectively). Among current smokers, heavy smokers also had a higher mean HbA1c level than light smokers (5.61 ± 0.02 vs. 5.53 ± 0.01%, p < 0.001). FPG levels were higher in current heavy smokers than current light smokers. However, there was no difference therein among never smokers, ex-smokers, and current light smokers (Table 1).
Age-, gender-, and FPG-adjusted HbA1c levels are shown in Table 2 (model 1). In all participants, including those with both normal and impaired FPG, mean HbA1c levels were slightly higher in current smokers than ex-smokers, even after adjusting for age, gender, and FPG. Furthermore, current heavy smokers demonstrated a higher mean HbA1c level than current light smokers, suggesting an exposure-dependent phenomenon. Subgroup analysis of individuals with normal FPG revealed similar results of a higher mean HbA1c level in current smokers than ex-smokers in a dose-dependent manner. Subgroup analysis of individuals with impaired FPG showed a similar trend, but without statistical significance.
A similar dose-dependent relationship between smoking exposure and HbA1c levels was identified in analyses using age, gender, FPG, heavy alcohol drinking, hematocrit, college graduation and waist circumference as covariates (Table 2, model 2; p < 0.001).
Factors associated with elevated HbA1c levels
Linear regression analysis to identify independent factors associated with HbA1c levels were performed using age, gender, current smoking, heavy alcohol drinking, college education, waist circumference, FPG, and hematocrit levels as confounding variables. In this model, current smoking was associated with HbA1c levels (coefficient 0.021, 95% CI 0.003–0.039, p = 0.019). (Table 3)
In the logistic regression analysis for ≥ 5.7% HbA1c levels, the current cutoff value for abnormal glucose regulation, using the above-mentioned variables as covariates, age, gender, heavy alcohol drinking, waist circumference, smoking habits, hematocrit, and FPG were associated with HbA1c levels. Using never smokers as a control, current light smoking (OR 1.29, 95% CI 1.04–1.60, p = 0.002) and current heavy smoking (OR 1.84, 95% CI 1.41–2.41, p < 0.001) were associated with ≥5.7% HbA1c levels. Ex smoking was not associated with ≥5.7% HbA1c levels. Furthermore, the OR for an HbA1c levels ≥6.1%, a FPG level of 126 mg/dl, and a 2-h plasma glucose level ≥200 mg/dl, according to an oral glucose tolerance test, in Korea[21], was 2.36 (95% CI 1.58–3.52, p < 0.001) in current heavy smokers. In addition, former smokers showed no significantly increased risk of elevated HbA1c levels compared with never smokers. (Table 4)
Since the definition of diabetes did not include HbA1c level criteria in this study, 215 patients who had HbA1c levels ≥ 6.5% and FPG <126 mg/dL were included in study population. Therefore, we performed additional logistic regression for HbA1c ≥ 5.7% or ≥ 6.1% using above- mentioned variables as covariates, after excluding subjects with HbA1c levels ≥ 6.5%. Using never smokers as a control, current light smoking (OR 1.29, 95% CI 1.04–1.61, p = 0.023) and current heavy smoking (OR 1.83, 95% CI 1.39–2.41, p < 0.001) were associated with HbA1c levels ≥5.7%. (Table 5)
Discussion
In the present study using KNHANES 2011–2012 data, we observed a significant association between smoking habits and HbA1c levels in the general adult population of Korea. Current smokers had higher HbA1c levels than former and never smokers in an exposure-dependent manner, even after adjusting for several clinical parameters that might affect the result.
Previously, an association between smoking and HbA1c levels was reported among healthy subjects without diabetes. Lincoln et al. discovered that mean HbA1c levels were lowest in never smokers, intermediate in former smokers, and highest in current smokers in a European multicenter International cohort study (the EPIC-Norfolk study) [15]. Their study also revealed an inverse association between years since smoking cessation and HbA1c levels. Jansen et al. also reported that smoking was independently associated with HbA1c levels in non-diabetic Dutch adults [16]. In the Scottish Health Survey, smokers had higher Hba1c levels than did non-smokers and were twice as likely to have HbA1c levels in the pre-diabetic range (5.7–6.4%) [22]. In a representative sample of the non-diabetic United States population, smokers had a 7% increase in HbA1c levels relative to never smokers[14].
All of the above studies were performed mainly in European and United States populations, which are both predominantly Caucasian. Furthermore, a recent meta-analysis revealed that higher HbA1c levels among current smokers are consistent in only Western population studies [17].
Nevertheless, this large population study of a racially homogeneous Korean population suggested that the association between smoking habits and HbA1c is also identifiable in non-Western populations without diabetes.
There are several hypotheses regarding the mechanism by which smoking increases HbA1c levels. Using data from the United States NHANES, Calore et al. reported that cotinine is associated with increased HbA1c levels [14]. Their study suggested that the link between smoking and HbA1c is at least partly due to the effects of nicotine. Consistent with this, a previous report showed that nicotine increased mTOR/P70S6K activity in cultured L6 myotubes, which was associated with increased IRS-1 Ser-636 phosphorylation, reduced insulin-stimulated glucose uptake, and subsequent insulin resistance [23]. Eliasson et al. found that the long-term use of nicotine-containing gum was associated with insulin resistance and hyperinsulinemia in subjects without diabetes [24]. However, in another study using euglycemic-clamps, nicotine infusion affected serum insulin levels in diabetic, but not healthy, subjects [25]. Nicotine also increased islet β-cell apoptosis via nicotinic acetylcholine receptors, leading to decreased insulin secretion. Mitochondrial dysfunction, oxidative stress, and inflammation all play a role in the direct toxicity induced by nicotine [26]. Taken together, these data suggest that nicotine exposure could negatively affect glucose metabolism by direct and/or indirect effects on insulin action, resulting in higher HbA1c levels even in non-diabetic smokers compared with never and former smokers.
Another hypothesis is that smoking could influence the formation of HbA1c indirectly, independent of its effect on blood glucose. Smoking might increase the passage of glucose across the erythrocyte membrane into cells, resulting in elevated HbA1c levels [27]. Soraya et al. suggested that smoking causes higher erythrocyte 2,3-diphosphoglycerate concentrations, conditions under which HbA1c formation is increased [17].
In addition, smoking causes increased oxidative stress and an associated increase in protein glycation, which can also increase HbA1c levels [22,28]. Conversely, antioxidant consumption and the levels of plasma antioxidants, such as vitamin C, were inversely correlated with HbA1c levels in non-diabetic individuals [29–31].
Although many clinical and experimental studies have reported an association between smoking and diabetes, it is unclear why smoking affects HbA1c levels, particularly in non-diabetic subjects. We excluded diabetic subjects in this study because use of anti-diabetes medications could have an effect on FPG and HbA1c levels. Furthermore, adjusting for confounding factors such as FPG had no effect on the statistical significance of the association between smoking and HbA1c. This supports the hypothesis that technical issues are associated with the HbA1c assay in samples from smokers, regardless of the mechanism involving smoking-induced glucose dysregulation.
In this study, we identified many non-glycemic determinants of HbA1c levels in non-diabetic subjects, in addition to smoking. The age-related increases in HbA1c levels reported in the current study were consistent with reports in large populations from China, Japan, and the United States [27,32–34]. These studies suggested altered rates of glycation and aging-associated renal function as possible explanations of age-related increases in HbA1c levels.
Alcohol consumption, a lifestyle parameter as important as smoking, was independently and negatively correlated with HbA1c levels, consistent with previous reports [29,35,36]. Previous studies suggested that moderate alcohol intake might have protective effects on glucose metabolism by lowering insulin resistance [37,38]. A meta-analysis also showed that moderate alcohol consumption decreased the relative risk for type 2 diabetes, compared to non-drinkers [39]. However, this positive effect of alcohol on glucose metabolism was attenuated in heavy drinkers, as heavy alcohol intake increased the risk of type 2 diabetes, compared to moderate alcohol intake. Such results suggest a “U-shaped” relationship between alcohol consumption and the risk of type 2 diabetes [39,40]. In the current study, the definition of heavy alcohol drinking was four or more drinks per week, which was less specific and more sensitive as a descriptor of high alcohol intake than the criteria of three or more drinks per day used in most previous studies [40]. Therefore, the correlation of heavy alcohol drinking with HbA1c levels between this and previous studies cannot be compared directly.
Weight circumference was also independently associated with HbA1c levels in this study. Because we included subjects with impaired FPG, some of them might have metabolic syndrome, which commonly includes central obesity and impaired FPG. Therefore, it is possible that there might be a residual confounding effect rather than a direct causal relationship.
We found that heavy smokers had higher BMI than current light smokers, never smokers, and ex-smokers, which is a contrast to the well documented effect for cigarette smokers to have lower BMI than non-smokers [41]. We speculate that this is due to clustering of risk behaviors potentially favoring weight gain in heavy smokers (e.g. unhealthy diet, physical inactivity) may outweigh the metabolic and anorectic effects of smoking. There are some studies showing similar results to our study. The Greek EPIC cohort study showed that tobacco smoking was positively associated with BMI and waist-to- hip ratio among smokers [42]. In the Swiss Healthy Survey, obesity was associated in a graded manner with the number of cigarettes smoked daily, particularly in men [43]. Another study also reported that moderate smokers had a decreased risk of obesity, compared with non-smokers (adjusted OR 0.4), but heavy smokers tended to have increased risk (OR 1.3) in Danish men 20 to 29 years of age [44]. Actually, heavy tobacco exposure exhibited the largest odds ratio (OR 2.4) for physical inactivity in the Danish study. Therefore, unhealthy life styles such as physical inactivity might account for the association between weight and smoking, especially in heavy smokers. The major strength of this study is that we included a large number of Korean adults and analyzed a nationally representative sample of adult Koreans without diabetes. To our knowledge, this is the first nationwide study that revealed a significant association between smoking habits and HbA1c levels in an Asian population. Although the difference in HbA1c between heavy smokers (5.60 ± 0.02%) and never smokers (5.52 ± 0.01%) was small in multivariable analysis, subjects with HbA1c levels around 5.7% (the current cutoff value for abnormal glucose regulation criteria) could be categorized as being at high risk for developing diabetes, depending on smoking status. The data showed that heavy current smokers were at a 1.83 times increased odds of belonging to the high risk group for developing diabetes, compared with non-smokers. Since this study included only subjects without diabetes, we could not confirm the effect of smoking status on diagnosis of diabetes with HbA1c of 6.5%. Nevertheless, this result suggested that smoking status needs to be taken into account when using HbA1c levels to screen for prediabetes or diabetes. In this respect, our findings hold clinical importance from a public health point of view.
This study has some limitations. First, we could not assess the accuracy of smoking exposure, because this study was based on self-reported smoking habits. Second, although we adjusted for many confounding factors, residual or hidden confounding variables could not be excluded, similar to other observational studies. We also could not draw an inference of causality due to the cross-sectional design of the study.
In conclusion, current smoking was independently associated with increased levels of HbA1c in a cigarette exposure-dependent manner in a nationally representative sample of Korean non-diabetic individuals. In this study, we observed an association between smoking status and HbA1c levels in non-diabetics from a non-Western population, consistent with previous findings in Western populations.
Data Availability
The raw data are owned by Korea Centers for Disease Control and Preservation. Anybody who signs up for membership can get raw data from the webpage of Korea Centers for Disease Control & Preservation(https://knhanes.cdc.go.kr/knhanes/index.do).
Funding Statement
The authors have no support or funding to report.
References
- 1. International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes care. 2009;32: 1327–1334. 10.2337/dc09-9033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Herman WH, Ma Y, Uwaifo G, Haffner S, Kahn SE, Horton ES, et al. Differences in A1C by race and ethnicity among patients with impaired glucose tolerance in the Diabetes Prevention Program. Diabetes care. 2007;30: 2453–2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ziemer DC, Kolm P, Weintraub WS, Vaccarino V, Rhee MK, Twombly JG, et al. Glucose-independent, black-white differences in hemoglobin A1c levels: a cross-sectional analysis of 2 studies. Annals of Internal Medicine. 2010;152: 770–777. 10.7326/0003-4819-152-12-201006150-00004 [DOI] [PubMed] [Google Scholar]
- 4. Wolffenbuttel BH, Herman WH, Gross JL, Dharmalingam M, Jiang H, Hardin DS. Ethnic differences in glycemic markers in patients with type 2 diabetes. Diabetes care. 2013;36: 2931–2936. 10.2337/dc12-2711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Eldeirawi K, Lipton RB. Predictors of hemoglobin A1c in a national sample of nondiabetic children: the Third National Health and Nutrition Examination Survey, 1988–1994. American journal of epidemiology. 2003;157: 624–632. [DOI] [PubMed] [Google Scholar]
- 6. Pani LN, Korenda L, Meigs JB, Driver C, Chamany S, Fox CS, et al. Effect of aging on A1C levels in individuals without diabetes: evidence from the Framingham Offspring Study and the National Health and Nutrition Examination Survey 2001–2004. 2008;31: 1991–1996. 10.2337/dc08-0577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Eliasson B. Cigarette smoking and diabetes. Progress in cardiovascular diseases. 2003;45: 405–413. [DOI] [PubMed] [Google Scholar]
- 8. Manson JE, Ajani UA, Liu S, Nathan DM, Hennekens CH. A prospective study of cigarette smoking and the incidence of diabetes mellitus among US male physicians. The American journal of medicine. 2000;109: 538–542. [DOI] [PubMed] [Google Scholar]
- 9. Cho NH, Chan JC, Jang HC, Lim S, Kim HL, Choi SH. Cigarette smoking is an independent risk factor for type 2 diabetes: a four-year community-based prospective study. Clinical endocrinology. 2009;71: 679–685. 10.1111/j.1365-2265.2009.03586.x [DOI] [PubMed] [Google Scholar]
- 10. Willi C, Bodenmann P, Ghali WA, Faris PD, Cornuz J. Active smoking and the risk of type 2 diabetes: a systematic review and meta-analysis. JAMA: the Journal of the American Medical Association. 2007;298: 2654–2664. [DOI] [PubMed] [Google Scholar]
- 11. Foy CG, Bell RA, Farmer DF, Goff DC, Wagenknecht LE. Smoking and incidence of diabetes among U.S. adults: findings from the Insulin Resistance Atherosclerosis Study. Diabetes care. 2005;28: 2501–2507. [DOI] [PubMed] [Google Scholar]
- 12. Nilsson PM, Lind L, Pollare T, Berne C, Lithell HO. Increased level of hemoglobin A1c, but not impaired insulin sensitivity, found in hypertensive and normotensive smokers. Metabolism, clinical and experimental. 1995;44: 557–561. [DOI] [PubMed] [Google Scholar]
- 13. Urberg M, Shammas R, Rajdev K. The effects of cigarette smoking on glycosylated hemoglobin in nondiabetic individuals. Journal of family practice. 1989;28: 529–531. [PubMed] [Google Scholar]
- 14. Clair C, Bitton A, Meigs JB, Rigotti NA. Relationships of cotinine and self-reported cigarette smoking with hemoglobin A1c in the U.S.: results from the National Health and Nutrition Examination Survey, 1999–2008. Diabetes care. 2011;34: 2250–2255. 10.2337/dc11-0710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sargeant LA, Khaw KT, Bingham S, Day NE, Luben RN, Oakes S, et al. Cigarette smoking and glycaemia: the EPIC-Norfolk Study. European Prospective Investigation into Cancer. International Journal of Epidemiology. 2001;30: 547–554. [DOI] [PubMed] [Google Scholar]
- 16. Jansen H, Stolk RP, Nolte IM, Kema IP, Wolffenbuttel BH, Snieder H. Determinants of HbA1c in nondiabetic Dutch adults: genetic loci and clinical and lifestyle parameters, and their interactions in the Lifelines Cohort Study. Journal of internal medicine. 2013;273: 283–293. 10.1111/joim.12010 [DOI] [PubMed] [Google Scholar]
- 17. Soulimane S, Simon D, Herman WH, Lange C, Lee CM, Colagiuri S, et al. HbA1c, fasting and 2 h plasma glucose in current, ex- and never-smokers: a meta-analysis. Diabetologia. 2014;57: 30–39. 10.1007/s00125-013-3058-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet (London, England). 2004;363: 157–163. [DOI] [PubMed] [Google Scholar]
- 19.Korea Centers for Disease Control and Prevention (KCDC). Korea National Health and Nutrition Examination Survey. Available: http://knhanes.cdc.go.kr/. Accessed 27 June 2013.
- 20. Lee H, Oh JY, Sung YA, Kim DJ, Kim SH, Kim SG, et al. Optimal hemoglobin A1C Cutoff Value for Diagnosing type 2 diabetes mellitus in Korean adults. Diabetes Res Clin Pract. 2013;99: 231–236. 10.1016/j.diabres.2012.09.030 [DOI] [PubMed] [Google Scholar]
- 21. Ko S, Kim S, Kim D, Oh S, Lee H, Shim K, et al. 2011 clinical practice guidelines for type 2 diabetes in Korea. Diabetes & Metabolism Journal. 2011;35: 431–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vlassopoulos A, Lean ME, Combet E. Influence of smoking and diet on glycated haemoglobin and 'pre-diabetes' categorisation: a cross-sectional analysis. BMC Public Health. 2013;13: 1013 10.1186/1471-2458-13-1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bajaj M. Nicotine and insulin resistance: when the smoke clears. Diabetes. 2012;61: 3078–3080. 10.2337/db12-1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Eliasson B, Taskinen MR, Smith U. Long-term use of nicotine gum is associated with hyperinsulinemia and insulin resistance. Circulation. 1996;94: 878–881. [DOI] [PubMed] [Google Scholar]
- 25. Axelsson T, Jansson PA, Smith U, Eliasson B. Nicotine infusion acutely impairs insulin sensitivity in type 2 diabetic patients but not in healthy subjects. Journal of internal medicine. 2001;249: 539–544. [DOI] [PubMed] [Google Scholar]
- 26. Xie X, Liu Q, Wu J, Wakui M. Impact of cigarette smoking in type 2 diabetes development. Acta pharmacologica Sinica. 2009;30: 784–787. 10.1038/aps.2009.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Higgins T, Cembrowski G, Tran D, Lim E, Chan J. Influence of variables on hemoglobin A1c values and nonheterogeneity of hemoglobin A1c reference ranges. Journal of diabetes science and technology. 2009;3: 644–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cerami C, Founds H, Nicholl I, Mitsuhashi T, Giordano D, Vanpatten S, et al. Tobacco smoke is a source of toxic reactive glycation products. Proceedings of the National Academy of Sciences of the United States of America. 1997;94: 13915–13920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Boeing H, Weisgerber UM, Jeckel A, Rose HJ, Kroke A. Association between glycated hemoglobin and diet and other lifestyle factors in a nondiabetic population: cross-sectional evaluation of data from the Potsdam cohort of the European Prospective Investigation into Cancer and Nutrition Study. The American journal of clinical nutrition. 2000;71: 1115–1122. [DOI] [PubMed] [Google Scholar]
- 30. Davie SJ, Gould BJ, Yudkin JS. Effect of vitamin C on glycosylation of proteins. Diabetes. 1992;41: 167–173. [DOI] [PubMed] [Google Scholar]
- 31. Krone CA, Ely JT. Ascorbic acid, glycation, glycohemoglobin and aging. Medical hypotheses. 2004;62: 275–279. [DOI] [PubMed] [Google Scholar]
- 32. Hashimoto Y, Futamura A, Ikushima M. Effect of aging on HbA1c in a working male Japanese population. Diabetes care. 1995;18: 1337–1340. [DOI] [PubMed] [Google Scholar]
- 33. Pani LN, Korenda L, Meigs JB, Driver C, Chamany S, Fox CS, et al. Effect of aging on A1C levels in individuals without diabetes: evidence from the Framingham Offspring Study and the National Health and Nutrition Examination Survey 2001–2004. Diabetes care. 2008;31: 1991–1996. 10.2337/dc08-0577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yang YC, Lu FH, Wu JS, Chang CJ. Age and sex effects on HbA1c. A study in a healthy Chinese population. Diabetes care. 1997;20: 988–991. [DOI] [PubMed] [Google Scholar]
- 35. Harding AH, Sargeant LA, Khaw KT, Welch A, Oakes S, Luben RN, et al. Cross-sectional association between total level and type of alcohol consumption and glycosylated haemoglobin level: the EPIC-Norfolk Study. European journal of clinical nutrition. 2002;56: 882–890. [DOI] [PubMed] [Google Scholar]
- 36. Kroenke CH, Chu N, Rifai N, Spiegelman D, Hankinson SE, Manson JE, et al. A cross-sectional study of alcohol consumption patterns and biologic markers of glycemic control among 459 women. Diabetes care. 2003;26: 1971–1978. [DOI] [PubMed] [Google Scholar]
- 37. Davies MJ, Baer DJ, Judd JT, Brown ED, Campbell WS, Taylor PR. Effects of moderate alcohol intake on fasting insulin and glucose concentrations and insulin sensitivity in postmenopausal women: a randomized controlled trial. JAMA: the Journal of the American Medical Association. 2002;287: 2559–2562. [DOI] [PubMed] [Google Scholar]
- 38. Facchini F, Chen YD, Reaven GM. Light-to-moderate alcohol intake is associated with enhanced insulin sensitivity. Diabetes care. 1994;17: 115–119. [DOI] [PubMed] [Google Scholar]
- 39. Koppes LL, Dekker JM, Hendriks HF, Bouter LM, Heine RJ. Moderate alcohol consumption lowers the risk of type 2 diabetes: a meta-analysis of prospective observational studies. Diabetes care. 2005;28: 719–725. [DOI] [PubMed] [Google Scholar]
- 40. Carlsson S, Hammar N, Grill V. Alcohol consumption and type 2 diabetes Meta-analysis of epidemiological studies indicates a U-shaped relationship. Diabetologia. 2005;48: 1051–1054. [DOI] [PubMed] [Google Scholar]
- 41. Albanes D, Jones DY, Micozzi MS, Mattson ME. Associations between smoking and body weight in the US population: analysis of NHANES II. Am J Public Health. 1987;77: 439–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bamia C, Trichopoulou A, Lenas D, Trichopoulos D. Tobacco smoking in relation to body fat mass and distribution in a general population sample. Int J Obes Relat Metab Disord. 2004;28: 1091–1096. [DOI] [PubMed] [Google Scholar]
- 43. Chiolero A, Jacot-Sadowski I, Faeh D, Paccaud F, Cornuz J. Association of cigarettes smoked daily with obesity in a general adult population. Obesity (Silver Spring). 2007;15: 1311–1318. [DOI] [PubMed] [Google Scholar]
- 44. Nielsen TL, Wraae K, Brixen K, Hermann AP, Andersen M, Hagen C. Prevalence of overweight, obesity and physical inactivity in 20- to 29-year-old, Danish men. Relation to sociodemography, physical dysfunction and low socioeconomic status: the Odense Androgen Study. Int J Obes (Lond). 2006;30: 805–815. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data are owned by Korea Centers for Disease Control and Preservation. Anybody who signs up for membership can get raw data from the webpage of Korea Centers for Disease Control & Preservation(https://knhanes.cdc.go.kr/knhanes/index.do).


