Abstract
Background
Candida is an important cause of bloodstream infections (BSI) in nosocomial settings causing significant mortality and morbidity. This study was performed to evaluate contemporary epidemiology, species distribution, antifungal susceptibility and outcome of candida BSI in an Italian hospital.
Methods
All consecutive patients who developed candidemia at Santa Maria della Misericordia University Hospital (Italy) between January 2009 and June 2014 were enrolled in the study.
Results
A total of 204 episodes of candidemia were identified during the study period with an incidence of 0.79 episodes/1000 admissions. C. albicans was isolated in 60.3% of cases, followed by C. parapsilosis (16.7%), C. glabrata (11.8%) and C. tropicalis (6.4%). Of all Candida BSI, 124 (60.8 %) occurred in patients admitted to IMW, 31/204 (15.2 %) in ICUs, 33/204 (16.2%) in surgical units and 16/204 (7.8%) in Hematology/Oncology wards. Overall, 47% of patients died within 30 days from the onset of candidemia. C. parapsilosis and C. glabrata candidemia were associated with the lowest mortality rate (36%), while patients with C. tropicalis BSI had the highest mortality rate (58.3%). Lower mortality rates were detected in patients receiving therapy within 48 hours from the time of execution of the blood cultures (57,1% vs 38,9%, P <0.05). At multivariate analysis, steroids treatment (OR= 0.27, p=0.005) and CVC removal (OR=3.77, p=0.014) were independently associated with lower and higher survival probability, respectively. Candidemia in patients with peripherally inserted central catheters (PICC) showed to be associated with higher mortality in comparison with central venous catheters (CVC, Short catheters and Portacath) and no CVC use. For each point increase of APACHE III score, survival probability decreased of 2%. Caspofungin (OR=3.45, p=0.015) and Amphothericin B lipid formulation (OR=15.26, p=0.033) were independently associated with higher survival probability compared with no treatment.
Introduction
Candida spp. is currently the main protagonist of fungal infections in hospitalized patients, representing the fourth and sixth leading cause of nosocomial sepsis in European and US studies, respectively [1,2]. A recent American study reported Candida as the first cause of bloodstream infections (BSI) in 180 medical centres [3]. Data are available from large series of laboratory-based [4] and population-based [5] surveillance studies, as well as studies focusing on specific patient populations such as neonates [6,7], cancer [8,9], surgical [10,11] and critically ill patients [12,13]. According to the data reported by the European Centre for Disease prevention and Control (ECDC) in 2013, Candida spp. is actually the fifth microorganism responsible of causing sepsis in patients admitted to the ICU. Recently, an increase in incidence has been related to the complex medical and surgical procedures undertaken to prolong the survival of critically ill patients and the change in the demographic characteristics of hospitalized populations [14,15].
The epidemiology of candidemia varies according to geographical regions [16,17]. For this reason, continuous surveillance studies to monitoring incidence, species distribution, and antifungal susceptibility profiles are mandatory.
Candidemia remains associated with high crude and attributable mortality rates along withincreased cost of care and duration of hospitalization [18]. Attributable mortality has been reported to range from 5% to 71% [13,19], and crude mortality rates can be as high as 76% [20]. The increase of the attributable mortality of candidemia has driven research into the role of early diagnosis and prompt treatment initiation in order to improve outcomes [21,22]. Inadequate initial antifungal treatment has been associated with increased mortality in patients with candidemia [23]. Furthermore, intrinsic and emerging resistance to azoles represents a major challenge for empirical therapeutic and prophylactic strategies [24,25].
This study was performed to evaluate contemporary epidemiology, species distribution, antifungal susceptibly and outcome of candida BSI in an Italian hospital.
Methods
This study was approved by the local institutional review board (Comitato Etico Unico Azienda Ospedaliera Universitaria Santa Maria della Misericordia) and written patient consent was not required because of the observational nature of this study. Patient characteristics were collected from an electronic database where records and information were anonymized and de-identified prior to analysis.
This is a retrospective, single-centre, observational cohort study conducted at Santa Maria della Misericordia hospital (Udine, Italy), a tertiary University Hospital with 960 beds and 44,000 admissions per year. All patients who developed candidemia, defined as patients with at least a positive blood culture for Candida spp. and a compatible clinical illness during the period January 2009—June 2014 were included in the study. Only the first episode of candidemia was recorded for each patient. Patients with “mixed candidemia”, identified as the isolation of two different Candida species from blood cultures performed on the same day or at a distance of at least 7 days, were also included. Clinical data were collected from the microbiological laboratory database and included underlying diseases, risk factors for candida infection (neutropenia, intravascular-devices, the administration of total parental nutrition, prior antimicrobial administration, prior antifungal therapy, recent abdominal/extra-abdominal surgery, major (surgery involving a risk to the life of the patient) and minor (an operation on the superficial structures of the body or a manipulative procedure that does not involve a serious risk) surgery, chemotherapy, radiation treatment, recent ICU admission, immunosuppressive treatment, bone marrow or solid-organ transplant), Candida species and susceptibility to antifungal agents, timing of antifungal administration (determined as the interval between the time when the first Candida-positive blood sample for culture was drawn and the time when the antifungal treatment was first administered to the patient) and outcome. Initial treatment was considered adequate when the infecting organism was ultimately shown to be susceptible and the dosage of antifungal used was adequate within the first 24 hours from culture positivity. The following antifungal dosages were considered adequate: fluconazole 800 mg loading dose followed by a daily dosage of at least 400 mg, liposomal amphotericin B (L-AmB) 3 mg/kg/day, amphotericin lipid complex (ABLC) 5mg/kg/day, caspofungin 70 mg loading dose followed by 50 mg/day, micafungin 100 mg/day, anidulafungin 200 mg loading dose followed by 100 mg/day.
Crude mortality rate was calculated at 30 days from blood cultures performing. No changes in the microbiological laboratory techniques at our hospital were undertaken during the study period. Candida spp. was isolated from blood samples using the Bactec 860 system (Becton, Dickinson, Inc., Sparks, MD). The species were identified using the API ID 32C system (bioMérieux, Marcy l'Etoile, France) or the Vitek 2 system (bioMérieux). In the cases of inconclusive results obtained by both systems, isolates were definitively identified using supplemental tests, e.g., by the presence or absence of well-formed pseudohyphae on cornmeal-Tween 80 agar and growth at 42 to 45°C. C. parapislosis strains were identified only at complex level.
Susceptibility to amphotericin B, caspofungin, fluconazole, itraconazole, and voriconazole was detected using the Sensititre YeastOne colorimetric plate (Trek Diagnostic Systems, Cleveland, OH). Until October 2011 MIC values were interpreted according to species-specific clinical breakpoints as established by the Clinical and Laboratory Standards Institute (CLSI) for amphotericin B, caspofungin, fluconazole, itraconazole (only for C. albicans), and voriconazole [26]. From November 2011 MIC values were interpreted according to species-specific clinical breakpoints as established by European Committee on Antimicrobial Susceptibility Testing (EUCAST) [27,28], updated to the last version available.
Continuous and categorical data were reported as median, 25° and 75° percentile and frequency distributions, respectively. The Wilcoxon test and χ2 test were used to determine if differences existed between groups for continuous and categorical variables, respectively. Multiple logistic regression analysis was performed to identify risk factors that were associated with hospital mortality at 3 months (JMP, SAS, NC, USA). Covariates that were significant at 0.10 in the univariate analysis were further evaluated for inclusion in multivariable regression models, using a stepwise algorithm. All tests were 2-tailed, and a p-value <0.05 was determined to represent statistical significance.
Results
A total of 204 episodes of candidemia, corresponding to an incidence of 0.79 cases / 1000 admissions, was recorded during the study period. The incidence of candidemia corresponded to 0.70–0.84–0.77–0.93–0.72 episodes /1000 admissions for 2009, 2010, 2011, 2012, and 2013, respectively. The demographic and clinical characteristics of the patients are summarized in Table 1. The most frequently represented age group was 61 to 80 years of age (46.9% of cases): more than 73% of candidemia were recorded in patients older than 60 years. During the study period, the mean age of the population rose from 61.2 years in 2009 to 73.5 years in 2014. Over 87% of the patients (178/204) had one or more comorbidities at the time of the diagnosis of candidemia. Specifically, 34.8% presented a solid organ malignancy, 27.9% were diabetics, 12.3% presented liver diseases, 8.3% renal failure, and 4.9% a haematological disease. Among immunosuppressive conditions, 55 (26.9%) received steroid therapy, 28 (13.2%) underwent chemo and / or radiotherapy, 7 (2.9%) received monoclonal antibodies and 9 (3.9%) received other immunosuppressive agents. Seventy patients (34.3%) underwent surgery in the two months prior to candidemia; of these, 31 had undergone major surgery, 18 a minor surgical procedures, and 21 had extra—abdominal operations. Nineteen/70 (24.3%) had a re-intervention in the two months preceding the diagnosis of candidemia. Forty-nine (24%), were hospitalized in the ICU in the month prior to the diagnosis of candidemia. A total of 140 (69.1%) patients received parental nutrition. An intravascular device was present in 84.4% of the patients on the candidemia was diagnosed. Ninety-one percent of the patients with candidemia had received at least one antibiotic for ≥ 7 days during the month before candidemia diagnosis. An episode of concomitant bacteraemia was detected in 18.8% of patients; of these, 72% was caused by Gram-positive bacteria, 25% by Gram-negative bacteria and by mixed bacterial flora in the remaining cases.
Table 1. Patients’ features and ward distribution among candida species (n = 204).
| Candida species | ||||||
|---|---|---|---|---|---|---|
| C. albicans (n = 123) | C. parapsilosis (n = 34) | C. glabrata (n = 24) | C. tropicalis (n = 13) | Others (n = 10) | All (n = 204) | |
| 60.2% | 16.7% | 11.8% | 6.4% | 5.2% | 100% | |
| Patients characteristics | ||||||
| Male sex, n (%) | 74 (60) | 20 (59) | 15 (63) | 7 (54) | 7 (70) | 118 (58) |
| Mean age (SD) | 67 (±21) | 71 (±18) | 71 (±22) | 68 (±16) | 53 (±18) | 67(±20) |
| Risk factors | ||||||
| Recent ICU admission | 31 (25.2) | 6 (17.6) | 8 (33.3) | 0 | 4 (40) | 49 (24) |
| Central venous catheter (CVC) | 105 (85.4) | 30 (88.2) | 17 (70.8) | 11 (84.6) | 9 (90) | 172 (84.4) |
| Parenteral nutrition | 87 (70.7) | 27(79.4) | 13 (54.2) | 8 (66.7) | 6 (60) | 141 (69.1) |
| Antibiotic treatment in the previous 30 days | 117 (95.1) | 25 (73.5) | 23 (95.8) | 12 (92.3) | 9 (90) | 186 (91.2) |
| Major abdominal surgery | 24 (19.5) | 5 (14.7) | 2 (8.3) | 1 (7.7) | 1(10) | 31 (16.2) |
| Minor abdominal surgery | 11 (8.9) | 1 (2.9) | 4 (16.7) | 2 (11) | 0 | 18 (8.8) |
| Extra-abdominal surgery | 15 (12.2) | 3 (8.8) | 1(4.2) | 2 (20) | 0 | 21 (10.3) |
| Solid tumor | 40 (32.5) | 11 (32.3) | 10 (41.7) | 6 (8) | 4 (40) | 71 (34.8) |
| Haematological malignancies | 5 (4,1) | 1 (2,9) | 0 | 3 (30) | 1 (10) | 10 (4.9) |
| Chemo/radio-therapy | 13 (10.6) | 5 (14.7) | 1 (4.2) | 5(19) | 3 (30) | 27 (13.2) |
| Diabetes mellitus | 34 (27.6) | 8 (23.5) | 8 (33.3) | 3 (5) | 4 (40) | 57 (27.9) |
| Haemodialysis | 14 (11.4) | 2 (5.9) | 1(4.2) | 0 | 1 (10) | 18 (8.8) |
| Steroid treatment | 30 (24,4) | 10 (29.4) | 8 (33.3) | 4 (7) | 3 (30) | 55 (26.9) |
| Hospital Units | ||||||
| Medical Units | 70 (56.9) | 23 (67.6) | 15 (62.5) | 7 (53.8) | 6 (60) | 121 (59.3) |
| Surgical Units | 25 (20.3) | 2 (5.9) | 4 (16.7) | 1 (7.7) | 1 (10) | 33 (16.2) |
| Hematology/Oncology | 5 (4) | 4 (11.8) | 1 (4.2) | 5 (38.5) | 2 (20) | 17 (8.3) |
| ICU | 23 (18.7) | 5 (14.7) | 4 (16.7) | 0 | 1 (10) | 33 (16.2) |
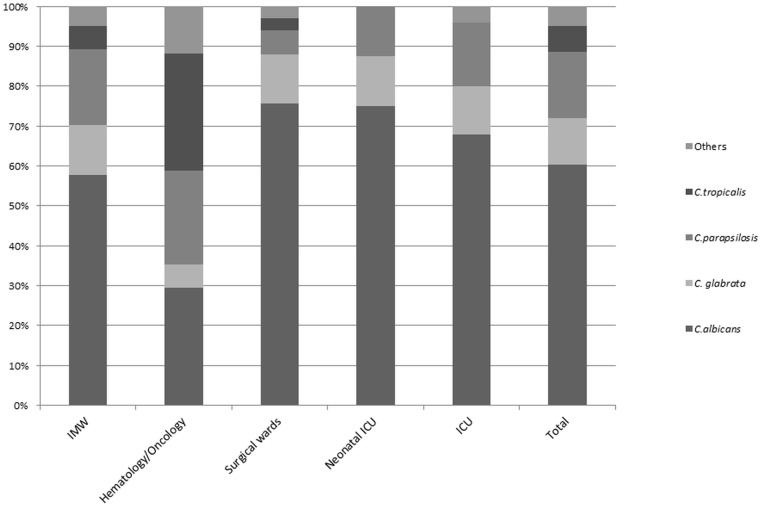
One hundred and twenty-four cases (60.8%) occurred in patients admitted to IMW, 31/204 (15.2%) in ICUs, 33/204(16.2%) in surgical units, and 16/204 (7.8%) in Hematology/Oncology wards. Candidemia distribution by hospital ward is shown in Fig 1.
Fig 1. Candida spp. distribution expressed as percentage of total candida species in various hospital wards.
Overall, C. albicans (CA) was the most common pathogen, accounting for 60% of total cases, followed by C. parapsilosis (17%), C. glabrata (12%), C. tropicalis (6%) and others (5%). The proportion of CA versus non-albicans Candida (CAN) changed over the years: CA was responsible for 66.7% of candidemia in 2009, 67.7% in 2010, 61.7% in 2011, 43.2% in 2012, 61.7% in 2013, and 68.4% in 2014.
Table 2 shows the results of the in vitro activity of 3 systemically active antifungal agents tested against BSI isolates of Candida spp. based on CLSI and EUCAST breakpoints. The rate of susceptibility to fluconazole was 98.4% and 99.2% for C. albicans according to CLSI and EUCAST breakpoints respectively, and 88.2% for C. parapsilosis according to CLSI breakpoints. Decreased susceptibility to fluconazole was mostly seen with C. glabrata (83.3%).
Table 2. MIC50, MIC90, and susceptibility of Candida strains to antifungals.
| Candida species N° (% isolates) | Antifungal agent | MIC range (μg/mL) | MIC 50 (μg/ml) | MIC 90 (μg/ml) | N° (%) of resistant or SDD isolates | |
|---|---|---|---|---|---|---|
| CLSI | EUCAST | |||||
| C. albicans 123 (60.3%) | Fluconazole | 0.12–256 | 0,25 | 1 | 2 (1.6) | 1 (0.8) |
| Caspofungin | 0.008–1 | 0,06 | 0,06 | 0 | N/A | |
| Amphotericin B | 0.25–1 | 0,125 | 0,5 | N/A | 0 | |
| C. parapsilosis 34 (16.7%) | Fluconazole | 0.25–256 | 1 | 4 | 1 (2.9) | 3 (8.8) |
| Caspofungin | 0.03–2 | 0.5 | 0.5 | 2 (5.9) | NR | |
| Amphotericin B | 0.016–0.5 | 0.06 | 0.25 | N/A | 0 | |
| C. glabrata 24 (11.8%) | Fluconazole | 1–32 | 8 | 16 | 0 | 4 (16.7) |
| Caspofungin Amphotericin B | 0.06–0.5 0.06–1 | 0.12 0.125 | 0.125 0.5 | 0 N/A | N/A 0 | |
| Amphotericin B | 0.06–1 | 0.125 | 0.5 | N/A | 0 | |
| C.tropicalis 13 (6.4%) | Fluconazole | 0.5–4 | 1 | 4 | 1(7.7) | 1(7.7) |
| Caspofungin | 0.03–0.25 | 0.06 | 0,125 | 0 | Na | |
| Amphotericin B | 0.12–0.5 | 0.25 | 0.5 | N/A | 0 | |
| Others 10 (5%) | Fluconazole | 0.12–32 | 1 | 32 | 2 (20) | 1(10) |
| Caspofungin | 0.12–05 | 0.25 | 0.25 | 0 | N/A | |
| Amphotericin B | 0,015–0,25 | 0,06 | 0,25 | N/A | 0 | |
| All Candida spp. 204 | Fluconazole | 0.12–256 | 0.5 | 8 | 7 (3.4) | 10 (4.9) |
| Caspofungin | 0.008–2 | 0.06 | 0.5 | 0 | N/A | |
| Amphotericin B | 0.015–1 | 0.125 | 0.5 | 0 | 0 | |
N/A: breakpoint not available, NR: not recommended
Type of treatment and outcome data were available for 190 patients. A total of 108 (56.8%) patients received adequate antifungal treatment within 48 hours after collection of blood cultures. Ninety patients (47.4%) died within 30 days following the diagnosis of candidemia.
When 30-day mortality was compared among different wards, significantly higher rates were observed in IMW compared to surgical wards (P = 0.002) and to all the other wards (P = 0.009). Thirty-day mortality rates for the population that received antifungal therapy within 48 hours from collection of blood cultures was 38.9% compared to 57.1% observed in the population in which the timing was > 48 hours (P = 0.03).
Empirical antifungal treatment was different in patients younger and older than 65 years (p = 0,001) (Table 3). A higher proportion of younger patients received caspofungin treatment compared to older patients [21/71 (29.6%) vs. 23/103 (17.3%) respectively, P = 0.001].
Table 3. Comparison of clinical characteristics between patients under and over 65 years old.
| Under 65 N° (%) (n. = 71) | Over 65 N° (%) (n. = 133) | p-value | |
|---|---|---|---|
| Gender male | 45 (63.4) | 78 (58.7) | 0.433 |
| Median age (range) | 53 (37–60) | 78 (71–84.5) | <0.001 |
| Hospital wards | |||
| Internal medicine Wars | 39 (54.9) | 85 (63.9) | NS |
| Surgical Wards | 14 (19.7) | 19 (14.3) | NS |
| Intensive Care Units | 13 (18.3) 5 (7) | 18 (13.5) 11 (8.3) | NS |
| Onco/hematological Units | 5 (7) | 11 (8.3) | NS |
| CVC | |||
| No CVC | 5 (7) | 27 (20.3) | <0.001 |
| Short catheter | 36 (50.7) | 49 (36.8) | <0.001 |
| Umbilical arterial catheter | 5 (7) | 0 | NS |
| Midline | 3 (4.2) | 13 (9.8) | NS |
| Peripherally Inserted Central Catheter (PICC) | 9 (12.7) | 27 (20.3) | <0.001 |
| Portacath | 13 (18.3) | 17 (12.8) | <0.001 |
| Total Parenteral Nutrition | 51 (71.8) | 89 (66.9) | 0.471 |
| Percutaneous Enteral Nutrition | 4 (5.6) | 12 (9) | 0.735 |
| Antibiotic treatment | 64 (90.1) | 121 (91) | 0.845 |
| Abdominal surgery | 9 (12.7) | 23 (17.3) | 0.57 |
| Extra-abdominal surgery | 10 (14.1) | 11 (8.3) | 0.193 |
| Reoperation | 10 (14.1) | 9 (6.8) | 0.087 |
| Solid cancer | 28 (39.4) | 43 (32.3) | 0.31 |
| Hematological cancer | 6 (8.5) | 4 (3) | 0.086 |
| Chemo/radiotherapy* | 20 (28.2) | 8 (6) | <0.001 |
| Monoclonal antibodies | 5 (7) | 2 (1.5) | 0.038 |
| Diabetes | 24 (33.8) | 33 (24.8) | 0.172 |
| Liver disease | 14 (19.7) | 13 (9.8) | 0.046 |
| Hemodialysis | 8 (11.3) | 10 (7.5) | 0.368 |
| Steroids | 24 (33.8) | 31 (23.3) | 0.108 |
| Other immunosuppressant therapy | 6 (8.5) | 3 (2.39 | 0.04 |
| Mechanical ventilation | 20 (28.2) | 11 (8.3) | <0.001 |
| Apache III | 36 (24.5–56.5) | 39 (35–52.75) | 0.12 |
| Vasopressor use | 15 (21.1) | 12 (9) | 0.015 |
| HIV | 4 (5.6) | 0 | 0.006 |
| CVC removal within 24h | 11 (15.5) | 21 (15.8) | 0.956 |
| Empiric antifungal treatment | 66 (93) | 102 (76.7) | 0.001 |
| Empirical treatment | |||
| Fluconazole | 37 (52.1) | 73(54.9) | NS |
| Voriconazole | 2(2.8) | 0 | NS |
| Amphotericin B lipid-formulations | 2 (2.8) | 1 (1.5) | NS |
| Caspofungin | 21 (29.6) | 23 (17.3) | 0.001 |
| Other echinocandins | 3 (2) | 4 (3) | NS |
| Empiric antifungal treatment | 66 (93) | 102 (76.7) | 0.001 |
| Definitive treatment | |||
| Fluconazole | 9(12.7) | 10 (7.5) | NS |
| Voriconazole | 2(2.8) | 2 (1.5) | NS |
| Amphotericin B lipid-formulations | 5 (7) | 1 (0.8) | NS |
| Caspofungin | 11 (15.5) | 18 (13.5) | NS |
| Other echinocandins | 2 (2.8) | 2 (1.5) | NS |
| Timing of therapy | |||
| Within 24h | 20 (28.2) | 37 (27.8) | 0.96 |
| Between 24 and 48h | 9 (12.7) | 16 (12) | 0.893 |
| More than 48 H | 2 (2.8) | 7 (5.3) | 0.418 |
| 30-day mortality | 30 (42.2) | 74 (55.6) | 0.071 |
CVC: central venous catheter
Table 4 summarizes the significant differences between patients with candidemia who died within 3 months from the diagnosis of candidemia compared with survivors at univariate analysis. Patients who died were significantly older than survivors (73 vs. 67.5 years, P = 0.005). Compared to patients who died, the survivors had significant lower APACHE III scores (median 37 vs. 41, p = 0.003). At univariate analysis, abdominal surgery (p<0,001), re-operation (p = 0,014) and CVC removal (p<0,001) were more frequent in survivors, while steroids treatment was more often present in patients who died (p = 0.006). Distribution of CVC was significantly different in patients with different mortality outcomes (p = 0.002): in particular, peripherally inserted central catheters (PICC) use was more frequently used in patients who died compared to survivors (25,8% vs. 4%
Table 4. Characteristics of survivors (N = 76) compared with patients with candidemia who died (N = 128).
| Alive N° (%) (n. = 76) | Deaths N° (%) (n. = 128) | p-value | |
|---|---|---|---|
| Gender (male) | 32 (42.1) | 49 (38.3) | 0.589 |
| Avarage age (range) | 67.5 (53–78) | 73 (61.25–83) | 0.005 |
| Over 65 years | 44 (57.9) | 89 (69.5) | 0.091 |
| Hospital wards | |||
| Internal medicine Wars | 39 (51.3) | 85 (66.4) | NS |
| Surgical Wards | 17 (22.4) | 16 (12.5) | NS |
| Intensive Care Units | 13 (17.1) | 18 (14.1) | NS |
| Onco/hematological Units | 7 (9.2) | 9 (7) | NS |
| CVC | |||
| No CVC | 13 (17.1) | 19 (14.8) | NS |
| Short catheter | 42 (55.3) | 43 (33.6) | 0.002 |
| Umbilical arterial catheteR | 2 (2.6) | 3 (2.3) | NS |
| Midline | 4 (5.3) | 12 (9.4) | NS |
| Peripherally Inserted Central Catheter | 3 (4) | 33 (25.8) | 0.002 |
| Portacath | 12 (15.8) | 18 (14.1) | NS |
| Total Parenteral Nutrition | 49 (64.5) | 91 (71.1) | 0.324 |
| Percutaneous Enteral Nutrition | 7 (9.2) | 9 (7) | 0.575 |
| Antibiotic treatment | 69 (90.8) | 116 (90.6) | 0.969 |
| Abdominal surgery | 21 (27.6) | 11 (8.6) | <0.001 |
| Extra-abdominal surgery | 6 (7.9) | 15 (11.7) | 0.385 |
| Re-operation | 12 (15.8) | 7 (5.5) | 0.014 |
| Solid cancer | 27 (35.5) | 44 (34.4) | 0.867 |
| Hematological cancer | 6 (7.9) | 4 (3.1) | 0.127 |
| Chemo/radiotherapy* | 8 (10.5) | 20 (15.6) | 0.21 |
| Monoclonal antibodies | 2 (2.6) | 5 (3.9) | 0.629 |
| Diabetes | 16 (21.1) | 41 (32) | 0.091 |
| Liver disease | 9 (11.8) | 18 (14.1) | 0.651 |
| Hemodialysis | 10 (13.2) | 8 (6.3) | 0.093 |
| Steroids | 12 (15.8) | 43 (33.6) | 0.006 |
| Other immunosuppressant therapy | 4 (5.3) | 5 (3.9) | 0.648 |
| Mechanical ventilation | 11 (14.5) | 20 (15.6) | 0.824 |
| Apache III | 37 (25.25–44.75) | 41 (33–56) | 0.003 |
| Vasopressor use | 7 (9.2) | 20 (15.6) | 0.191 |
| HIV | 2 (2.6) | 2 (1.6) | 0.59 |
| Bacteremia | 14 (18.4) | 24 (18.8) | 0.953 |
| CVC removal in 24h | 21 (27.6) | 11 (8.6) | <0.001 |
| Empirical treatment | |||
| No treatment | 7 (9.2) | 9 (22.7) | 0.075 |
| Fluconazole | 44 (57.9) | 29 (22.7) | NS |
| Voriconazole | 1 (1.3) | 1 (0.8) | NS |
| Caspofungin | 18 (23.7) | 26 (20.3) | NS |
| Other echinocandins | 3 (4) | 4 (3.1) | NS |
| Amphotericin B lipid formulation | 3 (4) | 1 (0.8) | NS |
| Definitive treatment | |||
| No treatment | 42 (55.3) | 100 (78.1) | 0.029 |
| Fluconazole | 10 (13.2) | 9 (7) | NS |
| Caspofungin | 16 (21.1) | 13 (10.2) | 0.029 |
| Amphotericin B lipid formulation | 3 (4) | 3 (2.3) | NS |
| Timing of therapy | |||
| Within 24h | 24 (31.6) | 33 (25.8) | 0.372 |
| Between 24 and 48h | 11 (14.5) | 14 (10.9) | 0.456 |
| More than 48 h | 4 (5.3) | 5 (3.9) | 0.648 |
The final logistic regression model included CVC type, steroids treatment, Apache III score, CVC removal and definitive treatment. Steroids treatment (OR = 0.27, p = 0.005) and CVC removal (OR = 3.77, p = 0.014) were independently associated with lower and higher survival probability, respectively (Table 5). PICC showed to be associated with higher mortality in comparison with more used CVCs (Short catheter, Portacath) and no CVC use. Fo each point increase of APACHE III score there was a 2% decrease of survival probability. Caspofungin (OR = 3.45, p = 0.015) and Amphothericin B lipid formulation (OR = 15.26, p = 0.033) were independently associated with significantly higher survival probability in comparison with no treatment.
Table 5. Multivariate analysis of risk factors for 30-day mortality.
| Effect likelihood ratio test | Survival Probability | ||||
|---|---|---|---|---|---|
| L-R chi square | p-value | Significant ratio | OR(95% C.I.) | ||
| CVC | 11.63 | 0.02 | Short catheter vs. PICC | 7 (1.8–38.17) | 0.004 |
| Portacath vs. PICC | 8.19 (1.86–48.27) | 0.005 | |||
| no CVC vs. PICC | 8.41 (1.85–50.86) | 0.005 | |||
| Steroids | 7.68 | 0.005 | Steroids vs. no steroids treatment | 0.27 (0.09–0.69) | |
| Apache III | 4.1 | 0.043 | Per unit increase | 0.98 (0.95–0.99) | |
| CVC removal | 6.04 | 0.014 | Removed vs. not removed | 3.77 (1.3–11.76) | |
| Definitive treatment | 15.81 | 0.007 | caspofungin vs no treatment | 3.49 (1.28–10) | 0.015 |
| amphotericin B lipid formulation vs. no treatment | 15.26 (1.25–366.13) | 0.033 | |||
Discussion
An increased incidence of candidemia in nosocomial settings has been shown by many studies in the last fifteen years [1,29]. Our study shows a substantial stability in the incidence over the past 5 years. Candidemia was more frequently diagnosed among patients aged 61 to 80 years, with a progressive increase of the mean age over time. We also observed a progressive, gradual rise of the population mean age, with an overall increase of more than 10 years during the 5 years of observation. The increase in mean age partially differs from other studies where a mean age 60 years was reported with no significant changes in the last decade [12,30]. However, our age population data aligns perfectly with other Italian studies [16,19,31,32]. As previously reported, our data suggests that the increase in the incidence of candidemia is related not only to an increased number of immunocompromised patients, but also to the aging of the population [31].
C. glabrata and C. parapsilosis BSI were more likely to occur in older patients. Conversely, candidemia caused by less common species, such as C. krusei and C. famata, were more often associated with younger age. We reported similar or higher proportions of immunocompromised patients compared with other studies [4,12,25]. Furthermore, high proportions of patients received broad spectrum antibiotics and steroid therapy prior to hospitalization with candidemia. As previously reported, we also observed high percentages of patients with parenteral nutrition and central vascular access, especially in C. parapsilosis fungemia [33]. As confirmed by our study, the incidence of candidemia in IMW is increasing [31,34] due to the increase in the mean age of hospitalized patients, a more extensive use of antibiotics, steroid therapy, and invasive procedures performed outside the ICUs [34].
Although there is an increasing evidence of the progressive rise of NCA, C. albicans still represents the most isolated species in Europe [19,35–37]. Although C. albicans was the main isolate in our hospital, the distribution varied considerably according to the ward. Similarly to other studies, C. albicans was the prevalent species in surgical wards, but not in haematology/oncology where CNA were prevalent [32]. The high proportion of C.albicans BSI observed in surgical units diverges from what shown by previous studies [2,32]. This could be related to a limited use of fluconazole prophylaxis performed in these units as previously reported [36]. Among CNA, C. parapsilosis was mostly isolated in IMW where an extensive use of intravascular-devices is widely practiced and C. krusei was more common among patients with hematologic disorders [38] who frequently receive azole-based prophylaxis or treatment.
Non-susceptibility to fluconazole (8.3%) was slightly higher than the one reported by European (6.3%) and North American (6.6%) studies, but appeared lower compared to other Italian studies where resistances up to 25% have been documented [16,34].
The presence of a concomitant bacteraemia in around 20% of cases was lower than the one reported in another study [39].
We showed 30-day mortality rates of 47.4%, similar to the ones reported by a recent study conducted in 5 Italian and Spanish academic hospitals [18]. During the study period, we did not observe substantial changes in both source control (e.g., intravascular device removal) and timing for antifungal treatment initiation, thus suggesting that the management of candidemia at our institution can still be improved. Antifungal therapy timing is crucial and is known to significantly impact the mortality of patients with candidemia [18,21,22]. We observed a significant difference in mortality between the patients who received antifungal therapy within 48 hours of collection of a blood culture and those who were treated after 48 hours. In particular, medical wards had the lowest rates of timely initiation of antifungal therapy and presented a significantly higher mortality if compared to surgical wards (P = 0.002).
Our study supports the importance of the source control (e.g., catheter removal) in patients with candidemia. Source control has previously been shown to be an important determinant of outcome for patients with serious infections attributed to Candida species [40]. Although controversy exists regarding the need to remove all central venous catheters in candidemic patients, the recent European guidelines support the early catheter removal [41].
Our results align with a recent patient-level review of candidemia trial that reported improved clinical outcomes in patients receiving an echinocandin [42].
In conclusion, this study confirms that candidemia is an important cause of morbidity and mortality, especially among elderly patients admitted to IMW. Early identification of risk factors associated with this disease is necessary to reduce its incidence, and a timely management is crucial to improve the outcome.
Data Availability
All relevant data are within the paper.
Funding Statement
The authors have no support or funding to report.
References
- 1. Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004; 39:309–317. [DOI] [PubMed] [Google Scholar]
- 2. Tortorano AM, Kibbler C, Peman J, Bernhardt H, Klingspor L, Grillot R. Candidaemia in Europe: epidemiology and resistance. Int J Antimicrob Agents. 2006; 27:359–66. [DOI] [PubMed] [Google Scholar]
- 3. Magill SS, Edwards JR, Fridkin SK, Emerging Infections Program Healthcare-Associated Infections and Antimicrobial Use Prevalence Survey Team. Survey of health care-associated infections. N Engl J Med. 2014;370(26):2542–3. 10.1056/NEJMc1405194#SA1 [DOI] [PubMed] [Google Scholar]
- 4. Montagna MT, Lovero G, Borghi E, Amato G, Andreoni S, Campion L, et al. Candidemia in intensive care unit: a nationwide prospective observational survey (GISIA-3 study) and review of the European literature from 2000 through 2013. Eur Rev Med Pharmacol Sci. 2014; 18(5):661–674. [PubMed] [Google Scholar]
- 5. Ma CF, Li FQ, Shi LN, Hu YA, Wang Y, Huang M, et al. Surveillance study of species distribution, antifungal susceptibility and mortality of nosocomial candidemia in a tertiary care hospital in China.BMC Infect Dis. 2013; 13:337 10.1186/1471-2334-13-337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chitnis AS, Magill SS, Edwards JR, Chiller TM, Fridkin SK, Lessa FC. Trends in Candida central line-associated bloodstream infections among NICUs, 1999–2009. Pediatrics. 2012; 130(1):46–52. 10.1542/peds.2011-3402 [DOI] [PubMed] [Google Scholar]
- 7. Blyth CC, Chen SC, Meyer W, Sorrell TC, Australian Candidemia Study. Not just little adults: candidemia epidemiology, molecular characterization, and antifungal susceptibility in neonatal and pediatric patients. Pediatrics. 2009; 123(5):1360–8. 10.1542/peds.2008-2055 [DOI] [PubMed] [Google Scholar]
- 8. Pagano L, Caira M, Candoni A, Offidani M, Fianchi L, Martino B, et al. The epidemiology of fungal infections in patients with hematologic malignancies: the SEIFEM-2004 study.Haematologica. 2006; 91(8):1068–75. [PubMed] [Google Scholar]
- 9. Tang HJ, Liu WL, Lin HL, Lai CC. Epidemiology and prognostic factors of candidemia in cancer patients. PLoS One. 2014; 9(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vincent JL, Anaissie E, Bruining H, Demajo W, el-Ebiary M, Haber J, et al. Epidemiology, diagnosis and treatment of systemic Candida infection in surgical patients under intensive care. Intensive Care Med.1998; 24(3):206–16. [DOI] [PubMed] [Google Scholar]
- 11. Montravers P, Dupont H, Eggimann P. Intra-abdominal candidiasis: the guidelines-forgotten non-candidemic invasive candidiasis. Intensive Care Med. 2013; 39(12):2226–30. 10.1007/s00134-013-3134-2 [DOI] [PubMed] [Google Scholar]
- 12. Colombo AL, Guimarães T, Sukienik T, Pasqualotto AC, Andreotti R, Nucci M, et al. Prognostic factors and historical trends in the epidemiology of candidemia in critically ill patients: an analysis of five multicenter studies. Intensive Care Med. 2014; 40:1489–1498. 10.1007/s00134-014-3400-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bouza E, Muñoz P. Epidemiology of candidemia in intensive care units. Int J Antimicrob Agents. 2008; 32 Suppl 2:87–91. 10.1016/S0924-8579(08)70006-2 [DOI] [PubMed] [Google Scholar]
- 14. Pfaller MA. Nosocomial candidiasis: emerging species, reservoirs, and modes of transmission. Clin Infect Dis. 1996; 22 Suppl 2:89–94. [DOI] [PubMed] [Google Scholar]
- 15. Dominic RM, Shenoy S, Baliga S. Candida biofilms in medical devices: Evolving trends. K Univ Med J (KUMJ). 2007; Vol. 5:19. [PubMed] [Google Scholar]
- 16. Tortorano AM, Prigitano A, Lazzarini C, Passera M, Deiana ML, Montagna MT, et al. A 1-year prospective survey of candidemia in Italy and changing epidemiology over one decade. Infection. 2013; 41:655–662. 10.1007/s15010-013-0455-6 [DOI] [PubMed] [Google Scholar]
- 17. Bassetti M, Trecarichi EM, Righi E, Sanguinetti M, Bisio F, Tumabrello M, et al. Incidence, risk factors, and predictors of outcome of Candidemia. Survey in 2 Italian universitaru hospitals. Diagn Microbiol Infect Dis. 2007; 58:3. [DOI] [PubMed] [Google Scholar]
- 18. Bassetti M, Righi E, Ansaldi F, Merelli M, Garnacho-Montero J, Tumbarello M, et al. A multicenter study of septic shock due to candidemia: outcomes and predictors of mortality. Intensive Care Med. 2014; 40(6):839–45. 10.1007/s00134-014-3310-z [DOI] [PubMed] [Google Scholar]
- 19. Bassetti M, Merelli M, Righi E, Sanguinetti M, Rello J, Tumbarello M, et al. Epidemiology, species distribution, antifungal susceptibility, and outcome of candidemia across five sites in Italy and Spain. J Clin Microbiol. 2013; 51(12):4167–72. 10.1128/JCM.01998-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Girão E, Levin AS, Basso M, Gobara S, Gomes LB, Medeiros EA, et al. Seven-year trend analysis of nosocomial candidemia and antifungal (fluconazole and caspofungin) use in Intensive Care Units at a Brazilian University Hospital. Med Mycol. 2008; 46(6):581–8. 10.1080/13693780802004996 [DOI] [PubMed] [Google Scholar]
- 21. Morrell M, Fraser VJ, Kollef MH. Delaying the empiric treatment of candida bloodstream infection until positive blood culture results are obtained: a potential risk factor for hospital mortality. Antimicrob Agents Chemother. 2005; 49(9):3640–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Garey KW, Rege M, Pai MP, Mingo DE, Suda KJ, Turpin RS, et al. Time to initiation of fluconazole therapy impacts mortality in patients with candidemia: a multi-institutional study. Clin Infect Dis. 2006; 43(1):25–31. [DOI] [PubMed] [Google Scholar]
- 23. Garnacho-Montero J, Díaz-Martín A, García-Cabrera E, Ruiz Pérez de Pipaón M, Hernández-Caballero C, Lepe-Jiménez JA. Impact on hospital mortality of catheter removal and adequate antifungal therapy in Candida spp. bloodstream infections. J Antimicrob Chemother. 2013; 68(1):206–13. 10.1093/jac/dks347 [DOI] [PubMed] [Google Scholar]
- 24. Slavin MA, Sorrell TC, Marriott D, Thursky KA, Nguyen Q, Ellis DH, et al. Australian Candidemia Study, Australasian Society for Infectious Diseases. Candidaemia in adult cancer patients: risks for fluconazole-resistant isolates and death. J Antimicrob Chemother. 2010; 65(5):1042–51. 10.1093/jac/dkq053 [DOI] [PubMed] [Google Scholar]
- 25. Ma CF, Li FQ, Shi LN, Hu YA, Wang Y, Huang M, et al. Surveillance study of species distribution, antifungal susceptibility and mortality of nosocomial candidemia in a tertiary care hospital in China. BMC Infect Dis. 2013; 13:337 10.1186/1471-2334-13-337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clinical and Laboratory Standards Institute. Reference method for broth dilution antifungal susceptibility testing of yeasts and supplement, 3 rd and 4th informational.2011- 2012, CLSI M27-S4. [PubMed]
- 27.European Committee on Antimicrobial Susceptibility test (2011) Antimicrobials for Candida infections—EUCAST clinical MIC breakpoints. 2011-04-27 (v 3.0).
- 28.European Committee on Antimicrobial Susceptibility test (2013) Antimicrobials for Candida infections—EUCAST clinical MIC breakpoints. 2013-03-11 (v 6.1).
- 29. Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2007; 348:1546–54. [DOI] [PubMed] [Google Scholar]
- 30. Lortholary O, Renaudat C, Dromer F, French Mycosis Study Group. Worrisome trends in incidence and mortality of candidemia in intensive care units (Paris area, 2002–2010). Intensive Care Med. 2014; 40(9):1303–12. 10.1007/s00134-014-3408-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Luzzati R, Cavinato S, Deiana ML, Rosin C, Maurel C, Borelli M. Epidemiology and outcome of nosocomial candidemia in elderly patients admitted prevalently in medical wards. Aging Clin Exp Res. 2014. [DOI] [PubMed] [Google Scholar]
- 32. Bassetti M, Taramasso L, Nicco E, Molinari MP, Mussap M, Viscoli C. Epidemiology, species distribution, antifungal susceptibility and outcome of nosocomial candidemia in a tertiary care hospital in Italy. PLoS One. 2011; 6(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stratman RC, Martin CA, Rapp RP, Berger R, Magnuson B. Candidemia Incidence in Recipients of Parenteral Nutrition. Nutr Clin Pract. 2010; 25:282 10.1177/0884533610368704 [DOI] [PubMed] [Google Scholar]
- 34. Bassetti M, Molinari MP, Mussap M, Viscoli C, Righi E. Candidaemia in internal medicine departments: the burden of a rising problem.Clin Microbiol Infect. 2013; 19(6):281–284. [DOI] [PubMed] [Google Scholar]
- 35. Wu Z, Liu Y, Feng X, Wang S, Zhu X, Chen Q, et al. Candidemia: incidence rates, type of species, and risk factors at a tertiary care academic hospital in China. Int J Infect Dis. 2014;22 10.1016/j.ijid.2013.12.008 [DOI] [PubMed] [Google Scholar]
- 36. Bassetti M, Righi E, Costa A, Fasce R, Molinari MP, Viscoli C, et al. Epidemiological trends in nosocomial candidemia in intensive care. BMC Infect Dis. 2006;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Marchetti O, Bille J, Flukiger U, Eggimann P, Garbino J, Pittet D et al. Epidemiology of candidemia in Swiss tertiary care hospitas: secular trends, 1991–2000. Clin Infect Dis. 2004;38(3):311–20. [DOI] [PubMed] [Google Scholar]
- 38. Pfaller MA, Diekema DJ, Gibbs DL, Newell VA, Nagy E, Dobiasova S, et al. Candida krusei, a Multidrug-Resistant Opportunistic Fungal Pathogen: Geographic and Temporal Trends from the ARTEMIS DISK Antifungal Surveillance Program, 2001 to 2005. J ClinMicrobiol. 2008;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kett DH, Azoulay E, Echeverria PM, Vincent JL. Candida bloodstream infections in intensive care units: Analysis of the extended prevalence of infection in intensive care unit study. Crit Care Med. 2011;39(4):665–70. 10.1097/CCM.0b013e318206c1ca [DOI] [PubMed] [Google Scholar]
- 40. Kollef M, Micek S, Hampton N, Doherty JA, Kumar A. Septic shock attributed to Candida infection: importance of empiric therapy and source control. Clin Infect Dis 2012, 54(12):1739–1746. [DOI] [PubMed] [Google Scholar]
- 41. Cornely OA, Bassetti M, Calandra T, Garbino J, Kullberg BJ, Lortholary O et al. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: non-neutropenic adult patients. Clin Microb Infect 2012, 18 Suppl 7:19–37 [DOI] [PubMed] [Google Scholar]
- 42. Andes DR, Safdar N, Baddley JW, Playford G, Reboli AC, Rex JH et al. Impact of treatment strategy on outcomes in patients with candidemia and other forms of invasive candidiasis: a patient-level quantitative review of randomized trials. Clin Infect Dis 2012, 54(8):1110–1122. 10.1093/cid/cis021 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.