Abstract
Background
A randomized multicenter phase II trial was conducted to assess the sequential treatment strategy using FOLFIRI.3 and gemcitabine alternately (Arm 2) compared to gemcitabine alone (Arm 1) in patients with metastatic non pre-treated pancreatic adenocarcinoma. The primary endpoint was the progression-free survival (PFS) rate at 6 months. It concludes that the sequential treatment strategy appears to be feasible and effective with a PFS rate of 43.5% in Arm 2 at 6 months (26.1% in Arm 1). This paper reports the results of the longitudinal analysis of the health-related quality of life (HRQoL) as a secondary endpoint of this study.
Methods
HRQoL was evaluated using the EORTC QLQ-C30 at baseline and every two months until the end of the study or death. HRQoL deterioration-free survival (QFS) was defined as the time from randomization to a first significant deterioration as compared to the baseline score with no further significant improvement, or death. A propensity score was estimated comparing characteristics of partial and complete responders. Analyses were repeated with inverse probability weighting method using the propensity score. Multivariate Cox regression analyses were performed to identify independent factors influencing QFS.
Results
98 patients were included between 2007 and 2011. Adjusting on the propensity score, patients of Arm 2 presented a longer QFS of Global Health Status (Hazard Ratio: 0.52 [0.31-0.85]), emotional functioning (0.35 [0.21–0.59]) and pain (0.50 [0.31 – 0.81]) than those of Arm 1.
Conclusion
Patients of Arm 2 presented a better HRQoL with a longer QFS than those of Arm 1. Moreover, the propensity score method allows to take into account the missing data depending on patients’ characteristics.
Trial registration information
Eudract N° 2006-005703-34. (Name of the Trial: FIRGEM).
Introduction
The results of a phase II trial concerning untreated patient with metastatic Pancreatic Cancer (mPC) have shown that sequential treatment using FOLFIRI.3 and gemcitabine was effective and safe [1].
In first line treatment, FOLFIRINOX protocol and the association of nab-paclitaxel + gemcitabine improve overall survival (OS) [2,3] and represent a new therapeutic option in first line. However, the less favorable toxicity profiles of these new strategies could limit this option to younger patients with a good Performance Status (0 or 1) [4]. A sequential association of chemotherapy protocol without cross-resistance may increase anti-tumor effects and limit toxicities, preserving patient’s Health-related Quality of Life (HRQoL).
Prognosis of patients with mPC remains extremely poor. In consequence, HRQoL is a major subject of concern for these patients who are often painful and symptomatic at the time of diagnosis. Moreover, HRQoL appears to be an independent prognostic factor for OS alongside classical clinical and demographic factors [5]. In metastatic settings, the current discussion is to consider HRQoL as a co-primary endpoint along with a tumor parameter such as progression-free survival (PFS) [6,7].
However, HRQoL results remain poorly used to modify therapeutic strategies, due to the complexity of its longitudinal analysis and to a lack of standardization. Moreover, results should have the ability to translate findings into information that decision makers find understandable and compelling.
In recent years, time to event models like time until definitive HRQoL score deterioration (TUDD) have been proposed as a modality of longitudinal HRQoL analysis in oncology, especially in metastatic setting [8]. The TUDD method produces clinically meaningful results for clinicians like Kaplan-Meier survival curves and hazard ratio (HR). TUDD including death as an event was defined as "HRQoL deterioration-free survival" (QFS) [9].
One other major concern of longitudinal HRQoL studies is missing data [10], specifically in advanced cancer where attrition is common [11]. Patients may dropout before the end of the study, generally due to a health status deterioration or death. In this case, missing data can bias the analysis and interpretation [10,12–14], and should be considered to ensure accuracy and robustness of the results. Several methods have been investigated to handle with missing data [15,16]. The most well-known is the pattern-mixture model [17] but it is rarely applied due to its complexity [17,18].
Then it would be interesting to develop a method to use in conjunction with QFS to handle with informative missing data. Methods using the propensity score are often used in observational studies in order to reduce the bias of the absence of randomization and to allow causal inference [19]. The propensity score is used to model the probability of receiving a treatment conditionally to the variables observed before treatment. The main methods used with propensity score are stratification, matching and inverse probability weighting (IPW) methods [20]. In survival analyses, IPW method is recommended [21]. Indeed, IPW method of the propensity score was already proposed to take into account missing data [22].
The objective of this study was to compare longitudinal HRQoL according to treatment arm using QFS in a metastatic setting and secondary to investigate the application of the IPW method based on the propensity score in conjunction with the TUDD in order to take into account missing data depending on patients’ characteristics.
Materials and Methods
Patients and eligibility criteria
This study was a multicenter, randomized, non-comparative, open phase II trial, conducted in French centers. Inclusion criteria were: histologically or cytologically proven mPC, no previous chemotherapy (adjuvant chemotherapy with gemcitabine was allowed if administered more than 12 months before inclusion) or radiotherapy (unless at least one measurable target lesion was present outside the irradiated area) and WHO performance status <2. Exclusion criteria were bile ducts adenocarcinoma, ampulloma and a history of another cancer. All patients were fully informed of the study and provided signed written informed consent (see S1 Informed Consent). The protocol was approved by the ethics committees (“Comité de Protection des Personnes”). This study FIRGEM was registered with EudraCT (https://eudract.ema.europa.eu/; N° 2006-005703-34) before the start date. The design of this study has been extensively described elsewhere [1]. The protocol for this trial and supporting CONSORT checklist are available as supporting information (see S1 and S2 Protocol; see S1 Checklist). List of Ethics Committees is also available in supporting information (see S1 Authorization).
Using minimization technique, patients were randomly (ratio 1:1) assigned to receive sequentially FOLFIRI.3 every 14 days during two months (four courses per cycle), followed by gemcitabine (6 courses at days 1, 8, 15, 29, 36 and 43 per cycle) (Arm 2) or gemcitabine alone (Arm 1). A deterministic minimization was employed and stratification criteria were center (10 centers), performance status (0 vs. 1) and the number of metastatic sites (one vs. more than one).
Health-related quality of life assessment
HRQoL was evaluated using the European Organisation for Research and Treatment of Cancer (EORTC) QLQ-C30 cancer specific questionnaire [23], at inclusion and every two months until progression, limiting toxicity, patient’s refusal or death. The QLQ-C30 includes 30 items and measures five functional scales (physical, role, emotional, cognitive and social functioning), global health status (GHS), financial difficulties and eight symptom scales (fatigue, nausea and vomiting, pain, dyspnea, insomnia, appetite loss, constipation, diarrhea) [23]. These scores vary from 0 (worst) to 100 (best) for the functional dimensions and GHS, and from 0 (best) to 100 (worst) for the symptom dimensions and were generated according to the EORTC Scoring Manual [24].
Statistical analysis
Sample size calculation
The primary endpoint was the 6-month PFS rate. Secondary endpoints were OS, safety/tolerability, tumor response, PFS and QFS. The trial was based on a Fleming one-step design [25]. The expected 6-month PFS rate with the sequential treatment was 45%. A PFS rate of 25% was chosen as uninteresting rate of effectiveness (H0: 6-month PFS 25% = unacceptable efficacy, H1: 6-month PFS 45% = expected efficacy). With a unilateral type I error of 5% and a type II error of 10%, it was necessary to include 46 patients in each arm, rounded to 49 to compensate for an anticipated 5% rate of loss to follow-up.
Based on Fleming decision criteria, experimental arm will be considered uninteresting if 15 or less than 15 alive patients were free of progression. It will be considered as promising if 16 or more than 16 alive patients were free of progression.
The analysis was performed on intent-to-treat principle (all randomized patients irrespective of treatment received and eligibility criteria). Analyses of primary endpoint were done on the first randomized 46 patients with available PFS data (to match with Fleming criteria decision rules) while all other analyses were done on all randomized patients. Tumour responses were defined using RECIST (version 1.1) [26] and determined by investigators.
Population
Randomized patients whatever eligibility criteria with at least one HRQoL score were included in the QFS analysis (modified intent to treat analysis). Pre-specified targeted HRQoL dimensions were GHS (mITT1), physical (mITT2) and emotional functioning (mITT3), fatigue (mITT4) and pain (mITT5).
Since this is a non comparative randomized phase II trial and HRQoL was an exploratory secondary endpoint, no p-value was provided while effect size was presented using hazard ratio with 95% confidence interval (CI95%). A five-point difference in HRQoL scores was considered as the Minimal Clinically Important Difference [27].
Descriptive analysis
Baseline variables were described using means and standard deviations for continuous variables and percentages for qualitative variables. Baseline HRQoL scores were described by treatment arm. The number of HRQoL questionnaires completed at each measurement time was reported. The Most Common Grade 3 or 4 Adverse Events occurring during the study according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 3.0) [28] were reported by treatment arm.
Missing data analysis
The missing data patterns were patients with at least one missing HRQoL score during the follow-up (partial responders) versus patients with all available scores until their drop-out of the study or death (complete responders). The number and percentage of patients according to the missing data profile (partial vs. complete responders) were described at each measurement time by treatment arm. The number and percentage of complete responders, partial responders and non responders (patients who did not complete any HRQoL questionnaire) were described by treatment arm and the difference between the two treatment arms was compared using Chi-square test. All baseline variables that could be associated with missing data patterns (partial vs. complete responders) were tested with an univariate logistic regression model. Variables with an univariate P-value ≤ 0.20 were eligible for multivariate analysis. To prevent collinearity, when two variables were significantly correlated, one variable was retained according to its clinical relevance. The final multivariate model was chosen according to the Akaike criteria and the area under the ROC curve and described with Odds-Ratio (OR) and its 95%CI. Fitted values were then extracted from the model and constituted the propensity score [29].
Longitudinal analysis
The QFS was defined as the time from randomization to a first deterioration with a 5-point Minimal Clinically Important Difference as compared to the baseline score with no further improvement of more than 5 points as compared to the baseline sore, or all-cause of death [8]. Patients with no baseline score were censored at baseline (Day 0). Patients with no follow-up measure were censored just after baseline (Day 1). Patients with no deterioration before their drop-out and those with a deterioration followed by a significant improvement are censored at the time of the last follow-up or the last HRQoL assessment. Each targeted dimensions of the QLQ-C30 was studied.
Based on the intention-to treat principle and according to the worst possible scenario, a sensitivity analysis was performed integrating non responders patients and considering these patients in deterioration since baseline (Day 1).
QFS curves were calculated using the Kaplan-Meier estimation method and described using median and its 95%CI. Univariate Cox analyses were done as exploratory analysis to estimate effect of treatment arm size with the HR and 95%CI. Follow-up was calculated using reverse Kaplan-Meier estimation.
To take into account missing data, analyses were repeated by assigning a weight to patients according to the IPW method of propensity score [21]. The weight equals to the inverse of the propensity score value for partial responders and to the inverse of the opposite of the propensity score value for complete responders [21].
Multivariate Cox regression model was also conducted as exploratory analysis in order to investigate parameters which seem to be associated with QFS. All variables collected at baseline were tested in univariate analysis. Some interaction effects between treatment arm and clinical variables were investigated. Variables with 1 not included in the 95%CI of the HR were eligible for multivariate analysis. The same variables were kept in multivariate analysis for unweighted and weighted QFS analyses. The variable treatment arm was forced in multivariate analysis.
All analyses were performed with R software [30].
Results
Study population
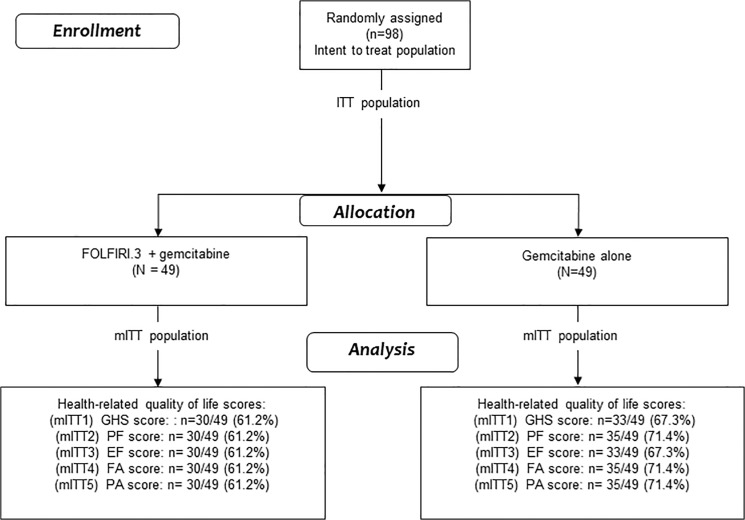
Between October 2007 and May 2011, 98 patients (49 in each treatment arm) were enrolled in 10 French centers (Fig 1). Baseline characteristics of patients are summarized in Table 1. The median age was 62 years (range 38–76) and 59 patients (60.20%) were men. At baseline, 34 patients (69.4%) completed the QLQ-C30 questionnaire in Arm 1 and 30 patients (61.2%) in Arm 2. No difference of baseline HRQoL level was observed between treatment arms (S1 Table).
Fig 1. CONSORT Diagram for health-related quality of life analysis.
ITT: intent to treat; mITT modified intent to treat; GHS global health status; PF: Physical functioning; EF: Emotional functioning; FA: Fatigue; PA: Pain.
Table 1. Baseline characteristics of patients included according to treatment arm.
| Variable | Response Category | Arm1 gemcitabine alone (N = 49) | Arm 2 FOLFIRI.3 + gemcitabine (N = 49) | ||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Sex | male | 28 | 57.1 | 31 | 63.3 |
| female | 21 | 42.9 | 18 | 36.7 | |
| WHO performance status | 0 | 16 | 32.6 | 16 | 32.7 |
| 1 | 33 | 67.4 | 33 | 67.3 | |
| Previous surgery | no | 40 | 81.6 | 38 | 77.6 |
| yes | 9 | 18.4 | 11 | 22.4 | |
| Surgery type | curative | 4 | 8.2 | 5 | 10.2 |
| palliative | 5 | 10.2 | 5 | 10.2 | |
| not applicable | 40 | 81.6 | 38 | 77.6 | |
| missing | 0 | 0.0 | 1 | 2.0 | |
| Number of metastatic sites | 1 | 35 | 71.4 | 33 | 67.3 |
| more than 1 | 14 | 28.6 | 16 | 32.7 | |
| Previous chemotherapy | no | 40 | 81.6 | 44 | 89.8 |
| yes | 3 | 6.1 | 2 | 4.1 | |
| missing | 6 | 12.2 | 3 | 6.1 | |
| Primary tumor location | head | 29 | 59.2 | 18 | 36.7 |
| body | 11 | 22.5 | 17 | 34.7 | |
| tail | 12 | 24.5 | 17 | 34.7 | |
| Sites of metastasis | liver | 35 | 71.4 | 39 | 79.6 |
| lung | 11 | 22.5 | 11 | 22.5 | |
| lymph node | 7 | 14.3 | 5 | 10.2 | |
| peritoneal | 10 | 20.4 | 16 | 33.7 | |
| other | 2 | 4.0 | 3 | 6.1 | |
| Age (years) a | 45 | 63 [41–76] | 48 | 62 [38–76] | |
| Leukocytes (/mm3) a | 49 | 7600 [3100–36500] | 49 | 8300 [85–21700] | |
| neutrophils (/mm3) a | 49 | 5000 [1800–32850] | 48 | 5591.5 [2300–19530] | |
| Creatinine (μmol/l) a | 48 | 71.0 [39–105] | 48 | 70 [45–108] | |
| Glycaemia (mmol/l) a | 29 | 6.2 [4.1–14] | 25 | 5.8 [0.7–15] | |
| Bilirubin (μmol/l) a | 48 | 11.6 [4–227] | 45 | 12 [1–154] | |
| LDH (UI/L) a | 23 | 271 [96–5022] | 22 | 340.5 [133–766] | |
| Hemoglobin (g/dl) a | 49 | 12.8 [7.9–16.5] | 48 | 12.9 [9.4–16] | |
| Platelet (10^3/mm3) a | 49 | 239 [94–570] | 48 | 278.5 [111–634] | |
| ASAT (UI/L) a | 49 | 26 [8–149] | 46 | 41.5 [10–187] | |
| ALAT(UI/L) a | 49 | 35 [8–155] | 46 | 53.5 [10–348] | |
| Prothrombin (%) a | 39 | 93 [26–109] | 41 | 86 [19–122] | |
aMedian [min-max] for continuous variables.
The median follow up was 32.5 months (95%CI 25.4–40.4).
The primary endpoint (6-month PFS rate), on 46 first randomized patients per arm using Fleming’s criterion was reached in Arm 2 with 20 patients alive and free of progression resulting in an observed 6-month PFS rate of 43.5% [95%CI 28.6–58.4] but not in Arm 1 with only 12 patients alive and free of progression resulting in a 6-month PFS rate of 26.1% [12.9–39.3].
Among all randomized patients, the estimated 6 months PFS rate was 25.7% [95%CI 14.4–38.6] for Arm 1 and 44.9% [30.7–58.0] for Arm 2. The objective response rate was 10.2% [1.4–19.0] for Arm 1 and 36.7% [22.7–50.7] for Arm 2. Median OS was 8.2 months [95%CI 5.3–9.2] in Arm 1 and 11 months [7.8–13.6] in Arm 2 [1].
The Most Common Grade 3 or 4 Adverse Events occurring during the study are reported in S2 Table.
Missing data analysis
Table 2 gives the number and percentage of complete, partial and non-responders in each treatment arm.
Table 2. Proportion of complete, partial and non responders for HRQoL assessment in each treatment arm.
| Arm gemcitabine alone (N = 49) | Arm FOLFIRI + gemcitabine (N = 49) | |
|---|---|---|
| Complete responders | 15 (30.6) | 11 (22.4) |
| Partial responders | 15 (30.6) | 25 (51.0) |
| Non responders | 19 (38.8) | 13 (26.5) |
Among the 66 patients (67.3%) who had completed at least one HRQoL questionnaire during the study, 40 (60.6%) were partial responders (15 in Arm 1 (37.5%), 25 in Arm 2 (62.5%)) and 26 (39.4%) were complete responders (15 in Arm 1 (57.7%), 11 in Arm 2 (42.3%)) during the follow-up. The details of the HRQoL questionnaire completed at each follow-up measurement time according to treatment arm and missing data profile are given in Table 3.
Table 3. Completion of HRQoL questionnaire at each follow-up measurement time according to treatment arm and missing data profile.
| Complete responders | Partial responders | |||||
|---|---|---|---|---|---|---|
| Arm 1 | Arm 2 | Total | Arm 1 | Arm 2 | Total | |
| N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | |
| cycle 1 | 14 (44.1) | 15 (50.0) | 29 (46.0) | 19 (55.9) | 15 (50.0) | 34 (54.0) |
| cycle 2 | 13 (46.4) | 7 (35.0) | 20 (41.2) | 15 (53.6) | 13 (65.0) | 28 (58.3) |
| cycle 3 | 7 (35.0) | 1 (10.0) | 8 (26.7) | 13 (65.0) | 9 (90.0) | 22 (73.3) |
| cycle 4 | 4 (28.6) | 1 (50.0) | 5 (31.3) | 10 (71.4) | 1 (50.0) | 11 (68.8) |
| cycle 5 | 3 (50.0) | 1 (50.0) | 4 (50.0) | 3 (50.0) | 1 (50.0) | 4 (50.0) |
| cycle 6 | 1 (33.3) | 0 (0.0) | 1 (25.0) | 2 (66.7) | 1 (100.0) | 3 (75.0) |
| cycle 7 | 1 (50.0) | 0 (0.0) | 1 (50.0) | 1 (50.0) | 0 (0.0) | 1 (50.0) |
| cycle 8 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (100.0) | 0 (0.0) | 1 (100.0) |
| cycle 9 | 1 (100.0) | 0 (0.0) | 1 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| cycle 10 | 1 (100.0) | 0 (0.0) | 1 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
Arm 1: gemcitabine alone; Arm 2: gemcitabine + FOLFIRI.3.
Based on the univariate analyses, variables associated with responder profiles and retained to build the propensity score were a primary tumor location at the pancreatic head (yes vs. no), presence of metastatic lymph node (yes vs. no), neutrophils, hemoglobin and platelet rates (dichotomized according to the median value). In multivariate analysis, a primary tumor location at the pancreatic head (OR = 2.72 [95%CI 0.86–9.16]), the presence of lymph node metastases (7.90 [95%CI 1.12–164.12]), a low neutrophils (2.13 [95%CI 0.64–7.25]) and platelets rate (2.77 [95%CI 0.84–9.72]) and a high hemoglobin rate (1.80 [95%CI 0.54–6.10]) were independently associated with partial responder profile but not statistically significant. The area under the ROC curve was equal to 0.76.
Longitudinal analysis
In Arm 1 (gemcitabine alone) and Arm 2 (gemcitabine + FOLFIRI.3) respectively:
- 18 and 17 patients experienced a QFS of GHS, among the 63 patients retained (30 in Arm 1, 33 in Arm 2).
- 19 and 17 patients experienced a QFS of physical functioning, among the 65 patients retained (30 in Arm 1, 35 in Arm 2)
- 19 and 18 patients experienced a QFS of emotional functioning, among the 63 patients retained (30 in Arm 1, 33 in Arm 2).
- 18 and 17 patients experienced a QFS of fatigue, among the 65 patients retained (30 in Arm 1, 35 in Arm 2).
- 18 and 16 patients experienced a QFS of pain, among the 65 patients retained (30 in Arm 1, 35 in Arm 2).
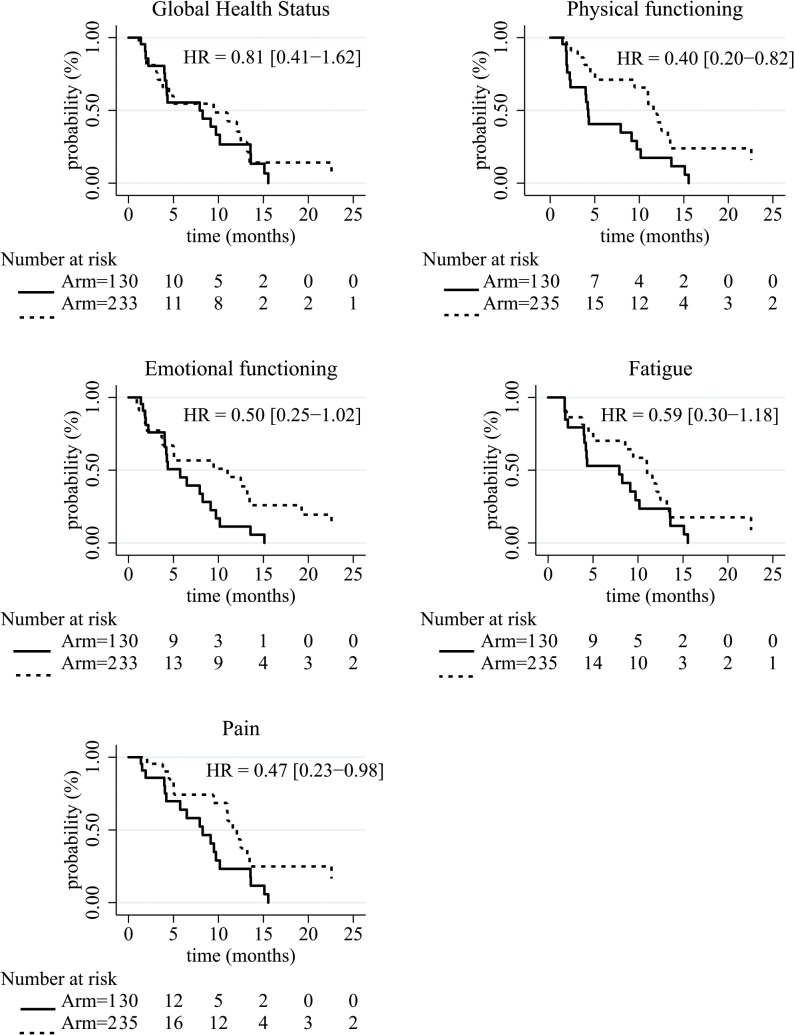
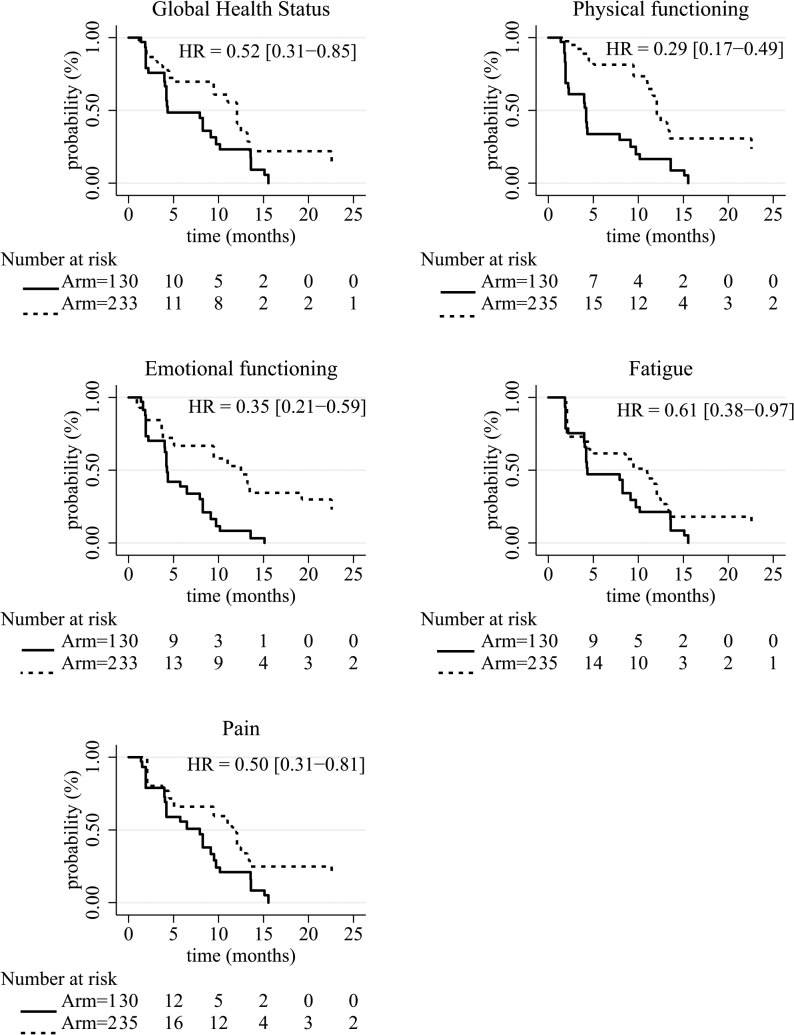
Regarding the unweighted analysis (Table 4), patients in FOLFIRI.3 + gemcitabine regimen tended to present a longer QFS than those of Arm 1 only for physical functioning (HR = 0.40 [95%CI 0.20–0.82]) with a median QFS of 7.92 months [95%CI 4.21–13.6] for Arm 1 and 9.42 [95%CI 3.81–13.47] for Arm 2. Regarding the weighted analysis, the same result was observed and patients in Arm 2 seemed to present a longer QFS of GHS (HR = 0.52 [95%CI 0.31–0.85]), emotional functioning (HR = 0.35 [95%CI 0.21–0.59]), and pain (HR = 0.50 [95%CI 0.31–0.81]). The median QFS of GHS was 4.34 months [95%CI 4.21–9.72] for Arm 1 and 12.06 [95%CI 9.46–13.47] for Arm 2. The median QFS of emotional functioning was 4.27 months [95%CI 4.04–7.92] for Arm 1 and 12.48 [95%CI 9.46–22.57] for Arm 2. Regarding pain, the median QFS was 7.92 months [95%CI 4.21–9.49] for Arm 1 and 11.60 [95%CI 9.46–13.21] for Arm 2. These QFS curves were described in Figs 2 and 3.
Table 4. Results of the Kaplan-Meier estimation of the health-related quality of life deterioration-free survival for a QLQ-C30 score and comparison between treatment arms.
| unweighted analysis | weighted analysis | |||||
|---|---|---|---|---|---|---|
| N (events) | median | HR [CI 95%] | median | HR [CI 95%] | ||
| [CI 95%] | [CI 95%] | |||||
| Global health status | Arm 1 a | 30 (18) | 7.92 |4.21–13.6] | 1 | 4.34 [4.21–9.72] | 1 |
| Arm 2 b | 33 (17) | 9.46 |3.81–13.47] | 0.81 (0.41–1.62) | 12.06 [9.46–13.47] | 0.52 [0.31–0.85] | |
| Physical functioning | Arm 1 | 30 (19) | 4.27 [2.27–10.15] | 1 | 4.21 [2.27–7.92] | 1 |
| Arm 2 | 35 (17) | 11.6 [9.46–26.25] | 0.40 (0.2–0.82) | 12.06 [11.6–22.57] | 0.29 [0.17–0.49] | |
| Emotional functioning | Arm 1 | 30 (19) | 5.75 [4.04–9.72] | 1 | 4.27 [4.04–7.92] | 1 |
| Arm 2 | 33 (18) | 11.01 [3.81–22.57] | 0.50 (0.25–1.02) | 12.48 [9.46–22.57] | 0.35 [0.21–0.59] | |
| Fatigue | Arm 1 | 30 (18) | 7.92 [4.21–13.57] | 1 | 4.34 [4.21–9.13] | 1 |
| Arm 2 | 35 (17) | 11.01 [8.57–13.47] | 0.59 (0.30–1.18) | 10.97 [5.03–12.06] | 0.61 [0.38–0.97] | |
| Pain | Arm 1 | 30 (18) | 8.25 [5.75–13.57] | 1 | 7.92 [4.21–9.49] | 1 |
| Arm 2 | 35 (16) | 11.6 [10.97-NA] | 0.47 (0.23–0.98) | 11.6 [9.46–13.21] | 0.50 |0.31–0.81] | |
a Arm 1: gemcitabine alone;
b Arm 2: gemcitabine+FOLRIRI.3.
Fig 2. Kaplan-Meier survival curves of the HRQoL deterioration-free survival by treatment arm for the raw and the weighted analysis.
Arm 1: gemcitabine alone, Arm 2: gemcitabine + FOLFIRI.3.
Fig 3. Kaplan-Meier survival curves of the HRQoL deterioration-free survival by treatment arm for the weighted analysis.
Arm 1: gemcitabine alone, Arm 2: gemcitabine + FOLFIRI.3.
Variables retained for the Cox multivariate analysis were treatment arm (Arm 2 vs. Arm 1), number of metastatic sites (2 or more vs. 1) and an interaction effect between treatment arm and the number of metastatic sites, according to the univariate Cox regression analysis (data not shown).
In the unweighted analysis, all the 95%CI contained the value of 1. Regarding the weighted analyses, the treatment arm (gemcitabine + FOLFIRI.3) and the number of metastatic sites (one site) seemed to be independently associated with longer QFS of physical functioning (Table 5). The number of metastatic sites (more than one vs. one) seemed to be associated with a shorter QFS of GHS, fatigue and pain.
Table 5. Results of the multivariate Cox regression analysis for the QFS analysis of each targeted score of the QLQ-C30 for the raw and the weighed analysis.
| without IPW | with IPW | |||
|---|---|---|---|---|
| N (events) | HR [CI 95%] | HR [CI 95%] | ||
| Global health status | 63 (35) | |||
| arm a | (arm 2) vs.(arm 1) | 0.86 [0.38–1.96] | 0.58 [0.31–1.07] | |
| number of metastatic sites | (2 or more) vs. 1 | 3.98 [1.21–13.71] | 4.39 [2.03–9.49] | |
| Interaction between arm and number of metastatic sites | 0.38 [0.08–1.88] | 0.41 [0.13–1.27] | ||
| Physical functioning | 65 (36) | |||
| arm a | (arm 2) vs.(arm 1) | 0.34 [0.14–0.82] | 0.25 [0.13–0.48] | |
| number of metastatic sites | (2 or more) vs. 1 | 2.80 [0.87–9.08] | 2.71 [1.28–5.75] | |
| Interaction between arm and number of metastatic sites | 0.86 [0.18–4.16] | 1.09 [0.36–3.30] | ||
| Emotional functioning | 63 (37) | |||
| arm a | (arm 2) vs.(arm 1) | 0.44 [0.19–1.03] | 0.29 [0.15–0.56] | |
| number of metastatic sites | (2 or more) vs. 1 | 2.72 [0.84–8.79] | 2.59 [1.24–5.41] | |
| Interaction between arm and number of metastatic sites | 0.91 [0.19–4.47] | 1.47 [0.50–4.37] | ||
| Fatigue | 65 (35) | |||
| arm a | (arm 2) vs.(arm 1) | 0.54 [0.23–1.24] | 0.71 [0.40–1.24] | |
| number of metastatic sites | (2 or more) vs. 1 | 3.27 [0.86–12.42] | 3.40 [1.58–7.30] | |
| Interaction between arm and number of metastatic sites | 0.58 [0.11–3.17] | 0.39 [0.13–1.11] | ||
| Pain | 65 (34) | |||
| arm a | (arm 2) vs.(arm 1) | 0.44 [0.18–1.07] | 0.57 [0.32–1.03] | |
| number of metastatic sites | (2 or more) vs. 1 | 3.04 [0.93–9.91] | 3.15 [1.51–6.57] | |
| Interaction between arm and number of metastatic sites | 0.66 [0.13–3.31] | 0.46 [0.16–1.33] |
a Arm 1: gemcitabine alone, Arm 2: gemcitabine + FOLFIRI.3.
As for the unweighted analysis, the same trends were observed for the sensitivity unweighted analysis integrating non-responders patients (see S1 Fig, S3 and S4 Tables).
Discussion
As previously reported, patients treated with sequential chemotherapy FOLFIRI.3 + gemcitabine presented a benefit in PFS at 6 months (44.9% (30.7–58.0) vs. 25.7% (14.4–38.6)), OS (64.7%(49.5–76.4) vs. 62.8% (47.6–74.7)) and objective response rate (36.7% vs. 10.2%) [1].
Meanwhile to the recent progress in the improvement of OS, preserving HRQoL is of paramount importance considering the symptom burden and the poor prognosis of mPC. If several Phase III trials attempted to show a clinical benefit or improvement in HRQoL, few have achieved their goals [31,32]. Recently, the clinical trial comparing FOLFIRINOX to gemcitabine shown an improvement in HRQoL for FOLFIRINOX arm [5].
In our trial, HRQoL results support the efficacy profile of FOLFIRI.3 + Gemcitabine regimen. Patients in FOLFIRI.3 + Gemcitabine arm presented a longer QFS than those of gemcitabine alone arm whatever the HRQoL score considered in both QFS analyses even if patients in FOLFIRI.3 + Gemcitabine arm presented twice as much as those of Gemcitabine alone arm occurrence of grade 3 or 4 neutropenia. In multivariate weighted analysis, treatment with sequential FOLFIRI.3 + gemcitabine seemed to be associated with longer QFS in each HRQoL score considered including pain and fatigue score, two symptoms commonly present at time of diagnosis. It would be interested to study the impact of the occurrence of at least one grade 3–4 toxicity on the QFS.
Median QFS for each domain was shorter than median PFS irrespective of the use of IPW method. It is noteworthy that survival estimates depend on the QFS definition. Contrary to our definition, all-cause death was not integrated as an event in the definition of TUDD chosen by Gourgou-Bourgade et al. [5]. In consequence, median TUDD was not reached after a 26.6 months follow-up while the median PFS was 6.4 months in the FOLFIRINOX arm [2,5] which was not in agreement with clinical profiles of these patients. Moreover it is underlined that comparison across trials is not possible, stressing the need to adopt a common definition of TUDD or QFS [9].
If QFS is increasingly used in clinical trials, consensual methods to optimize management of missing data are still lacking [5,33–35]. In FOLFIRINOX trial, little information was provided on the method used to deal with missing data, except when authors declared that the two groups did not differ in terms of rate of missing data [5].
In our study, in both unweighted and weighted analyses, patients in Arm 2 presented a longer QFS than patients in Arm 1. In multivariate analyses, treatment arm (gemcitabine + FOLFIRI.3) and number of metastatic sites (one site) tended to be associated with longer QFS of physical functioning in the weighted analysis. The same trends were observed for the unweighted analysis.
In this way, using the IPW method of the propensity score influences the results of the multivariate analysis by underlining more significant associations. A high weight is assigned to patients with no missing data (mainly patients of Arm 1) and a low weight to partial responders (mainly patients of Arm 2). As in unweighted analysis, a longer QFS was yet observed for patients of Arm 2 as compared to those of Arm 1 for most HRQoL dimensions, the HR increased with the use of the IPW method. The use of the propensity score in conjunction with the TUDD method allowed reducing the bias due to the occurrence of missing data depending on patients’ characteristics during the follow-up. This bias cannot be totally eliminated because missing data can also depend on unobserved data. However, some logistic problems could explain the reasons for partial and non-responders because these patients were followed in the study for other endpoints. Another statistical approach to use in conjunction of the QFS method should be proposed to adequately take into account missing not at random data.
Besides primary prevention procedures for limiting missing data rate, additional work on statistical methods to handle with missing data is still needed. Multiple imputations on the HRQoL scores could also be performed but this method requires a larger sample and can only retain one or two factors associated with missing data [36], more variable can be retained in the propensity score. Then this approach could be suggested for the trials with limited sample size. Contrary to the pattern mixture models, the IPW method in conjunction with the TUDD approach is more appropriate to the design of oncology clinical trials, for which a lot of HRQoL measures are done. In fact, the number of possible patterns increases with the number of HRQoL measures. Austin et al. recommend to use IPW for time to event data [21]. Propensity score matching could also be performed for survival analysis but a higher sample size is needed. Finally, the IPW method is easy understandable (weighting observations according to the presence or absence of missing data) [21].
In conclusion, analyses of QFS supports that sequential strategy with FOLFIRI.3 followed by gemcitabine in patients with untreated mPC is feasible and, despite more toxicities, delayed the HRQoL deterioration. Moreover, using the propensity score allows controlling the imbalance of informative missing data between the two arms and provides more precise estimation of the treatment effect. This sequential treatment strategy will now be compared with FOLFIRINOX in a phase III trial (French study). This phase III clinical trial will allow to confirm or not these results raised from an exploratory analysis.
Supporting Information
(DOC)
(DOCX)
aArm 1: gemcitabine alone, Arm 2: gemcitabine + FOLFIRI.3.
(EPS)
(DOCX)
(DOC)
(DOC)
(DOC)
(DOC)
(DOC)
(DOC)
Data Availability
Data are unsuitable for public deposition due to ethical and legal restrictions and are therefore available upon request with the signature of a data privacy form. To request the data, the readers may contact Prof. Julien Taieb (julien.taieb@egp.aphp.fr) and Prof. Franck Bonnetain (franck.bonnetain@univfcomte.fr).
Funding Statement
Financial support for this research was provided by Pfizer, AGEO and AROLD (Association pour la Recherche en Oncologie Digestive). This study was also supported by a grant from the French Public Health Research Institute (http://www.iresp.net) under the 2012 call for projects as part of the 2009-2013 Cancer Plan. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Trouilloud I, Dupont-Gossard AC, Malka D, Artru P, Gauthier M, Lecomte T et al. Fixed-dose rate gemcitabine alone or alternating with FOLFIRI.3 (irinotecan, leucovorin and fluorouracil) in the first-line treatment of patients with metastatic pancreatic adenocarcinoma: An AGEO randomised phase II study (FIRGEM). Eur J Cancer. 2014; 50: 3116–3124. 10.1016/j.ejca.2014.09.015 [DOI] [PubMed] [Google Scholar]
- 2. Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011; 364: 1817–1825. 10.1056/NEJMoa1011923 [DOI] [PubMed] [Google Scholar]
- 3. Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Eng J Med. 2013; 18: 1691–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Seufferlein T, Bachet JB, Van Cutsem E, Rougier P, Esmo Guidelines Working Group. Pancreatic adenocarcinoma: ESMO-ESDO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012; 23 Suppl 7: vii33–40. [DOI] [PubMed] [Google Scholar]
- 5. Gourgou-Bourgade S, Bascoul-Mollevi C, Desseigne F, Ychou M, Bouche O, Guimbaud R et al. Impact of FOLFIRINOX compared with gemcitabine on quality of life in patients with metastatic pancreatic cancer: results from the PRODIGE 4/ACCORD 11 randomized trial. J Clin Oncol. 2013; 31: 23–29. 10.1200/JCO.2012.44.4869 [DOI] [PubMed] [Google Scholar]
- 6. Fiteni F, Westeel V, Pivot X, Borg C, Vernerey D, Bonnetain F. Endpoints in cancer clinical trials. J Visc Surg. 2014; 151: 17–22. 10.1016/j.jviscsurg.2013.10.001 [DOI] [PubMed] [Google Scholar]
- 7. Bonnetain F, Bosset JF, Gerard JP, Calais G, Conroy T, Mineur L et al. What is the clinical benefit of preoperative chemoradiotherapy with 5FU/leucovorin for T3-4 rectal cancer in a pooled analysis of EORTC 22921 and FFCD 9203 trials: surrogacy in question? Eur J Cancer 2012; 48: 1781–1790. 10.1016/j.ejca.2012.03.016 [DOI] [PubMed] [Google Scholar]
- 8. Bonnetain F, Dahan L, Maillard E, Ychou M, Mitry E, Hammel P et al. Time until definitive quality of life score deterioration as a means of longitudinal analysis for treatment trials in patients with metastatic pancreatic adenocarcinoma. Eur J Cancer. 2010; 46: 2753–2762. 10.1016/j.ejca.2010.07.023 [DOI] [PubMed] [Google Scholar]
- 9. Anota A, Hamidou Z, Paget-Bailly S, Chibaudel B, Bascoul-Mollevi C, Auquier P et al. Time to health-related quality of life score deterioration as a modality of longitudinal analysis for health-related quality of life studies in oncology: do we need RECIST for quality of life to achieve standardization? Qual Life Res. 2015; 1: 5–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Little RJ, Rubin DB. Statistical analysis with missing data New York: John Wiley & Sons; 1987. [Google Scholar]
- 11. Sherman DW, McSherry CB, Parkas V, Ye XY, Calabrese M, Gatto M. Recruitment and retention in a longitudinal palliative care study. Appl Nurs Res. 2005; 18: 167–177. [DOI] [PubMed] [Google Scholar]
- 12. Fairclough DL, Peterson HF, Chang V. Why are missing quality of life data a problem in clinical trials of cancer therapy? Stat Med. 1998; 17: 667–677. [DOI] [PubMed] [Google Scholar]
- 13. Ross L, Thomsen BL, Boesen EH, Johansen C. In a randomized controlled trial, missing data led to biased results regarding anxiety. J Clin Epidemiol. 2004; 57: 1131–1137. [DOI] [PubMed] [Google Scholar]
- 14. Curran D, Bacchi M, Schmitz SF, Molenberghs G, Sylvester RJ. Identifying the types of missingness in quality of life data from clinical trials. Stat Med. 1998; 17: 739–756. [DOI] [PubMed] [Google Scholar]
- 15. Liao K, Freres DR, Troxel AB. A transition model for quality-of-life data with non-ignorable non-monotone missing data. Stat Med. 2012; 31: 3444–3466. 10.1002/sim.5359 [DOI] [PubMed] [Google Scholar]
- 16. Fairclough DL, Peterson HF, Cella D, Bonomi P. Comparison of several model-based methods for analysing incomplete quality of life data in cancer clinical trials. Stat Med. 1998; 17: 781–796. [DOI] [PubMed] [Google Scholar]
- 17. Pauler DK, McCoy S, Moinpour C. Pattern mixture models for longitudinal quality of life studies in advanced stage disease. Stat Med. 2003; 22: 795–809. [DOI] [PubMed] [Google Scholar]
- 18. Little RJ, Wang Y. Pattern-mixture models for multivariate incomplete data with covariates. Biometrics. 1996; 52: 98–111. [PubMed] [Google Scholar]
- 19. Trojano M, Pellegrini F, Paolicelli D, Fuiani A, Di Renzo V. observational studies: propensity score analysis of non-randomized data. Int MS J. 2009; 16: 90–97. [PubMed] [Google Scholar]
- 20. D'Agostino RB Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998; 17: 2265–2281. [DOI] [PubMed] [Google Scholar]
- 21. Austin PC. The performance of different propensity score methods for estimating marginal hazard ratios. Stat Med. 2013; 32: 2837–2849. 10.1002/sim.5705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Seaman SR, White IR. Review of inverse probability weighting for dealing with missing data. Stat Methods Med Res. 2013; 22: 278–295. 10.1177/0962280210395740 [DOI] [PubMed] [Google Scholar]
- 23. Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993; 85: 365–376. [DOI] [PubMed] [Google Scholar]
- 24. Fayers PM, Aaronson NK, Bjordal K, Groenvold M, Curran D, Duez NJ et al. EORTC QLQ-C30 Scoring Manual (3rd edition). Brussels: EORTC; 2001. ed2001. [Google Scholar]
- 25. Fleming TR. One-sample multiple testing procedure for phase II clinical trials. Biometrics. 1982; 38: 143–151. [PubMed] [Google Scholar]
- 26. Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000; 92: 205–216. [DOI] [PubMed] [Google Scholar]
- 27. Osoba D, Rodrigues G, Myles J, Zee B, Pater J. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol. 1998; 16: 139–144. [DOI] [PubMed] [Google Scholar]
- 28.NCI Common Terminology Criteria for Adverse Events v3.0. 2006.
- 29. Little RJ, Rubin DB. Causal effects in clinical and epidemiological studies via potential outcomes: concepts and analytical approaches. Annual review of public health. 2000; 21: 121–145. [DOI] [PubMed] [Google Scholar]
- 30. Team RDC. R: A Language and Environment for Statistical Computing R Foundation for Statistical Computing, Vienna, Austria: ISBN 3-900051-07-0. 2010. URL http://www.R-project.org/. [Google Scholar]
- 31. Moinpour CM, Vaught NL, Goldman B, Redman MW, Philip PA, Millwood B, et al. Pain and emotional well-being outcomes in Southwest Oncology Group-directed intergroup trial S0205: a phase III study comparing gemcitabine plus cetuximab versus gemcitabine as first-line therapy in patients with advanced pancreas cancer. J Clin Oncol. 2010; 28: 3611–3616. 10.1200/JCO.2009.25.8285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bernhard J, Dietrich D, Scheithauer W, Gerber D, Bodoky G, Ruhstaller T, et al. Clinical benefit and quality of life in patients with advanced pancreatic cancer receiving gemcitabine plus capecitabine versus gemcitabine alone: a randomized multicenter phase III clinical trial—SAKK 44/00-CECOG/PAN.1.3.001. J Clin Oncol. 2008; 26: 3695–3701. 10.1200/JCO.2007.15.6240 [DOI] [PubMed] [Google Scholar]
- 33. Cortes J, Baselga J, Im YH, Im SA, Pivot X, Ross G, et al. Health-related quality-of-life assessment in CLEOPATRA, a phase III study combining pertuzumab with trastuzumab and docetaxel in metastatic breast cancer. Ann Oncol. 2013; 10: 2630–2635. 10.1093/annonc/mdt274 [DOI] [PubMed] [Google Scholar]
- 34. Yang JC, Hirsh V, Schuler M, Yamamoto N, O'Byrne KJ, Mok TS, et al. Symptom Control and Quality of Life in LUX-Lung 3: A Phase III Study of Afatinib or Cisplatin/Pemetrexed in Patients With Advanced Lung Adenocarcinoma With EGFR Mutations. J Clin Oncol. 2013; 27: 3342–3350. 10.1200/JCO.2012.46.1764 [DOI] [PubMed] [Google Scholar]
- 35. Burris HA 3rd, Lebrun F, Rugo HS, Beck JT, Piccart M, Neven P, et al. Health-related quality of life of patients with advanced breast cancer treated with everolimus plus exemestane versus placebo plus exemestane in the phase 3, randomized, controlled, BOLERO-2 trial. Cancer. 2013; 119: 1908–1915. 10.1002/cncr.28010 [DOI] [PubMed] [Google Scholar]
- 36. Fairclough DL. Design and analysis of quality of life studies in clinical trials: CRC press; 2010. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOCX)
aArm 1: gemcitabine alone, Arm 2: gemcitabine + FOLFIRI.3.
(EPS)
(DOCX)
(DOC)
(DOC)
(DOC)
(DOC)
(DOC)
(DOC)
Data Availability Statement
Data are unsuitable for public deposition due to ethical and legal restrictions and are therefore available upon request with the signature of a data privacy form. To request the data, the readers may contact Prof. Julien Taieb (julien.taieb@egp.aphp.fr) and Prof. Franck Bonnetain (franck.bonnetain@univfcomte.fr).