Abstract
Persistent smoking following stroke is associated with poor outcomes including development of secondary stroke and increased mortality risk. This study uses longitudinal data from the U.S. Health and Retirement Study (1992–2008) to investigate whether depression and duration of inpatient hospital care impact smoking outcomes among stroke survivors (N = 745). Longer duration of care was associated with lower likelihood of persistent smoking. Depression was associated with greater cigarette consumption. Interaction effects were also significant, indicating that for survivors who experienced longer inpatient care there was a weaker association between depression and cigarette consumption. Implications for practice and research are discussed.
Keywords: stroke, smoking, depression, inpatient care
INTRODUCTION
Each year, over 600,000 people in the United States experience a first stroke (Roger et al., 2011). For some individuals, critical health events like stroke are experienced as a “wake up call” that leads to a reappraisal of health values and beliefs and an examination of current health practices. Changes in health behaviors, including cigarette smoking, are critical following stroke in order to mitigate the consequences of the illness and reduce the risk of reoccurrence. Studies have shown that a substantial portion of survivors report quitting smoking following stroke with, and to a lesser extent, without formal intervention (Falba, 2005; Ives, Heuschmann, Wolfe, & Redfern, 2008; Twardella, et al, 2006; Ovbiagele, et. al, 2004). However, it is estimated that over three-quarters of individuals who smoke prior to stroke continue to smoke up to two years after stroke with little improvement in smoking habits in subsequent years (Newsom et al., 2011). The Centers for Disease Control and Prevention estimate that smoking costs the U.S. economy $75.5 billion in health-care-related expenditures every year (Rock et al., 2007). For stroke survivors, persistent smoking has been associated with a variety of poor outcomes including increased risk of secondary stroke and other cardiovascular illnesses, as well as increased mortality risk (Kammersgaard, 2006; Lightwood & Glantz, 1997). Given the serious consequences when survivors continue to smoke, it is important to identify the factors associated with persistent smoking and to identify those most in need of smoking cessation assistance.
Although it is well established that sustained abstinence from smoking results in substantial economic and health benefits (Bjartveit, 2009; Hurley & Matthews, 2007), a recent review concluded that reducing cigarette consumption also can lead to improvements in cardiovascular biomarkers (e.g., hemoglobin concentration, pulse rate) and respiratory functioning as well as decreased risk for hospitalization from myocardial infarction (Pisinger and Godtfredsen, 2007). As the transtheoretical model would suggest (Prochaska & DiClemente, 1984), a reduction in cigarette consumption following stroke may also be an initial step toward quitting. Thus, in addition to examining factors that predict persistent smoking after stroke, it is important to investigate factors that contribute to the number of cigarettes survivors consume so that individuals with the willingness and capacity to reduce their smoking can be targeted for cessation programs.
Correlates of Smoking Status after Chronic Illness
Past studies have shown that an array of sociodemographic and physical factors affect the smoking habits of individuals experiencing chronic illness. Among stroke survivors, for example, characteristics like younger age, male sex, Caucasian ethnicity, lower socioeconomic status, and better physical functioning have been found to be associated with persistent smoking (Bak et. al, 2002; Ives, et al., 2008; Redfern, McKevitt, Dundas, Rudd, & Wolfe, 2000; Sienkiewicz-Jarosz, Zatorski, Baranowska, Ryglewicz, & Bienkowski., 2009). However, other psychological and social factors are also relevant.
Depressive symptoms have been associated with less immediate cessation, as well as greater risk for smoking relapse, among individuals diagnosed with chronic illness. Among patients with heart disease, Gravely-Witte, Stewart, Suskin, and Grace (2009) found a significant relationship between greater depressive symptoms and persistent smoking after diagnosis. Studies have documented that, even though individuals may initially quit smoking following the immediate health crisis, patients with greater depressive symptoms are more likely to relapse to smoking several months after hospital discharge (Dawood, et. al, 2008; Perez, Nicolau, Romano, & Laranjeira, 2008).
Few studies have investigated the role of depressive symptoms in smoking outcomes among survivors of neurological health crises like stroke, with their attendant physical, psychological, and social sequela. In a prospective study of first-ever ischemic stroke survivors, Sienkiewicz-Jarosz et al. (2009) found that greater depression was associated with continued smoking three months after diagnosis. In a focus group study, stroke survivors and family members consistently reported how the effects of stroke, including depression, impaired survivors’ ability to make positive changes in health behaviors that could mitigate the risk for additional health problems (Lawrence, Kerr, Watson, & Ellis, 2010).
Although there is broad agreement about the potential for critical health events like stroke to serve as “teachable moments” and catalysts for behavior change, very little empirical work has been conducted to examine the relationship between exposure to potentially teachable moments through routine interactions with healthcare providers during inpatient hospital care and smoking outcomes. Physicians are encouraged to ask patients with smoking-related illnesses about their tobacco use and to offer a range of interventions including brief advice to quit smoking, pharmacotherapy, or behavioral counseling (Hall & Lorence, 2010). In a recent systematic review that focused on the effectiveness of physician advice in smoking cessation among general practice patients, Stead, Bergson, and Lancaster (2008) reported that even brief advice significantly increased patients’ rate of quitting.
Unfortunately, many individuals may not be receiving adequate encouragement to quit smoking or reduce cigarette consumption, or may not be receiving such information when they are cognitively and emotionally prepared to digest it. Among patients with heart disease, pulmonary disease, and stroke, Buckland and Connolly (2005) reported that only 17% of patient documentation reflected the provision of smoking cessation advice. Gall, Dewey, and Thrift (2009) found that only 40% of stroke survivors recalled receiving advice to quit smoking and only 8% recalled being told to reduce the amount that they were smoking. In a study about the effects of information provision on stroke survivors’ health beliefs, one survivor remarked “… when you’ve first had your stroke, you’re in no condition to absorb anything” (Lawrence, Kerr, Watson, & Ellis, 2010; p. 4).
Lack of appropriate encouragement to quit smoking may be an even greater issue for older patients. Maguire, et al. (2000) found that, irrespective of an individual’s physical or mental health, hospital-based physicians were significantly less likely to advise patients over the age of 65 years old to quit smoking. This is problematic considering that about three-quarters of all strokes occur in people over the age of 65 (Centers for Disease Control, 2011).
These findings are compelling and point to a need for increased awareness among physicians about the importance of advising hospitalized patients to quit or reduce smoking. However, conclusions should be drawn with caution given that existing studies are based on relatively small convenience samples (e.g., Buckland & Connolly, 2005; N = 100; Gall, Dewey, & Thrift, 2009; N = 40; Maguire, et al., 2000, N = 142; Lawrence, Kerr, Watson, & Ellis, 2010, N = 49). More importantly, existing research focused primarily on the role of physicians in smoking cessation after chronic illness does not reflect the multitude of allied healthcare providers, each with opportunities to capitalize on teachable moments, that stroke survivors interact with during inpatient hospitalization. A growing shortage of hospital-based physicians in the U.S. (Association of American Medical Colleges, 2010) has resulted in allied health professionals increasingly being charged with various aspects of patient care. These professionals, including social workers, may be in a key position to encourage patients to adopt healthy behaviors such as quitting smoking. Efforts like the present study are needed to better understand the extent to which routine exposure to allied health professionals that takes place during inpatient hospitalization after stroke may be related to smoking outcomes and whether this routine exposure could moderate the effects of other factors like depression.
In addition to helping to clarify how depression and duration of inpatient hospitalization may impact smoking outcomes, the present study goes beyond prior work by using a large dataset and a prospective design that allows us to measure smoking habits and other factors prior to and after the occurrence of the stroke. We hypothesize that:
greater depressive symptoms will be predictive of continued smoking and higher cigarette consumption among stroke survivors; and
longer duration of hospital care will be associated with lower likelihood of continued smoking and lower cigarette consumption.
We also propose that duration of hospital care following stroke will moderate the effects of depression on smoking outcomes. Inpatient hospitalization and the associated contact with medical and allied health professionals may serve as a stabilizing force for survivors coping with concurrent mental health challenges and the behavioral and lifestyle changes associated with quitting smoking. Inpatient care may give survivors the time they need to deal simultaneously with depression and nicotine withdrawal symptoms in order to maintain stability, focus, and motivation to quit smoking.
METHODS
Sample
This paper uses data from the U.S. Health and Retirement Study (HRS; 2011), an ongoing biannual longitudinal study of 11,191 U.S. residents aged 50 or older that began in 1992. The study sample consisted of 745 non-institutionalized survivors of first stroke who began the HRS study without a history of stroke. Further details of the HRS design, sampling procedures, data collection, and response rates at each wave are available in Heeringa and Connor (1995).
Research design
The present study includes HRS interviews conducted between 1992 and 2008. These prospective data allowed us to measure smoking habits and other factors prior to and after the occurrence of the stroke, thereby assessing changes between pre-diagnosis and post-diagnosis. As indicated for specific measures below, analyses were based on all available waves for which HRS items were worded consistently. We defined pre- and post-stroke time points individually for each case. The pre-stroke time point was defined as the last wave of interview before respondents reported a diagnosis of stroke. The post-stroke time point was defined as the same wave at which respondents reported a diagnosis of stroke and so occurred between 0 and 2 years after the pre-stroke time-point.
Measures
Stroke status and smoking outcomes
Stroke status was assessed at each wave with the question “Since [wave #], has a doctor ever told you that you had a stroke?” The dependent variables in this study were current smoking status and average number of cigarettes smoked per day. Smoking status was assessed with the question, “Do you smoke cigarettes now?” (yes/no) and number of cigarettes was assessed with the question, “About how many cigarettes or packs do you usually smoke in a day now?” Those who quit smoking after stroke were assigned a value of zero for their post cigarette consumption.
Sociodemographic and health-related variables
Sociodemographic variables included participant gender (male, female), race (Caucasian, other), educational attainment (less than high school diploma, high school diploma or greater), and marital status (married or partnered, other) reported at the baseline wave of the HRS, and age, medications taken for stroke, and self-reported number of comorbid health conditions (cancer, diabetes, heart disease, lung disease, high blood pressure or hypertension, arthritis or rheumatism) reported at the post-stroke time point. Pre-stroke smoking status and number of cigarettes consumed were based on participants’ responses to the questions described above at the pre-stroke time point.
Functional limitations were measured at the post-stroke time point by participant responses to six items (no = 0, yes = 1) about activities done during a typical day (e.g., walking across a room; getting in or out of bed, bathing or showering). Individual scores were based on a summary of “yes” responses. Consistently worded items were available for HRS waves three through nine.
Predictor variables
Depressive symptomatology was measured at the post-stroke time point by the 8-item Centers for Epidemiological Studies – Depression (CES-D) scale (Steffick, 2000). This shortened version of the original 20-item scale (Radloff, 1977) contains eight yes/no items about symptoms of depression (e.g., “enjoyed life”, “felt sad”, “couldn’t get going”) and has been used in studies with a variety of chronic illness populations including stroke (e.g., Glymour, et al., 2010).
Duration of hospital care was measured at the post-stroke time point with one item: “Altogether, how many nights were you a patient in the hospital in the last two years?” Depression and duration of care items were available for HRS waves two through nine.
Analysis
By including measures of smoking prior to stroke, this longitudinal model allowed us to assess the change in smoking patterns between pre-diagnosis and post-diagnosis. Logistic and ordinary least squares (OLS) regression analyses were used to test hypotheses one and two, respectively. A logistic regression model was estimated to predict the probability of persistent smoking after stroke while controlling for smoking status prior to stroke. This model included sex, race, age at diagnosis, education, marital status, smoking status before stroke (yes/no), medications for stroke, number of health conditions, functional limitations, depressive symptoms, number of hospital overnights, and the interaction between depressive symptoms and hospital overnights. Based on available data from waves of consistently worded items, 611 participants who either smoked or did not smoke prior to stroke were included in the logistic analyses.
Using the same predictor variables, an OLS regression model was then estimated to predict the average number of cigarettes smoked per day among those who continued to smoke after stroke, controlling for the number of cigarettes smoked prior to the stroke. The OLS model was based on 163 participants who had values for number of cigarettes smoked prior to and after stroke. Simple slopes tests were conducted to examine the specific nature of the interaction effects (Aiken & West, 1991). All statistical tests were adjusted for the HRS’s complex sampling design using SAS PROCSURVEY version 9.2 (SAS Institute Inc., 2009). Complete data were required on smoking variables and report of stroke, but multiple imputation was used to maximize sample size where data were missing on covariates (Little & Rubin, 2002).
RESULTS
Participant characteristics
Table 1 presents characteristics of the study participants. At HRS baseline (i.e., 1992), 55.8% of the sample was male, 75.0% was Caucasian, 67.5% had at least a high school diploma, and 74.1% reported being married or partnered. At the post-stroke wave, 61.4% reported taking medications for stroke and the average age of participants was 67 years old, ranging from 53 to 86. Participants reported experiencing an average of over 2 additional comorbidities (mean = 2.64, SD = 1.29, range 0 to 6), had an average functional limitation score of 1.27 (SD = 1.88, range 0 to 6), and had an average depression severity score of 2.36 (SD = 2.40, range 0 to 8). Participants reported having spent an average of approximately 16 nights in the hospital in the previous two years (mean 15.95, SD = 3.88, range 0 to 550). About 19.2% of the total sample reported smoking after stroke and, of these individuals, the average number of cigarettes consumed per day was approximately 12.81 (SD = 14.81, range 0 to 100).
Table 1.
Characteristics of Study Sample (Percentages, Means, and Standard Deviations)
| N | % | Mean (SD) | |
|---|---|---|---|
| Sex (% male) | 745 | 55.8 | - |
| Race (% Caucasian) | 745 | 75.0 | - |
| Education (% HS or greater) | 745 | 67.5 | - |
| Marital status (% married or partnered) | 745 | 74.1 | - |
| Medications taken for stroke (% yes) | 745 | 61.4 | - |
| Age at diagnosis (range 53 – 86) | 745 | - | 66.86 (6.24) |
| Health conditions (range 0 – 6) | 698 | - | 2.64 (1.29) |
| Functional limitations (range 0 – 6) | 643 | - | 1.27 (1.88) |
| Depressive symptomatology (range 0 – 8) | 613 | - | 2.36 (2.40) |
| Hospital overnights in past two years (range 0 – 550) | 725 | - | 15.95 (3.88) |
| Smoking status (% yes) | 745 | 19.2 | - |
| Number of daily cigarettes (range 0 – 100) | 188 | - | 12.81 (14.81) |
Note: Sex, race, education, and marital status measured at HRS baseline (i.e., 1992). All others measured at post- stroke time point.
Post-stroke smoking status
Table 2 presents results from the logistic and OLS models predicting persistent smoking and the average number of daily cigarettes post-stroke. The logistic regression analyses included 611 participants. In the model, pre-stroke smoking status (OR= 73.60, 95%CI 33.77–160.38), medications taken for stroke (OR= 0.35, 95%CI 0.17–0.70), and number of hospital overnights (OR= 0.99, 95%CI 0.98–0.99) predicted persistent smoking following stroke, with survivors who experienced more hospital overnights being less likely to continue smoking. Depressive symptoms and the interaction of depression and hospital care were not significant predictors (OR= 1.11, 95%CI 0.93–1.33; OR= 1.00, 95%CI 0.99–1.00, respectively).
Table 2.
Logistic and OLS Regressions for Continued Smoking and Average Daily Cigarettes after Stroke
| Logistic Model (N = 611) | OLS Model (N = 163) | |||
|---|---|---|---|---|
|
| ||||
| Variable | OR | 95% CI | β | p-value |
| sex | 1.30 | 0.66–2.57 | 1.93 | 0.228 |
| race | 0.82 | 0.37–1.82 | −4.31 | 0.170 |
| age at diagnosis | 0.97 | 0.91–1.03 | −0.02 | 0.885 |
| education | 1.49 | 0.72–3.04 | 5.37 | <0.001 |
| marital status | 0.63 | 0.30–1.31 | 0.39 | 0.838 |
| pre-stroke smoking | 73.60 | 33.77–160.38 | 0.36 | <0.001 |
| medications for stroke | 0.35 | 0.17–0.70 | −8.22 | <0.001 |
| health conditions | 1.23 | 0.95–1.59 | 0.23 | 0.594 |
| functional limitations | 0.88 | 0.70–1.12 | −0.11 | 0.879 |
| depressive symptoms | 1.11 | 0.93–1.33 | 1.15 | 0.005 |
| hospital overnights | 0.99 | 0.98–0.99 | −0.04 | 0.198 |
| depression X hospital | 1.00 | 0.99–1.00 | −0.03 | 0.003 |
| Model R-square | 22.4 | |||
Post-stroke average cigarettes
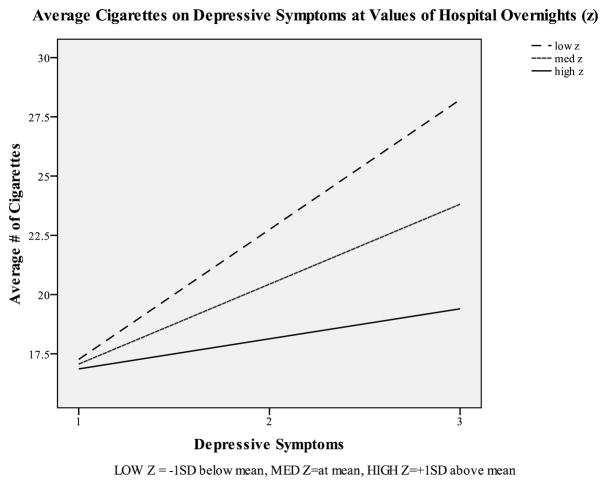
The OLS analysis included 163 participants. After controlling for sociodemographic factors, number of cigarettes consumed prior to stroke, medications received for stroke, other health conditions, and functional limitations, depressive symptoms remained a significant predictor of post-stroke cigarette consumption (β = 1.15, p = .005), with greater symptomatology associated with higher average cigarettes. Although the main effect for hospital overnights was not significant (β = −0.04, p = .198), the interaction term for depression and hospital overnights was significant (β = −0.03, p = .003), such that participants with more hospital overnights (i.e., at, or one standard deviation above, the mean) exhibited a weaker relationship between depressive symptoms and post-stroke cigarette consumption than participants with fewer hospital overnights (i.e., one standard deviation below the mean). Simple slopes tests illustrating these results are presented in Figure 1, although these tests are not based on imputed data nor adjusted for complex sampling design.
Figure 1.
Simple Slopes Test of Depressive Symptoms Moderated by Number of Hospital Overnights
DISCUSSION AND IMPLICATIONS
Although it is widely recognized that complete abstinence from cigarette smoking is valuable for mitigating the consequences of stroke, preventing future adverse health events, and achieving good overall health, a large percentage of stroke survivors continue to smoke following diagnosis (Newsom, et al., 2011; Newsom et al., in press). This study investigated predictors of persistent smoking, as well as average cigarettes consumed by survivors who continued to smoke following stroke.
Several findings are consistent with past studies but others add substantially to our understanding or present new questions for future investigations. First, although participants’ level of education was not examined as a primary variable in this study, after controlling for a range of factors, greater education was significantly associated with greater average cigarette consumption after stroke in the OLS model. This finding contrasts somewhat with past research that indicates that lower socioeconomic status (SES), including education and employment status, is associated with persistent smoking (Bak et al., 2000). Our findings raise the question of whether more highly educated people who do not successfully quit smoking after stroke may become more entrenched in the habit. Future research should delve into this issue to further clarify how education and other SES indicators impact dichotomous (i.e., current status) as well as continuous (i.e., number of cigarettes consumed) smoking outcomes.
Second, this study adds to our understanding about how depression may contribute to smoking outcomes after a physically, psychologically, and socially complex condition like stroke and does so by using a large, nationally representative dataset and a prospective design. Contrary to our hypothesis, depressive symptoms were not related to persistent smoking among stroke survivors in the logistic model. Survivors with higher depressive symptoms were no more or no less likely to quit smoking following stroke. However, depressive symptoms were strongly associated with greater cigarette consumption among survivors who continued to smoke. Depression among stroke survivors has been associated with numerous adverse outcomes including increased reoccurrence of stroke, and a three times greater risk of early mortality (OR=3.4, 95%CI 1.4–8.4; Morris, Robinson, Andrzejewski, Samuels, & Price, 1993; Pan et al., 2011). Given the connection between smoking and adverse health outcomes, our findings about the link between depression and greater cigarette consumption suggest that mediational effects may be at play. This study should raise awareness among social workers and other allied health professionals about the importance of addressing patient mental health in order to facilitate changes in critical health behaviors like smoking and, consequently, prevent more severe distal outcomes like early mortality.
Third, this study found much weaker main effects than anticipated from duration of inpatient hospital care on smoking outcomes. Although findings were statistically significant, survivors with a greater number of hospital overnights were only slightly less likely to continue smoking. For individuals who persisted in smoking following stroke, the number of cigarettes they consumed was not significantly affected by the number of nights they spent in the hospital. This suggests that from a transtheoretical perspective (Prochaska & DiClemente, 1984) little if any movement towards quitting resulted from longer inpatient care.
Although a number of formal smoking cessation interventions have been reported to be effective for hospitalized patients (Rigotti, Munafo, & Stead, 2007), routine practitioner-patient interactions may not, by themselves, be enough to facilitate quitting. In other words, as suggested by the literature (e.g., Buckland & Connolly, 2005; Gall, Dewey, & Thrift, 2009; Lawrence, Kerr, Watson, & Ellis, 2010; Maguire, et al., 2000), medical and allied health care providers may not be capitalizing on “teachable moments” to the full extent that they could.
In a systematic review of interventions for smoking cessation in hospitalized patients, Rigotti, Munafo, and Stead (2007) conclude that the most effective interventions delivered during hospitalization include physician advice combined with follow-up for at least one month after discharge. Given hospital social workers’ frequent involvement in discharge planning and follow-up (Judd & Sheffield, 2010), they may be in an ideal position to intervene and assist patients to quit or reduce smoking by reinforcing interventions delivered during inpatient care. Other brief interventions that do not include follow up also may be promising for improving smoking outcomes. For example, Hajek, Taylor, & Mills (2002) employed a novel intervention in which patients were given a carbon monoxide reading to illustrate the health benefits of quitting, written materials that challenged the belief in the stress-reducing properties of cigarettes and a subsequent quiz that was discussed with the provider, peer support through the facilitation of a relationship with a quitting “buddy”, a sticker on their chart to remind staff to reinforce the need to quit smoking at every opportunity, and a signed declaration of intent to quit smoking to serve as a reminder of their commitment. Although the full intervention was equally as effective as only receiving verbal advice and written materials (i.e., no significant difference between intervention and control groups on smoking outcomes), certain components of the intervention stood out and may be worth incorporating in practice. For example, participants who declared their intent to quit in writing were almost twice as likely to have remained abstinent from cigarettes 12 months after discharge (OR = 1.6, p = .002). This strategy may be especially effective for patients experiencing immediate cognitive effects from conditions like stroke.
Research suggests that including family members and caregivers in interventions to assist survivors to quit smoking may improve survivor outcomes (Lawrence, Kerr, Watson, & Ellis, 2010; Rizzo, 2006) and social workers are in a key position to facilitate family member involvement. Moreover, family members and caregivers may be dealing with their own trauma driven by the acute illness and may, themselves, be in need of a range of emotional, psychosocial, instrumental, spiritual, and health information support (Kim & Moon, 2007; MacIsaac, Harrison, & Godfrey, 2010). By practicing from a person-in-environment perspective, social workers can work to improve survivor outcomes directly and, at the same time, facilitate the well-being of families and caregivers.
Finally, we found an interesting, and potentially clinically informative interaction between survivor depression and duration of inpatient hospital care, suggesting that the relationship between depression and smoking outcomes may be more complex than previously thought. For survivors with a longer duration of care, greater depression was less associated with higher cigarette consumption. It may be that hospitalization for a longer period of time after stroke provides medical and allied health professionals with more opportunities to identify and treat depression with antidepressants with known smoking cessation benefits (Buchhalter, Fant, & Henningfield, 2008). Practitioners should remain aware of the high incidence of depression among stroke survivors (i.e., 33% as reported by meta-analyses; Hacket, Yapa, Parag, & Anderson, 2005) and look for opportunities to simultaneously address mental health issues and health behaviors, through pharmacological and non-pharmacological interventions. It may also be that longer inpatient care gives survivors the time to develop coping strategies for depressive symptoms other than cigarette smoking. Future research should focus on the specific mechanisms by which duration of inpatient care impacts the association between depression and smoking.
Study strengths and limitations
This study had a number of strengths that bear mentioning. The sample was population-based and representative, as opposed to only consisting of patients enrolled in stroke registries or receiving specific medical services. The study also used a prospective design that allowed us to control for real-time reports of smoking status and number of cigarettes consumed prior to stroke, as opposed to retrospective report.
Readers should exercise caution in interpretation of study findings, though, due to study limitations. First, measures of stroke and smoking were based on self report. Fortunately, studies have shown that self-report of chronic conditions is highly accurate (e.g., Giles, Croft, Keenan, Lane, & Wheeler, 1994; Vargas, Burt, Gillum, & Pamuk, 1995) and that self-report of smoking is generally unbiased (Patrick et al., 1994). Second, the HRS does not provide information about whether study participants took part in formal smoking cessation programs during inpatient hospitalization. Participation in such programs would likely attenuate the effects of hospital care and, therefore, it would be valuable to control for this variable. Similarly, the HRS does not provide information about the exact time of stroke diagnosis, a potentially important covariate in examinations of the relationship between depression, duration of inpatient care, and smoking outcomes.
Despite these limitations, this study provides valuable information about how sociodemographic, physical, psychological, and social factors individually and collectively influence stroke survivors’ smoking patterns. It highlights how depressive symptoms can contribute to higher cigarette consumption, how routine contact with medical and allied healthcare professionals during inpatient hospitalization may not, by itself, be adequate to facilitate smoking cessation, and how brief but thoughtful interventions may help survivors quit smoking and avoid longer term health problems. These findings shed light on the complex relationship between psychological and social factors influencing health behavior change and may aid in the prevention and treatment of secondary complications associated with stroke and other chronic illnesses.
Acknowledgments
The HRS is sponsored by the National Institute on Aging (U01 AG009740) and is conducted by the University of Michigan. This research was supported by funding from the National Institute on Aging (Grant # [omitted for review]). We would also like to thank our collaborators at [omitted for review], for their input throughout the preparation of this report.
Contributor Information
Michael J. McCarthy, School of Social Work, University of Cincinnati, Cincinnati, Ohio, USA.
Nathalie Huguet, School of Community Health, Portland State University, Portland, Oregon, USA.
Jason T. Newsom, School of Community Health, Portland State University, Portland, Oregon, USA.
Mark S. Kaplan, School of Community Health, Portland State University, Portland, Oregon, USA.
Bentson H. McFarland, Oregon Health and Science University, Portland, Oregon, USA.
References
- Aiken LS, West SG. Multiple Regression: Testing and interpreting interactions. Newbury Park, CA: Sage; 1991. [Google Scholar]
- Association of American Medical Colleges. [Accessed April 4, 2012];AAMC releases new physician shortage estimates post-reform. 2010 from https://www.aamc.org/newsroom/newsreleases/2010/150570/100930.html.
- Bak S, Sindrup SH, Alslev T, Kristensen O, Christensen K, Gaist D. Cessation of smoking after first-ever stroke: A follow-up study. Stroke. 2002;33:2263–2269. doi: 10.1161/01.str.0000027210.50936.d0. [DOI] [PubMed] [Google Scholar]
- Bjartveit K. Health consequences of sustained smoking cessation. Tobacco Control. 2009;18(3):197–205. doi: 10.1136/tc.2008.026898. [DOI] [PubMed] [Google Scholar]
- Buchhalter AR, Fant RV, Henningfield JE. Novel pharmacological approaches for treating tobacco dependence and withdrawal: Current status. Drugs. 2008;68(8):1067–1088. doi: 10.2165/00003495-200868080-00005. [DOI] [PubMed] [Google Scholar]
- Buckland A, Connolly MJ. Age-related differences in smoking cessation advice and support given to patients hospitalised with smoking-related illness. Age and Ageing. 2005;34(6):639–642. doi: 10.1093/ageing/afi199. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control. [Accessed April 5, 2012];Stroke Facts. 2011 from http://www.cdc.gov/stroke/facts.htm.
- Covinsky KE, Yaffe K, Lindquist K, Cherkasova E, Yelin E, Blazer DG. Depressive Symptoms in Middle Age and the Development of Later-Life Functional Limitations: The Long-Term Effect of Depressive Symptoms. JAGS. 2010;58:551–556. doi: 10.1111/j.1532-5415.2010.02723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawood N, Vaccarino V, Reid KJ, Spertus JA, Hamid N, Parashar S. Predictors of smoking cessation after a myocardial infarction: the role of institutional smoking cessation programs in improving success. Archives of Internal Medicine. 2008;168(18):1961–1967. doi: 10.1001/archinte.168.18.1961. [DOI] [PubMed] [Google Scholar]
- Falba T. Health events and the smoking cessation of middle aged Americans. Journal of Behavioral Medicine. 2005;28(1):21–33. doi: 10.1007/s10865-005-2560-1. [DOI] [PubMed] [Google Scholar]
- Hajek P, Taylor TZ, Mills P. Brief intervention during hospital admission to help patients to give up smoking after myocardial infarction and bypass surgery: randomised controlled trial. BMJ. 2002;324(12):87–89. doi: 10.1136/bmj.324.7329.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacket ML, Yapa C, Parag V, Anderson CS. Frequency of depression after stroke: A systematic review of observational studies. Stroke. 2005;36:1330–1340. doi: 10.1161/01.STR.0000165928.19135.35. [DOI] [PubMed] [Google Scholar]
- Hall SL, Lorence T. Secondary prevention of coronary artery disease. American Family Physician. 2010;81(3):289–296. [PubMed] [Google Scholar]
- Hayes RB, Dunsiger S, Borrelli B. The influence of quality of life and depressed mood on smoking cessation among medically ill smokers. Journal of Behavioral Medicine. 2010;33:209–218. doi: 10.1007/s10865-010-9254-z. [DOI] [PubMed] [Google Scholar]
- Health and Retirement Study. Public use dataset. Ann Arbor, MI: 2011. Produced and distributed by the University of Michigan with funding from the National Institute on Aging (grant number NIA U01AG009740) [Google Scholar]
- Hurley SF, Matthews JP. The Quit Benefits Model: a Markov model for assessing the health benefits and health care cost savings of quitting smoking. Cost Effectiveness and Resource Allocation. 2007;5(2):1–20. doi: 10.1186/1478-7547-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gall SL, Dewey HM, Thrift AG. Smoking cessation at 5 years after stroke in the North East Melbourne Stroke Incidence Study. Neuroepidemiology. 2009;32(3):196–200. doi: 10.1159/000195689. [DOI] [PubMed] [Google Scholar]
- Giles WH, Croft JB, Keenan NL, Lane MJ, Wheeler FC. The validity of self-reported hypertension and correlates of hypertension awareness among Blacks and Whites within the stroke belt. American Journal of Preventive Medicine. 1995;11:163–169. [PubMed] [Google Scholar]
- Glymour MM, Maselko J, Gilman SE, Patton KK, Avendaño M. Depressive symptoms predict incident stroke independently of memory impairments. Neurology. 2010;75(23):2063–2070. doi: 10.1212/WNL.0b013e318200d70e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravely-Witte S, Stewart DE, Suskin N, Grace SL. The association among depressive symptoms, smoking status and antidepressant use in cardiac outpatients. Journal of Behavioral Medicine. 2009;32:478–490. doi: 10.1007/s10865-009-9218-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ives SP, Heuschmann PU, Wolfe CDA, Redfern J. Patterns of smoking cessation in the first 3 years after stroke: the South London Stroke Register. European Journal of Cardiovascular Prevention & Rehabilitation. 2008;15(3):329–335. doi: 10.1097/HJR.0b013e3282f37a58. [DOI] [PubMed] [Google Scholar]
- Judd RG, Sheffield S. Hospital social work: contemporary roles and professional activities. Social Work in Health Care. 2010;49:856–871. doi: 10.1080/00981389.2010.499825. [DOI] [PubMed] [Google Scholar]
- Kammersgaard LP. Cardiovascular risk factors and 5-year mortality in the Copenhagen Stroke Study. Cerebrovascular Diseases. 2006;21(3):187–193. doi: 10.1159/000090531. [DOI] [PubMed] [Google Scholar]
- Kapson HS, Haaga DAF. Depression vulnerability moderates the effects of cognitive behavior therapy in a randomized controlled trial for smoking cessation. Behavior Therapy. 2010;41(4):447–460. doi: 10.1016/j.beth.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JW, Moon SS. Needs of family caregivers caring for stroke patients: based on the rehabilitation treatment phase and the treatment setting. Social Work in Health Care. 2007;45(1):81–97. doi: 10.1300/J010v45n01_06. [DOI] [PubMed] [Google Scholar]
- Lawrence M, Kerr S, Watson H, Paton G, Ellis G. An exploration of lifestyle beliefs and lifestyle behaviour following stroke: findings from a focus group study of patients and family members. BMC Family Practice. 2010;1197:1–11. doi: 10.1186/1471-2296-11-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lightwood JM, Glantz SA. Short-term economic and health benefits of smoking cessation: Myocardial infarction and stroke. Circulation. 1997;96:1089–1096. doi: 10.1161/01.cir.96.4.1089. [DOI] [PubMed] [Google Scholar]
- Little RJA, Rubin DB. Statistical Analysis with Missing Data. 2. New York: Wiley; 2002. [Google Scholar]
- MacIsaac L, Harrison MB, Godfrey C. Supportive care needs of caregivers of individuals following stroke: a synopsis of research. Canadian Journal of Neuroscience Nursing. 2010;32(1):39–46. [PubMed] [Google Scholar]
- Maguire CP, Ryan J, Kelly A, O’Neil D, Coakley D, Walsh JB. Do patient age and medical condition influence medical advice to stop smoking? Age and Ageing. 2000;29(3):264–266. doi: 10.1093/ageing/29.3.264. [DOI] [PubMed] [Google Scholar]
- Morris LP, Robinson RG, Andrzejewski P, Samuels J, Price TR. Association of depression with 10-year poststroke mortality. American Journal of Psychiatry. 1993;150(1):124–129. doi: 10.1176/ajp.150.1.124. [DOI] [PubMed] [Google Scholar]
- Newsom JT, Huguet N, McCarthy MJ, Ramage-Morin P, Kaplan MS, Bernier J, McFarland BH, Oderkirk J. Health behavior change following chronic illness in middle and later life. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences. 2011 doi: 10.1093/geronb/gbr103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsom JT, Huguet N, Ramage-Morin P, McCarthy MJ, Bernier J, Kaplan MS, McFarland BH. Health behaviour changes after diagnosis of chronic illness among Canadians aged 50 or older. Health Reports. in press. [PMC free article] [PubMed] [Google Scholar]
- Ovbiagele B, Saver JL, Fredieu A, Suzuki S, Selco S, Rajajee V, McNair N, Razinia T, Kidwell CS. In-hospital initiation of secondary stroke prevention therapies yields high rates of adherence at follow-up. Stroke. 2004;35:2879–2883. doi: 10.1161/01.STR.0000147967.49567.d6. [DOI] [PubMed] [Google Scholar]
- Ovbiagele B, Saver JL, Fredieu A, Suzuki S, Selco S, Rajajee V, McNair N, Razinia T, Kidwell CS. In-hospital initiation of secondary stroke prevention therapies yields high rates of adherence at follow-up. Stroke. 2004;35:2879–2883. doi: 10.1161/01.STR.0000147967.49567.d6. [DOI] [PubMed] [Google Scholar]
- Pan A, Okereke OI, Sun Q, Logroscino G, Manson JE, Willett WC, Ascherio A, Hu FB, Rexrode KM. Depression and incident stroke in women. Stroke. 2011;42(10):2770–2775. doi: 10.1161/STROKEAHA.111.617043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick DL, Cheadle A, Thompson DC, Diehr P, Koepsell T, Kinne S. The validity of self-reported smoking: A review and meta-analysis. American Journal of Public Health. 1994;84(7):1086–1093. doi: 10.2105/ajph.84.7.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez GH, Nicolau JC, Romano BW, Laranjeira R. Depression: a predictor of smoking relapse in a 6-month follow-up after hospitalization for acute coronary syndrome. European Journal of Cardiovascular Prevention and Rehabilitation. 2008;15:89–94. doi: 10.1097/HJR.0b013e3282f4b212. [DOI] [PubMed] [Google Scholar]
- Pisinger C, Godtfredsen NS. Is there a health benefit of reduced tobacco consumption? A systematic review. Nicotine & Tobacco Research. 2007;9(6):631–646. doi: 10.1080/14622200701365327. [DOI] [PubMed] [Google Scholar]
- Prochaska J, DiClemente C. The transtheoretical approach: Crossing traditional boundaries of therapy. Homewood, Ill: Dow Jones-Irwin; 1984. [Google Scholar]
- Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Redfern J, McKevitt C, Dundas R, Rudd AG, Wolfe CDA. Behavioral risk factor prevalence and lifestyle change after stroke; A prospective study. Stroke. 2000;31:1877–1881. doi: 10.1161/01.str.31.8.1877. [DOI] [PubMed] [Google Scholar]
- Rigotti N, Munafo’ MR, Stead LF. Interventions for smoking cessation in hospitalised patients. Cochrane Database of Systematic Reviews. 2007;2007:3, Art. No.: CD001837. doi: 10.1002/14651858.CD001837.pub2. [DOI] [PubMed] [Google Scholar]
- Rock VJ, Malarcher A, Kahende JW, Asman K, Husten C, Caraballo R. Cigarette smoking among adults – United States, 2006. MMWR Weekly. 2007;56(44):1157–1161. Retrieved July 6, 2011, from http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5644a2.htm. [PubMed] [Google Scholar]
- Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Ho PM, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, McDermott MM, Meigs JB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Rosamond WD, Sorlie PD, Stafford RS, Turan TN, Turner MB, Wong ND, Wylie-Rosett J on behalf of the American Heart Association Statistics Committee Stroke Statistics Subcommittee. Heart disease and stroke statistics—2011 update: a report from the American Heart Association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAS Institute Inc. SAS/STAT User’s Guide, Version 9.2. Cary, NC: SAS Institute Inc; 2009. [Google Scholar]
- Schnoll RA, Martinez E, Langer C, Miyamoto C, Leone F. Predictors of smoking cessation among cancer patients enrolled in a smoking cessation program. Acta Oncologica. 2011;50(5):678–584. doi: 10.3109/0284186X.2011.572915. [DOI] [PubMed] [Google Scholar]
- Sienkiewicz-Jarosz H, Zatorski P, Baranowska A, Ryglewicz D, Bienkowski P. Predictors of Smoking Abstinence After First-Ever Ischemic Stroke: A 3-Month follow-up. Stroke. 2009;40:2592–2593. doi: 10.1161/STROKEAHA.108.542191. [DOI] [PubMed] [Google Scholar]
- Stead LF, Bergson G, Lancaster T. Physician advice for smoking cessation (Review) Cochrane Database of Systematic Reviews. 2008;2008;2:Art. No.: CD000165. doi: 10.1002/14651858.CD000165.pub3. [DOI] [PubMed] [Google Scholar]
- Steffick DE. Documentation of affective functioning measures in the Health and Retirement Study. HRS Documentation Report. 2000:DR-005. Retrieved July 8, 2011, from http://hrsonline.isr.umich.edu/sitedocs/userg/dr-005.pdf.
- Twardella D, Loew M, Rothenbacher D, Stegmaier C, Ziegler H, Brenner H. The diagnosis of a smoking-related disease is a prominent trigger for smoking cessation in a retrospective cohort study. Journal of Clinical Epidemiology. 2006;59:82–89. doi: 10.1016/j.jclinepi.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Vargas CM, Burt VL, Gillum RF, Pamuk ER. Validity of self-reported hypertension in the National Health and Nutrition Examination Survey III, 1988–1991. Preventive Medicine. 1997;26:678–685. doi: 10.1006/pmed.1997.0190. [DOI] [PubMed] [Google Scholar]