Abstract
The effect of vitamin D supplementation and caloric restriction (CR) on glycemic indices and osteocalcin (OC) is not clear. In this randomized controlled double blind trial, we examined whether vitamin D3 supplementation at 2500 IU/d (D) or placebo has differential effects on markers of insulin sensitivity and bone turnover in overweight/obese postmenopausal women during 6 weeks of caloric restriction (weight loss; WL, n = 39) compared to weight maintenance (WM, n = 37). Seventy-six women (57 ± 6 years) completed this study and the WL groups lost 4 ± 1% of body weight. Baseline serum 25-hydroxyvitamin D (25OHD) was 24.8 ± 5.6 ng/mL at baseline; the rise was greatest in WL-D group (p < 0.05). There was an interaction between vitamin D intake and weight on serum OC, insulin, glucose and markers of insulin sensitivity (p < 0.05). The change in OC was explained by changes in serum 25OHD and insulin (model R 2 = 25.6%). Overall, vitamin D supplementation and CR influence serum osteocalcin levels and modestly favor improvements in insulin sensitivity.
Keywords: Caloric restriction, Bone turnover, Osteocalcin, 25-hydroxyvitamin D (25OHD), Vitamin D supplementation
1. Introduction
The effect of vitamin D on calcium metabolism and bone health are well established and recommended dietary intakes (RDA) were established based on this outcome (IOM, 2011). There are also numerous purported nonskeletal effects of vitamin D such as amelioration of insulin resistance, cardiovascular disease, and neo-plastic diseases, but studies for these outcomes either show inconsistent results or cannot establish a cause and effect relationship (IOM, 2011; Rosen et al., 2012). There is strong evidence for a role of calcitriol in the synthesis and secretion of insulin in beta islet cells as shown in rodent and cell studies (Cade and Norman, 1987; Norman et al., 1980; Pittas et al., 2007) and therefore, should influence insulin sensitivity outcomes in diabetes. Several observational studies have shown that serum 25-hydroxy-vitamin D (25OHD) is inversely associated with the prevalence of diabetes, although the associations remain inconclusive (Mitri et al., 2011; Seida et al., 2014). Randomized studies using vitamin D supplementation in individuals without diabetes show mixed results. Most studies indicate that vitamin D supplementation in persons with established diabetes has no effect on insulin resistance (Kampmann et al., 2014), although some studies show that vitamin D supplementation is associated with improvements in parameters of glucose tolerance in patients without a prior diagnosis of diabetes (Belenchia et al., 2013; Dutta et al., 2014). There are a few reasons that vitamin D supplementation may attenuate insulin resistance, such as a paracrine effect to upregulate 1,25-hydroxyvitamin D synthesis in the beta cell, or attenuate inflammation or fat infiltration in skeletal muscle, but the mechanism is not clear (Muscongiuri et al., 2014).
Osteocalcin (OC) is a 5700 K da extracellular bone matrix Gla protein and a marker of bone formation. Recent reports suggest that OC may influence the regulation of energy metabolism and glucose homeostasis (Lee et al., 2007). The synthesis of OC and the carboxylation of its glutamyl residues are dependent on vitamin K (Booth et al., 2013; Gundberg et al., 2012). It has been shown that PTH and 1,25-dihydroxyvitamin D increase the expression of OC (Carvallo et al., 2008; Jiang et al., 2004). In addition, lower 25OHD levels are associated with lower OC concentrations (Fonseca et al., 1988). The levels of OC are low in subjects with glucose intolerance or in those at increased risk of type 2 diabetes (Díaz-López et al., 2013; Dobnig et al., 2006). Osteocalcin increases again with improved glycemic control, whether this is achieved by medications or weight loss alone (Rosato et al., 1998; Sayinalp et al., 1995). Multiple mechanisms have been proposed whereby OC may influence insulin sensitivity, but it is still not clear (Ferron et al., 2008; Gower et al., 2013; Lee et al., 2007).
Weight reduction leads to improvements in insulin sensitivity and causes alterations in bone regulating hormones (Shapses et al., 2013). For example, it is well known that serum levels of 25OHD are low in obesity, and increase with weight loss (Ekwaru et al., 2014; Shapses et al., 2013; Zittermann et al., 2014). Furthermore previous studies show that with a greater and long-term weight reduction there is also a modest improvement in osteocalcin levels that are associated with improved insulin sensitivity in both animal and human studies (Gower et al., 2013; Lee et al., 2007). Because vitamin D increases gene expression of OC, a rise in serum 25OHD due to weight loss and/or vitamin D supplementation may positively affect OC and metabolic outcomes. To test this hypothesis, we used a randomized double-blind controlled trial to examine whether vitamin D supplementation influences OC and other markers of bone turnover and insulin sensitivity in older overweight/obese women during short term caloric restriction or weight maintenance.
2. Subjects and methods
2.1. Subjects
Postmenopausal women who reported no menstruation for at least 2 years prior to the study were recruited. The inclusion/ exclusion criteria are described in the parent study (Shapses et al., 2013) with primary outcome on calcium absorption. Participants (ages of 50–70 yrs) were free from any disease states or medications known to influence bone metabolism or glucose/insulin. Subjects were screened for serum 25OHD levels and were excluded if levels were >30 ng/mL, or had a fasting blood glucose >126 mg/dL. Other exclusion criteria included abnormal creatinine clearance, blood urea nitrogen, calcium and phosphorus levels, as described previously (Shapses et al., 2013). For patients who were taking thyroid medications, a stable dose for the past 2 or more years was required for inclusion, and none reported any change in medication when questioned during the intervention. Subjects between BMI 25 and 40 kg/ m 2 were included. Before initiation of any study procedures, subjects signed an informed consent approved by the Institutional Review Board at Rutgers University and an external advisory board. This trial was registered at clinical trials.gov (NCT00473031).
2.2. Study design
Postmenopausal women were enrolled in a 6 week intervention trial using a standard life style modification program to lose weight (WL) or maintain weight (WM). Briefly, women in the WL group followed a standard nutrition education and behavior modification weight-reduction program with the goal to reduce energy by 500–600 kcal/day while maintaining habitual exercise levels. Women in the WM group were asked to maintain their weight for 6 weeks and then offered a couple of free weight loss counseling sessions with the dietitian as incentive to improve adherence during the maintenance period. Subjects were stabilized to 1.2 g calcium and 400 IU vitamin D/day, beginning 1 month prior to baseline measurements using a multivitamin (NatureMade Multi 50+) and a calcium supplement without added vitamin D (200 mg calcium citrate/tablet, Citracal, Bayer, NJ) as needed, based on dietary intake, and continued throughout the intervention. The multivitamin contained 200 mg Ca, 10 μg (400 IU) vitamin D3, 10 μg vitamin K, 100 mg magnesium, and 48 mg phosphorus, in addition to other standard micro-nutrients. The extra Ca supplement was only given if Ca intake was less than 1.2 g from habitual dietary intake and the daily multivitamin/mineral supplement. After baseline measurements, all subjects continued to take the multivitamin, but were also randomly assigned in a double blind manner to either vitamin D3 supplements (15,000 IU) or matching placebos (Bio Tech Pharmacal, AR, USA) were given once weekly for 6 weeks. Subjects in the higher vitamin D3 group averaged 2500 IU/d (2100 IU from vitamin D3 supplement + 400 IU from multivitamin) and those in the placebo received 400 IU/d of vitamin D3 (placebo + 400 IU from multivitamin). This study was designed to ensure that all participants received the RDA for vitamin D (at the time of the intervention), and hence even the placebo group received a 400 IU supplement of vitamin D3. The higher dose of vitamin D was calculated to raise serum 25OHD levels to serum 25OHD levels >30 ng/mL in this overweight/ obese population based on preliminary data in the laboratory. In addition, this is consistent with recent estimations for the response of serum 25OHD based on a given vitamin D intake in the obese (Ekwaru et al., 2014; Zittermann et al., 2014).
2.3. Weight, height and food records
Weight and height were measured with a balance beam scale and stadiometer, respectively (Detecto, Webb City, MO). At each morning visit, weight was recorded with minimal clothing. Food records were collected for at least 3 days during the month prior to the study, and also during the study. In addition, a Ca and vitamin D food frequency questionnaire was used to estimate intake at baseline. These data were analyzed and reported previously (Shapses et al., 2013).
2.4. Blood and urine analysis
Fasting blood and urine samples were collected at baseline, and weeks 3 and 6. Serum and urine were stored at −80 °C, in our laboratory until further analysis. Serum 25OHD was measured by radioimmunoassay (RIA) from Diasorin (DiaSorin, Stillwater, MI, CV < 12.5%). Our laboratory participates in a vitamin D external quality assessment scheme (DEQAS) that monitors the performance of our 25(OH)D assay. Intact PTH was determined by immunoradioassay (Scantibodies, Santee, CA, inter- and intra-assay CV < 6.8%). Bone formation markers such as osteocalcin (BTI, Sloughton, MA, CV < 9%), pro-peptide of type 1 collagen (P1NP, Orion Diagnostica, Finland, CV < 10.2%), serum N-telopeptide of type I collagen (NTx) (Scarborough, ME, USA with CV < 6.9%) and deoxypyridinoline (DPD, CV < 10%) were measured in the urine by reverse-phase HPLC and fluorescence detection and normalized for creatinine excretion. All CV are for the inter and intra assay differences as reported in the assay kit. Serum insulin and glucose were measured using ELISA and hexokinase reagent, respectively. Homeostatic model assessment (HOMA)-IR [glucose (mg/dL) × insulin (μU/mL)/405] was used to estimate insulin resistance. The quantitative insulin sensitivity check index (QUICKI) was calculated using the formula: 1/log fasting insulin (μU/mL) + log (fasting glucose, mg/dL) as an index of insulin sensitivity.
2.5. Statistical analysis
The goal in this study was to determine whether vitamin D supplementation and weight loss influence bone turnover and insulin sensitivity. Power analysis is based on a previous study that showed changes in osteocalcin levels due to weight reduction (Riedt et al., Q9 2005). Based on this study, in order to detect a moderate effect size for changes in osteocalcin between groups, at a power of 80% power (alpha = 0.05), we needed at least 15 participants per group. Additional participants were recruited to account for potential drop-outs in the study. Differences between groups at baseline values were assessed by 1-way ANOVA. The influence of treatment (D or placebo) and weight (WL or WM) on the hormones, bone turnover markers and insulin sensitivity was measured using two-factor factorial ANOVA. We further used race as a covariate in an analysis of covariance (ANCOVA) model . Post-hoc analysis using Tukey's test was done when the model F ratio was significant. Multiple regression analysis was performed with the change in OC as the dependent variable and changes in insulin, glucose, 25OHD and PTH as independent variables. SAS Statistical Package (version 9.2; SAS Institute, Cary, NC) was used for the analysis. Values are expressed as mean ± SD. P values <0.05 were considered significant.
3. Results
The mean age of the participants was 57 ± 6 years and the BMI was 31 ± 4 kg/m2. Fig. 1 shows the enrollment of participants in the study. The baseline characteristics of the participants who completed this trial are given in Table 1. Women were primarily Caucasians and the race distribution was different between groups, with 4 African Americans (AA) in the D-WL group, 3 AA, 1 other and 1 Asian in the D-WM group, 1 AA and 1 Hispanic (H) in the Pl-WL group and 2 AA and 1 H in the Pl-WM group. There was 1 participant with pre-diabetes in each group (WL-D, WM-D and WL-Pl) except for 2 with pre-diabetes in the WM-Pl groups. At baseline, 23% of women had a serum 25OHD < 20 ng/mL (ranging from 11.4 to 19.8 ng/mL), that were equally distributed between groups. Compliance was monitored at weekly visits, using pill counts. Participants were asked to bring back their pillboxes and we examined the number of remaining pills in the container. There was no evidence of non-compliance to vitamin D or other supplements over the 6 weeks.
Fig. 1.
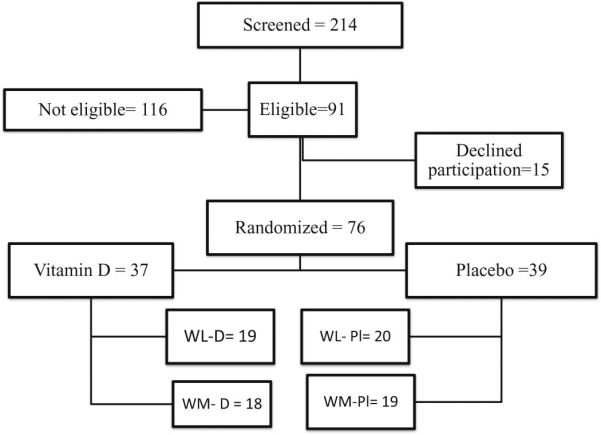
Participant flowchart for study. WL, weight loss; WM, weight maintenance. Eligibility for this study required sample availability for glycemic indices and bone turnover markers analysis that were secondary outcomes in for the original study design.
Table 1.
Baseline characteristics and change in body weight, insulin sensitivity and bone turnover markers after 6 weeks with vitamin D3 supplementation or placebo1234567.
| Baseline | Weight loss change (%) |
Weight maintenance change (%) |
Vit D |
Weight | 1nteraction | |||
|---|---|---|---|---|---|---|---|---|
| WL-D(N = 19) | WL-Pl (N = 20) | WM-D (N = 18) | WM-Pl (N = 19) | P value | ||||
| Weight (kg) | 79.2 ± 12.5 | –3.7 ± 1.2a | –3.9 ± 1.1a | –0.43 ± 1.7b | –0.1 ± 1.6b | 0.909 | <0.001 | 0.4001 |
| 25OHD(ng/mL) | 24.8 ± 5.6 | 32.4 ± 23.5a | 6.9 ± 17.6bcd | 17.0 ± 21.7bc | 4.1 ± 15.7bd | <0.001 | 0.051 | 0.172 |
| PTH (pg/mL) | 41.5 ± 19.6 | –2.8 ± 30.8 | 1.5 ± 43.9 | –4.8 ± 34.7 | 10.5 ± 27.2 | 0.2237 | 0.664 | 0.493 |
| HOMA-1R | 0.51 ± 0.20 | –18.8 ± 26.0a | 4.6 ± 28.7b | 11.0±31.3b | –0.75 ±33.9ab | 0.432 | 0.102 | 0.020 |
| QUICKI | 0.5 ± 0.04 | 4.9 ± 6.5a | 0.02 ± 4.7b | –1.1 ±5.3b | 1.4 ± 7.5ab | 0.428 | 0.118 | 0.013 |
| Osteocalcin (ng/mL) | 8.4 ± 2.2 | 1.4 ± 20.1a | –13.5 ± 15.6b | –8.3 ± 16.7ab | –4.2 ± 14.7ab | 0.169 | 0.957 | 0.017 |
| PINP (ug/L) | 48.2 ± 18.4 | 1.9 ± 20.7 | 3.3 ± 15.7 | 4.2 ± 9.5 | 0.6 ± 15.6 | 0.789 | 0.939 | 0.508 |
| NTx (BCE) | 12.6 ± 4.4 | –5.8 ± 22.5 | –9.6 ± 19.5 | 7.7 ± 28.9 | –3.9 ± 26.6 | 0.198 | 0.106 | 0.513 |
| DPD(nmol/mmol) | 6.4 ± 2.6 | 1.5 ± 36.6 | –0.4 ± 34.5 | 14.2 ± 28.3 | 7.3 ± 35.9 | 0.549 | 0.166 | 0.732 |
Values represent the mean ± SD.
Abbreviations: 25OHD, 25-hydroxyvitamin D; Pl, placebo; PTH, parathyroid hormone; NTx, N-telopeptide of type-1 collagen; DPD, deoxypyridinium crosslinks/creatinine; P1NP, propeptide of type 1 collagen, HOMA: homeostatic model assessment, QUICKI : quantitative insulin sensitivity check index.
Vitamin D3 includes 2 levels of intake (the vitamin D group received 15,000 IU once weekly from a supplement plus 400 IU/d from a multivitamin equivalent to 2500 IU/d; the Placebo group received once weekly placebo tablet plus 400 IU vitamin D/d from a multivitamin).
Baseline values did not differ significantly between groups as assessed using a one way ANOVA and is presented as a combined mean for 4 groups.
P value is for a two-way factorial ANOVA for weight (WL/WM) * vitamin D (vit D/placebo) treatment. Post-hoc analysis (Tukey's test) was done when the model F ratio was significant. Different superscripts in the same row indicate significant differences between groups.
All baseline blood samples were taken between January and February and all endpoint blood samples were taken between March and the first week of April.
An ANCOVA analysis was done using race as a covariate in the analysis. This analysis did not change any findings including significant findings (OC = 0.013), Ins 0.046, HOMA = 0.020, QUICKI = 0.015.
Women lost 6.9 ± 2.6 kg in the WL group and −0.43 ± 2.7 kg in WM group and with no differences between vitamin D and Pl groups (Table 1). There was a main effect of vitamin D supplementation to increase serum 25OHD, and post-hoc analysis indicated that the greatest rise was in WL-D group (+32.4 ± 23.5%) compared to other groups (p < 0.05) (Fig. 2). There were no differences in the change in PTH concentrations between the groups (Table 1). Interestingly, markers of bone formation and resorption did not differ between groups, except for OC concentrations. The interaction between vitamin D intakes and weight change on serum OC was significant (p < 0.02). Serum OC concentrations did not change significantly from baseline in any group. There was an interaction between vitamin D and weight loss for insulin (p < 0.05) and glucose (p = 0.02) (Fig. 2), and for HOMA and QUICKI (Table 1). For all glycemic indices, the decline in serum concentrations of insulin, glucose, HOMA and QUICKI was greatest in the WL-D group compared to the WM-D group (p < 0.05) (Table 1, Fig. 2).
Fig. 2.
Changes in serum 25-hydroxyvitamin D (25OHD), insulin and glucose due to 6 week intervention with vitamin D supplementation. All groups received a multivitamin with 400 IU/d and were then randomized to 2500 IU vitamin D/day (D) or placebo (Pl) in weight loss (WL) and weight maintenance (WM) groups of postmenopausal women. The four groups are WL-D (n = 19), WM-D (n = 20), WL-Pl (n = 18) and WM-Pl (n = 19). Values are mean ± SEM. P values are shown for two-factor ANOVA for vitamin D (D or Pl) and weight (WL or WM).
A multiple regression analysis examining the relationship between changes in osteocalcin and other variables is shown in Table 2. Changes in 25OHD, PTH, insulin and glucose are used as independent variables and OC as the dependent variable. The change in serum OC was positively and negatively predicted by the change in 25OHD and insulin, respectively (model R2 = 25.6%) and this was observed only during weight loss (Table 2).
Table 2.
Multiple regression model for the change in serum osteocalcin levels and the relative influence of hormones and glycemic indices in women over 6 weeks of caloric restrictiona.
|
Osteocalcin Model R2 (%) = 25.6 |
|||
|---|---|---|---|
| R2 | Std β | P | |
| 25OHD | 15.6 | 0.386 | 0.032 |
| PTH | 0.02 | –0.016 | 0.927 |
| Insulin | 9.2 | –0.339 | 0.062 |
| Glucose | 0.8 | 0.093 | 0.607 |
Abbreviations: 25OHD, 25-hydroxyvitamin D; PTH, parathyroid hormone; Std β, standardized beta coefficient.
These data are for the women in the weight loss group (n = 39). There were no significant predictors for changes in osteocalcin in the weight maintenance group (n = 37) or in the entire group (n = 76).
4. Discussion
Vitamin D supplementation (2500 IU/d) compared to 400 IU/d had no effect on markers of insulin resistance after a 6 week intervention in postmenopausal obese/overweight women. However, the higher dose of vitamin D reduced serum glucose, insulin and HOMA in women during caloric restriction. In addition, vitamin D supplementation compared to placebo did not influence markers of bone turnover. This is consistent with findings that weight loss increases bone turnover, but only under conditions of inadequate calcium intake (Jensen et al., 2001; Riedt et al., 2005). The change in OC showed a positive association with the change in serum 25OHD and a negative trend with insulin and HOMA. The effects of vitamin D supplementation on markers of insulin sensitivity have not been consistent. Similar to previous findings (Seida et al., 2014), these data indicate that short term vitamin D supplementation did not improve insulin sensitivity in healthy women. However, we found that the combination of vitamin D supplementation and caloric restriction significantly influenced insulin sensitivity, and that this is partially explained by serum OC that showed an interaction between groups.
Serum osteocalcin levels have traditionally been considered a marker of bone formation. The γ-carboxylation of OC, a bone matrix GLA protein, is dependent on vitamin K, however vitamin D increases transcription and gene expression of total OC (Patti et al., 2013). Osteocalcin has been shown to have a positive role in glucose metabolism and to increase with improved glucose control. This is possibly due to its role in inducing insulin production by pancreatic β cells and adiponectin secretion from adipocytes (Lee et al., 2007). Also, OC has been shown to increase basal and insulin stimulated glucose transport in adipocytes (Ferron et al., 2008). In animal models and cell studies, OC that is not γ-carboxylated (ucOC) is reported to affect glucose metabolism (Lee et al., 2007). In most clinical trials, there is a consistent effect of total OC influencing glucose metabolism, although the role of ucOC remains inconclusive (Booth et al., 2013; Díaz-López et al., 2013; Douglas et al., 1995; Gower et al., 2013). It has been suggested that the discrepancy between studies may be because the effect requires the presence of impaired glucose tolerance (Booth et al., 2013; Gower et al., 2013). In addition, studies using vitamin K or warfarin that shift the ratio of carboxylated OC and ucOC have no effect on glucose homeostasis (Gundberg et al., 2012). This study was not designed to address this question. However, because vitamin K intakes were similar in the 4 groups due to diet and supplementation (~90 ug/d), carboxylation of OC should not have been influenced by differences in intake. In the current study, we examined the role of vitamin D on total circulating OC since it is known to increase OC gene expression (Stein et al., 1996). Others have shown that vitamin D supplementation does not alter serum total OC (Je et al., 2011; Von Hurst et al., 2010) or ucOC concentrations (O'Connor et al., 2010). In the current study, vitamin D supplementation also had no effect on total OC under any of the conditions, although it correlates with changes serum 25OHD during caloric restriction.
The mechanism whereby vitamin D may be acting on OC is not clear, but 1,25(OH)2D3 has shown to increase the gene expression of OC (Carvallo et al., 2008; Jiang et al., 2004). In addition, vitamin D promotes osteoblast maturation, and OC is released by the mature osteoblast (Stein et al., 1996). Other bone formation markers, P1NP and BAP, are released during early phases of osteoblast cell cycle, while OC is released during later osteoblast maturation. Therefore, since vitamin D mediates progression of osteoblast differentiation, it is possible that it promotes OC synthesis in the more mature osteoblast (and OC synthesis) without an effect on early markers (PINP) of osteoblast maturation. Our study does not support the hypothesis that vitamin D supplementation increases serum levels of total OC, although it influenced changes in OC during caloric restriction. It is interesting that only OC and no other bone markers differed between groups. This supports the findings by other researchers (Lee et al., 2007), that osteocalcin may be a unique marker of bone formation due to its role in energy metabolism and also changing differently compared to other markers of bone turnover. Other studies have shown that bone resorption markers Q10 increase with weight loss (Shapses and Sukumar, 2012), but the absence of change here may be due to the individualized calcium intake that met the recommended intakes.
Strengths and limitations of this study are the following. The study employs a randomized double blinded approach to assess the influence of vitamin D supplementation and weight loss on bone turnover markers and regulating hormones. Furthermore, the performance of the 25OHD assay in our laboratory is monitored using DEQAS, which is an international quality assurance group. Furthermore, to avoid the influence of sun on 25OHD measures, these studies were carried out in the winter and early spring. Women who were enrolled in the study were primarily Caucasians and the potential generalizability of the data may also be a limitation, since and thus results may not apply to other ethnic groups. Another limitation is that our study design did not include measuring undercarboxylated form of OC, which has been shown to have an effect on glycemic indices in murine studies (Ferron et al., 2008; Lee et al., 2007), and should be included in a future study that could further examine the role of vitamin K in addition to vitamin D supplementation.
The effect of vitamin D supplementation on insulin resistance is significant when overweight/obese individuals are losing weight. In contrast, vitamin D supplementation alone or caloric restriction alone did not elicit an OC or glucose response. This may be simply due to the attenuated rise in serum 25OHD in the weight stable women compared to those adhering to caloric restriction. Alternatively, it is possible that the change in OC is additive with vitamin D treatment. In conclusion, this randomized controlled trial indicates that vitamin D supplementation in healthy overweight/obese older women shows a modest favorable effect on insulin sensitivity, but this is only significant in the presence of caloric restriction.
Acknowledgments
We thank the laboratory and clinical staff for their invaluable technical and clinical assistance. In addition, we appreciate the assistance of Yvette Schlussel, PhD for her statistical expertise and LC Pop, MD, and M Watford, DPhil, in the review of these data and the manuscript.
Funding Source: National Institutes of Health grant RO1-AG12161 to SA Shapses.
References
- Belenchia AM, Tosh AK, Hillman LS, Peterson CA. Correcting vitamin D insufficiency improves insulin sensitivity in obese adolescents: a randomized controlled trial. Am. J. Clin. Nutr. 2013;97(4):774–781. doi: 10.3945/ajcn.112.050013. [DOI] [PubMed] [Google Scholar]
- Booth SL, Centi A, Smith SR, Gundberg C. The role of osteocalcin in human glucose metabolism: marker or mediator? Nat. Rev. Endocrinol. 2013;9(1):43–55. doi: 10.1038/nrendo.2012.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cade C, Norman AW. Rapid normalization/stimulation by 1,25-dihydroxyvitamin D3 of insulin secretion and glucose tolerance in the vitamin D-deficient rat. Endocrinology. 1987;120:1490–1497. doi: 10.1210/endo-120-4-1490. [DOI] [PubMed] [Google Scholar]
- Carvallo L, Henríquez B, Paredes R, Olate J, Onate S, van Wijnen AJ, et al. 1alpha,25-dihydroxy vitamin D3-enhanced expression of the osteocalcin gene involves increased promoter occupancy of basal transcription regulators and gradual recruitment of the 1alpha,25-dihydroxy vitamin D3 receptor-SRC-1 coactivator complex. J. Cell. Physiol. 2008;214(3):740–749. doi: 10.1002/jcp.21267. [DOI] [PubMed] [Google Scholar]
- Díaz-López A, Bulló M, Juanola-Falgarona M, Martínez-González MA, Estruch R, Covas MI, et al. Reduced serum concentrations of carboxylated and undercarboxylated osteocalcin are associated with risk of developing type 2 diabetes mellitus in a high cardiovascular risk population: a nested case-control study. J. Clin. Endocrinol. Metab. 2013;98(11):4524–4531. doi: 10.1210/jc.2013-2472. [DOI] [PubMed] [Google Scholar]
- Dobnig H, Piswanger-Sölkner JC, Roth M, Obermayer-Pietsch B, Tiran A, Strele A, et al. Type 2 diabetes mellitus in nursing home patients: effects on bone turnover, bone mass, and fracture risk. J. Clin. Endocrinol. Metab. 2006;91(9):3355–3363. doi: 10.1210/jc.2006-0460. [DOI] [PubMed] [Google Scholar]
- Douglas AS, Robins SP, Hutchison JD, Porter RW, Stewart A, Reid DM. Carboxylation of osteocalcin in post-menopausal osteoporotic women following vitamin K and D supplementation. Bone. 1995;17(1):15–20. doi: 10.1016/8756-3282(95)00133-x. [DOI] [PubMed] [Google Scholar]
- Dutta D, Mondal SA, Choudhuri S, Maisnam I, Hasanoor Reza AH, Bhattacharya B, et al. Vitamin-D supplementation in prediabetes reduced progression to type 2 diabetes and was associated with decreased insulin resistance and systemic inflammation: an open label randomized prospective study from Eastern India. Diabetes Res. Clin. Pract. 2014;103(3):e18–e23. doi: 10.1016/j.diabres.2013.12.044. [DOI] [PubMed] [Google Scholar]
- Ekwaru JP, Zwicker JD, Holick MF, Giovannucci E, Veugelers PJ. The importance of body weight for the dose response relationship of oral vitamin d supplementation and serum 25-hydroxyvitamin d in healthy volunteers. PLoS ONE. 2014;9(11):e111265. doi: 10.1371/journal.pone.0111265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferron M, Hinoi E, Karsenty G, Ducy P. Osteocalcin differentially regulates beta cell and adipocyte gene expression and affects the development of metabolic diseases in wild-type mice. Proc. Natl. Acad. Sci. U.S.A. 2008;105(13):5266–5270. doi: 10.1073/pnas.0711119105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca VA, D'Souza V, Houlder S, Thomas M, Wakeling A, Dandona P. Vitamin D deficiency and low osteocalcin concentrations in anorexia nervosa. J. Clin. Pathol. 1988;41(2):195–197. doi: 10.1136/jcp.41.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gower BA, Pollock NK, Casazza K, Clemens TL, Goree LL, Granger WM. Associations of total and undercarboxylated osteocalcin with peripheral and hepatic insulin sensitivity and β-cell function in overweight adults. J. Clin. Endocrinol. Metab. 2013;98(7):E1173–E1180. doi: 10.1210/jc.2013-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundberg CM, Lian JB, Booth SL. Vitamin K-dependent carboxylation of osteocalcin: friend or foe? Adv. Nutr. 2012;3(2):149–157. doi: 10.3945/an.112.001834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Je SH, Joo NS, Choi BH, Kim KM, Kim BT, Park SB, et al. Vitamin K supplement along with vitamin D and calcium reduced serum concentration of undercarboxylated osteocalcin while increasing bone mineral density in Korean postmenopausal women over sixty-years-old. J. Korean Med. Sci. 2011;26(8):1093–1098. doi: 10.3346/jkms.2011.26.8.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen LB, Kollerup G, Quaade F, Sørensen OH. Bone minerals changes in obese women during a moderate weight loss with and without calcium supplementation. J. Bone Miner. Res. 2001;16(1):141–147. doi: 10.1359/jbmr.2001.16.1.141. [DOI] [PubMed] [Google Scholar]
- Jiang D, Franceschi RT, Boules H, Xiao G. Parathyroid hormone induction of the osteocalcin gene. Requirement for an osteoblast-specific element 1 sequence in the promoter and involvement of multiple-signaling pathways. J. Biol. Chem. 2004;279(7):5329–5337. doi: 10.1074/jbc.M311547200. [DOI] [PubMed] [Google Scholar]
- Lee NK, Sowa H, Hinoi E, Ferron M, Ahn JD, Confavreux C, et al. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007;130(3):456–469. doi: 10.1016/j.cell.2007.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitri J, Muraru MD, Pittas AG. Vitamin D and type 2 diabetes: a systematic review. Eur. J. Clin. Nutr. 2011;65(9):1005–1015. doi: 10.1038/ejcn.2011.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman AW, Frankel JB, Heldt AM, Grodsky GM. Vitamin D deficiency inhibits pancreatic secretion of insulin. Science. 1980;209:823–825. doi: 10.1126/science.6250216. [DOI] [PubMed] [Google Scholar]
- O'Connor E, Mølgaard C, Michaelsen KF, Jakobsen J, Cashman KD. Vitamin D-vitamin K interaction: effect of vitamin D supplementation on serum percentage undercarboxylated osteocalcin, a sensitive measure of vitamin K status, in Danish girls. Br. J. Nutr. 2010;104(8):1091–1095. doi: 10.1017/S0007114510001935. [DOI] [PubMed] [Google Scholar]
- Patti A, Gennari L, Merlotti D, Dotta F, Nuti R. Endocrine actions of osteocalcin. Int. J. Endocrinol. 2013;2013:846480. doi: 10.1155/2013/846480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittas AG, Lau J, Hu FB, Dawson-Hughes B. The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2007;92:2017–2029. doi: 10.1210/jc.2007-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedt CS, Cifuentes M, Stahl T, Chowdhury HA, Schlussel Y, Shapses SA. Overweight postmenopausal women lose bone with moderate weight reduction and 1 g/day calcium intake. J. Bone Miner. Res. 2005;20(3):455–463. doi: 10.1359/JBMR.041132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosato MT, Schneider SH, Shapses SA. Bone turnover and insulin-like growth factor I levels increase after improved glycemic control in noninsulin-dependent diabetes mellitus. Calcif. Tissue Int. 1998;63(2):107–111. doi: 10.1007/s002239900498. [DOI] [PubMed] [Google Scholar]
- Rosen CJ, Adams JS, Bikle DD, Black DM, Demay MB, Manson JE, et al. The nonskeletal effects of vitamin D: an Endocrine Society scientific statement. Endocr. Rev. 2012;33(3):456–492. doi: 10.1210/er.2012-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayinalp S, Gedik O, Koray Z. Increasing serum osteocalcin after glycemic control in diabetic men. Calcif. Tissue Int. 1995;57(6):422–425. doi: 10.1007/BF00301944. [DOI] [PubMed] [Google Scholar]
- Seida JC, Mitri J, Colmers IN, Majumdar SR, Davidson MB, Edwards AL, et al. Effect of vitamin d3 supplementation on improving glucose homeostasis and preventing diabetes: a systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2014;99(10):3551–3560. doi: 10.1210/jc.2014-2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapses SA, Sukumar D, Schneider SH, Schlussel Y, Sherrell RM, Field MP, et al. Vitamin D supplementation and calcium absorption during caloric restriction: a randomized double-blind trial. Am. J. Clin. Nutr. 2013;97(3):637–645. doi: 10.3945/ajcn.112.044909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein GS, Lian JB, Stein JL, Van Wijnen AJ, Montecino M. Transcriptional control of osteoblast growth and differentiation. Physiol. Rev. 1996;76(2):593–629. doi: 10.1152/physrev.1996.76.2.593. [DOI] [PubMed] [Google Scholar]
- Von Hurst PR, Stonehouse W, Kruger MC, Coad J. Vitamin D supplementation suppresses age-induced bone turnover in older women who are vitamin D deficient. J. Steroid Biochem. Mol. Biol. 2010;121(1–2):293–296. doi: 10.1016/j.jsbmb.2010.03.054. [DOI] [PubMed] [Google Scholar]
- Zittermann A, Ernst JB, Gummert JF, Börgermann J. Vitamin D supplementation, body weight and human serum 25-hydroxyvitamin D response: a systematic review. Eur. J. Nutr. 2014;53(2):367–374. doi: 10.1007/s00394-013-0634-3. [DOI] [PubMed] [Google Scholar]
- Pollock NK, Bernard PJ, Gower BA, Gundberg CM, Wenger K, Misra S, et al. Lower uncarboxylated osteocalcin concentrations in children with prediabetes is associated with beta-cell function. J. Clin. Endocrinol. Metab. 2011;96(7):E1092–E1099. doi: 10.1210/jc.2010-2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehpour A, Shidfar F, Hosseinpanah F, Vafa M, Razaghi M, Amiri F. Does vitamin D3 supplementation improve glucose homeostasis in overweight or obese women? A double-blind, randomized, placebo-controlled clinical trial. Diabet. Med. 2013;30(12):1477–1481. doi: 10.1111/dme.12273. [DOI] [PubMed] [Google Scholar]
- Wongwiwatthananukit S, Sansanayudh N, Phetkrajaysang N, Krittiyanunt S. Effects of vitamin D(2) supplementation on insulin sensitivity and metabolic parameters in metabolic syndrome patients. J. Endocrinol. Invest. 2013;36(8):558–563. doi: 10.3275/8817. [DOI] [PubMed] [Google Scholar]



