Abstract
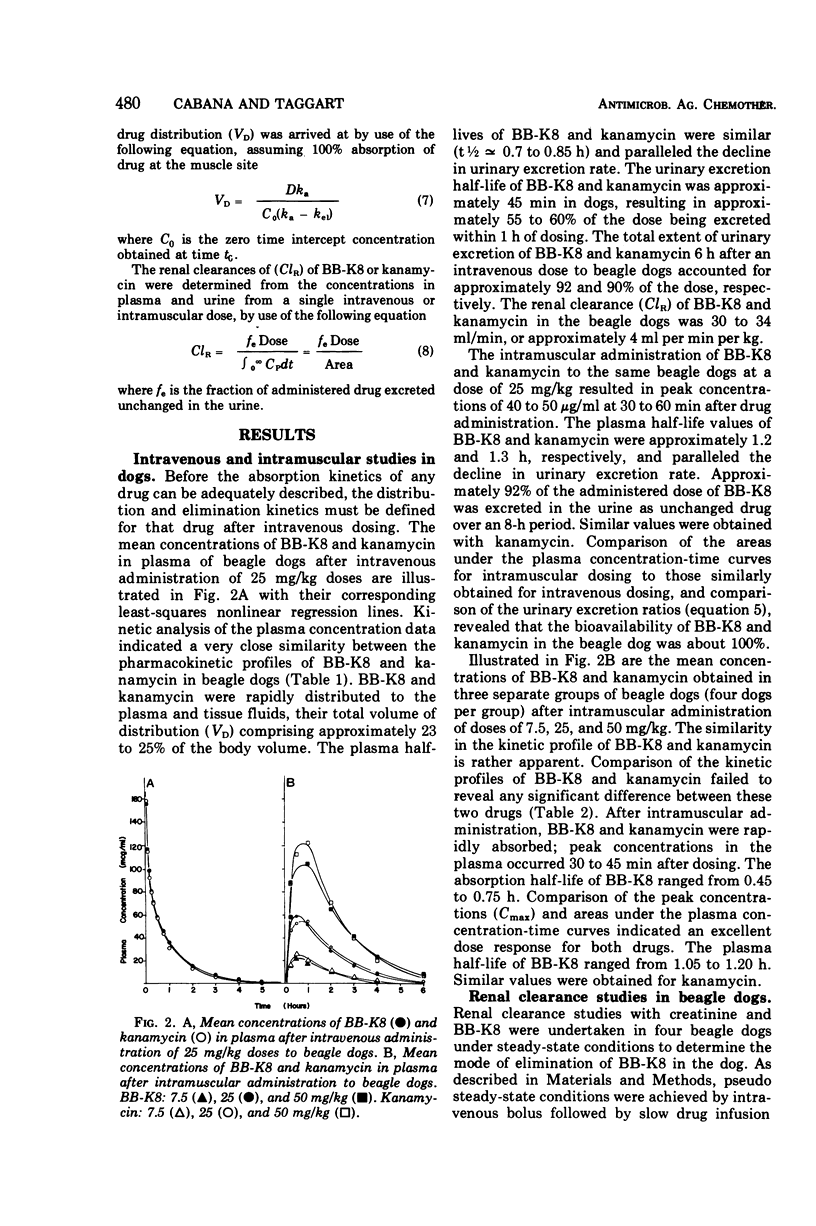
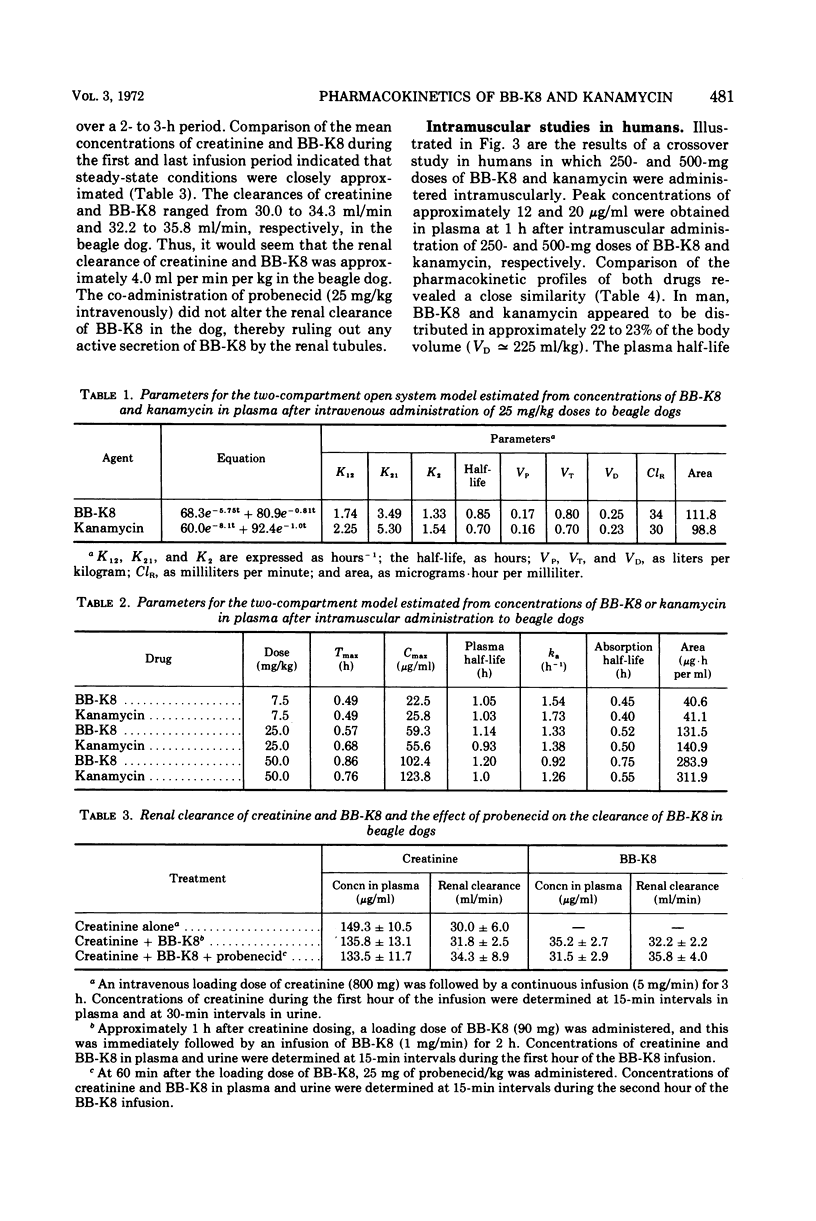
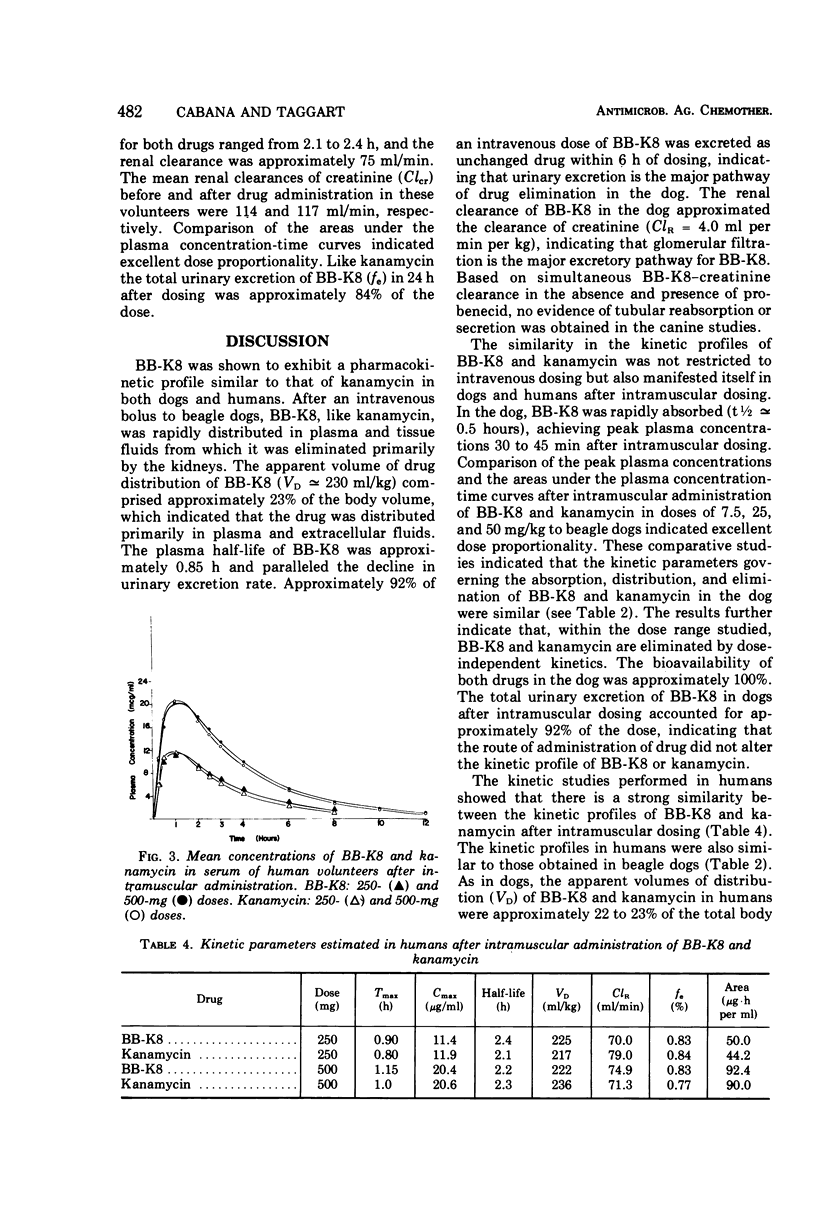
Comparative studies in beagle dogs suggested that the pharmacokinetic profiles of BB-K8 and kanamycin are similar. After intravenous (iv) administration to dogs, BB-K8 and kanamycin were rapidly distributed in plasma and tissue fluids; their “apparent” volumes of distribution comprised approximately 23% of the total body volume. Like kanamycin, BB-K8 had a plasma half-life of about 0.8 h which paralleled the urinary excretion rate. Approximately 92% of the dose was excreted as unchanged drug within 6 h of dosing, and clearance appeared to be primarily by glomerular filtration. After intramuscular (im) administration to dogs, BB-K8 and kanamycin were totally and rapidly absorbed; peak concentrations in the plasma occurred 0.5 to 1.0 h after dosing. The kinetic parameters governing the distribution and elimination of BB-K8 and kanamycin after an im dose were similar to those obtained for iv dosing and indicate desirable dose-independent kinetics. A human pharmacokinetic study indicated that the kinetic profiles of BB-K8 and kanamycin are similar in man after im dosing. Like kanamycin, BB-K8 is rapidly absorbed, yielding peak serum concentrations of about 20 μg/ml at 1 h after a 500-mg im dose. The plasma half-life of these two drugs was approximately 2.3 h. Clearance in man was primarily by glomerular filtration, and the urinary excretion of BB-K8 and kanamycin accounted for 83% of the dose.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cabana B. E., Willhite L. E., Bierwagen M. E. Pharmacokinetic evaluation of the oral absorption of different ampicillin preparations in beagle dogs. Antimicrob Agents Chemother (Bethesda) 1969;9:35–41. [PubMed] [Google Scholar]
- Loo J. C., Riegelman S. New method for calculating the intrinsic absorption rate of drugs. J Pharm Sci. 1968 Jun;57(6):918–928. doi: 10.1002/jps.2600570602. [DOI] [PubMed] [Google Scholar]
- Orme B. M., Cutler R. E. The relationship between kanamycin pharmacokinetics: distribution and renal function. Clin Pharmacol Ther. 1969 Jul-Aug;10(4):543–550. doi: 10.1002/cpt1969104543. [DOI] [PubMed] [Google Scholar]
- Riegelman S., Loo J. C., Rowland M. Shortcomings in pharmacokinetic analysis by conceiving the body to exhibit properties of a single compartment. J Pharm Sci. 1968 Jan;57(1):117–123. doi: 10.1002/jps.2600570123. [DOI] [PubMed] [Google Scholar]
- Riegelman S., Loo J., Rowland M. Concept of a volume of distribution and possible errors in evaluation of this parameter. J Pharm Sci. 1968 Jan;57(1):128–133. doi: 10.1002/jps.2600570125. [DOI] [PubMed] [Google Scholar]
- Wagner J. G., Northam J. I. Estimation of volume of distribution and half-life of a compound after rapid intravenous injection. J Pharm Sci. 1967 Apr;56(4):529–531. doi: 10.1002/jps.2600560424. [DOI] [PubMed] [Google Scholar]
- Wagner J. G., Novak E., Leslie L. G., Metzler C. M. Absorption, distribution, and elimination of spectinomycin dihydrochloride in man. Int Z Klin Pharmakol Ther Toxikol. 1968 Apr;1(4):261–285. [PubMed] [Google Scholar]



