Abstract
Physician-modified endovascular grafts, with fenestrations added to accommodate major branch vessels, provide a means for endovascular treatment of abdominal aortic aneurysms that are adjacent to the renal arteries. Manual measurements of vessel origin locations from CT images, however, take time and can lead to errors in the positions of the fenestrations. To make the fenestration process faster and more accurate, we have developed a procedure to create custom templates that serve as patient-specific guides for graft fenestration. We use a 3D printer to create a clear rigid sleeve that replicates the patient’s aorta and includes holes placed precisely at the locations of the branch vessels. The sleeve is slipped over the graft, the locations of the openings are marked with a pen, and the fenestrations are created after removing the sleeve. Custom fenestration templates can potentially save procedural costs and make minimally-invasive aortic aneurysm repair available to more patients.
INTRODUCTION
Minimally-invasive endovascular repair of abdominal aortic aneurysms (AAAs) has been shown to reduce mortality and morbidity associated with open repair.1 Unfortunately, not all patients presenting with AAAs are candidates for endovascular repair.2 Reasons for exclusion predominantly involve lack of a suitable proximal aortic neck, which is required for stable fixation without covering major branch vessels. Multi-branched or “fenestrated” endografts3–5 offer one solution to this problem, but generally with increased cost and long manufacture/delivery times.
On-site physician modification of endovascular grafts for branch vessel preservation is an alternative treatment strategy for juxtarenal AAAs.6,7 In these cases, fenestrations are added to standard commercial stent grafts to accommodate patient-specific vessel origin sites. The opening locations are determined from the vessel origins visualized in CT images. Longitudinal distances are measured on the graft, and the relative angular locations are specified as ‘clock’ positions relative to a reference point. However, potential barriers to wider use of physician-modified endovascular grafts are 1) pre-operative planning time for measuring the fenestration locations from CT data, 2) time in the operating room required to map the opening locations to the endograft, 3) accuracy of the placement of the openings, and 4) optimum location of all openings to avoid stent struts. To address these issues we have developed a method to create patient-specific fenestration templates that allow for faster and more accurate endograft modification.
METHODS
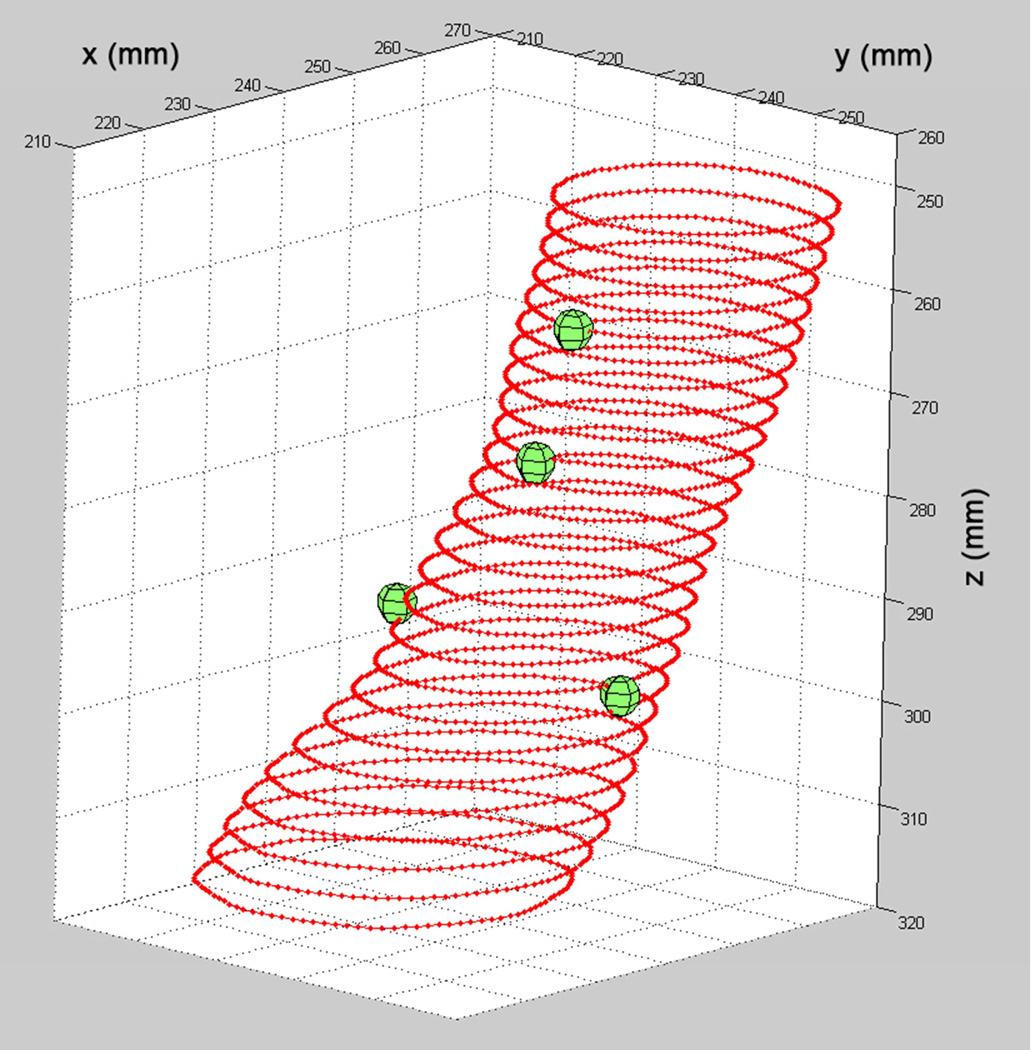
We use a patient’s CT image data to create a 3D-printed rigid sleeve that replicates the proximal neck of their aorta, including openings at the locations of the branch vessel origins. The creation of a custom fenestration template for a patient with a juxtarenal AAA is presented in Figs 1 through 3. The protocol was approved by the University of Washington Institutional Review Board and the subject gave informed consent. We use custom software developed at the University of Washington Cardiovascular Research and Training Center to outline the aorta in a patient’s CT image dataset. 8,9 The aorta lumen is manually traced and point markers are placed at specific 3D locations to indicate the origins of the branch vessels (Fig 1).
Fig 1.
3D display of the traced lumen contours of the AAA proximal neck for the demonstration data set. The branch vessel origin locations are displayed as spheres.
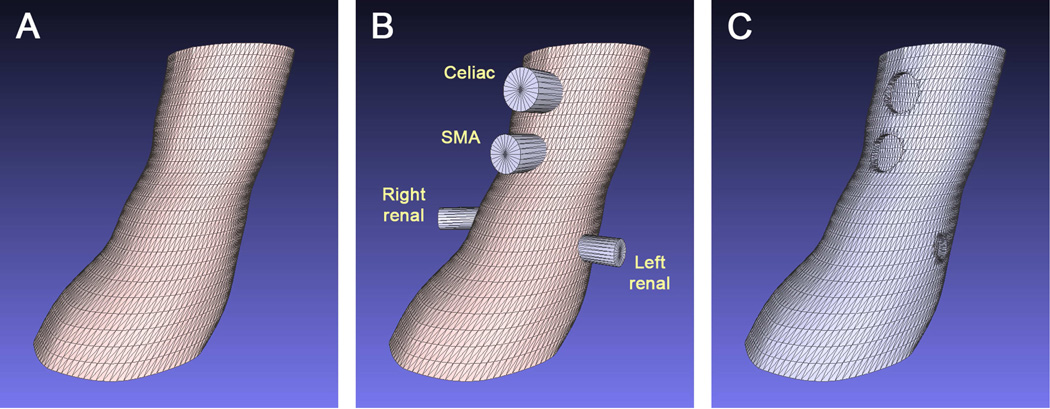
Next, custom software10 developed in the MATLAB programming environment (The MathWorks, Natick, MA) is used to convert the lumen outlines into a computer model that defines a sleeve whose inner surface represents the aorta lumen (Figure 2A). The holes at the locations of the branch vessels are created by first constructing cylindrical objects in 3D space that are centered at the branch origin points (Fig 2B). A Computer-Aided Design (CAD) software package (Rhinoceros 5, Robert McNeel & Associates, Seattle, WA) is then used to subtract the sections of the aorta mesh that are intersected by the cylinders, effectively ‘punching holes’ in the aorta mesh model. The result is a computer mesh model with openings at the branch vessel origin locations (Fig 2C).
Fig 2.
Fenestration template CAD model. The lumen contours shown in Fig 1 were converted to a solid model format compatible with a 3D printer. A, Polygon model of the aneurysm proximal neck. B, Combined display of the proximal neck model and cylinders at each of the branch vessel origins. The cylinder diameters are set to match the branch vessel opening diameters as measured from the original CT images. C, Result of the Boolean difference between the proximal neck object and each cylinder. The result is a hole in the aorta model at the location of each of the branch vessel origins.
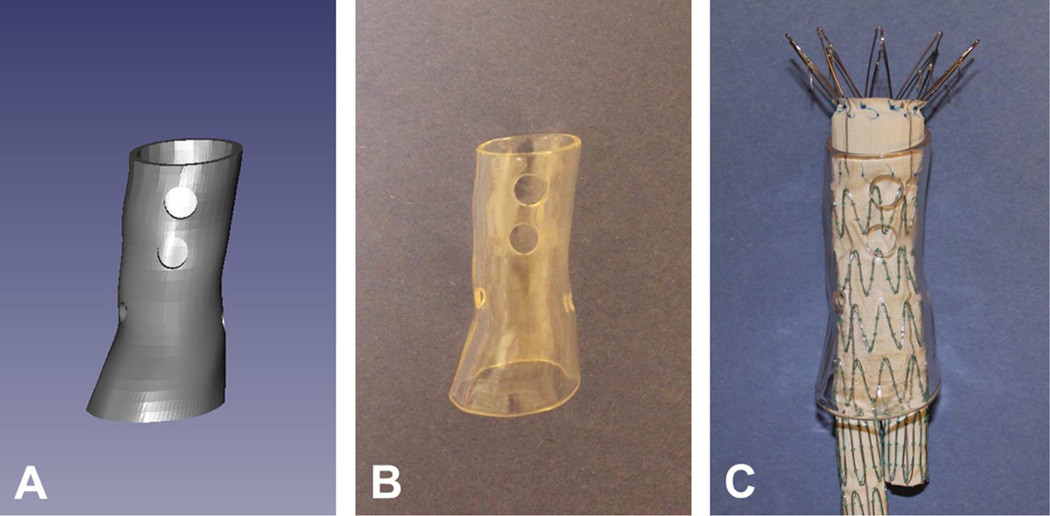
Finally, a stereolithography 3D printer (4D Parts Direct, Broadview Heights, Ohio) is used to produce a clear rigid sleeve that includes holes placed precisely at the locations of the branch vessels (Fig 3). The clear printing material allows visualization of the stent struts during positioning of the graft in the template (Fig 3C).
Fig 3.
3D-printed fenestration template. A, Computer model of the template. B, Solid printed model of the template created using a stereolithography 3D printer with clear resin. C, An endovascular graft inserted in the template.
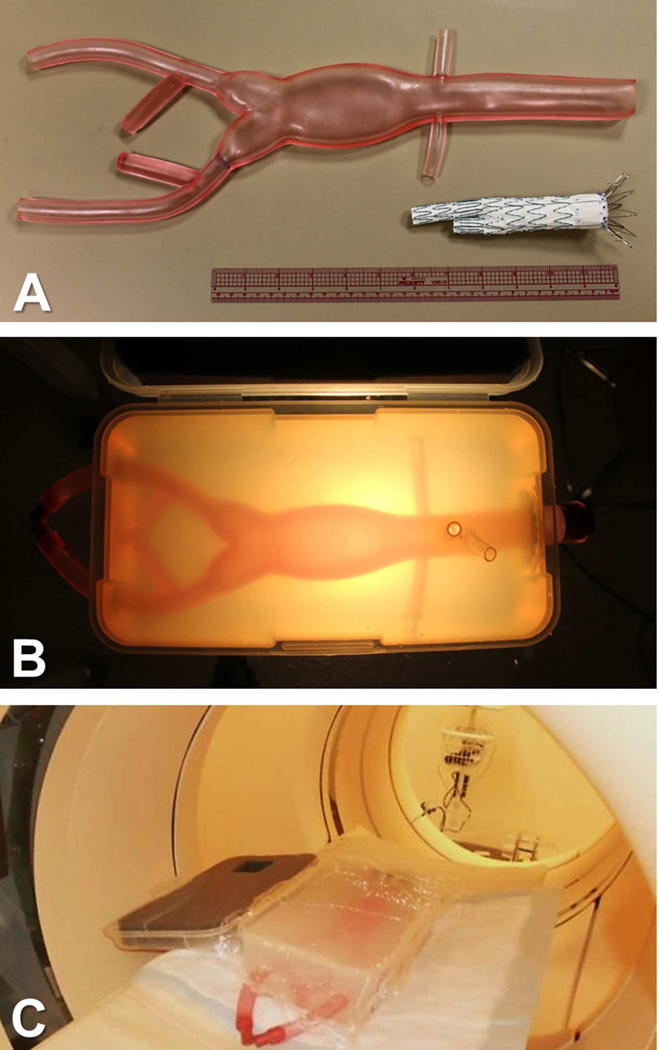
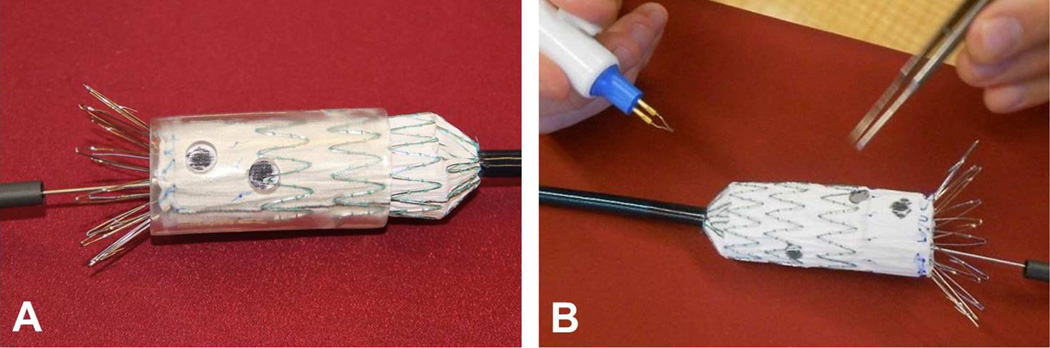
Our template-based fenestration procedure was validated by deploying a custom fenestrated graft in an aorta phantom. The phantom was created by embedding a commercially-available flexible AAA model (Aortic Aneurysm 1808-2, Pacific Research Laboratories, Vashon Island, WA) in an agar block (Fig 4A, 4B). The phantom was scanned by CT (Fig 4C) and a template was designed and printed to match the four branch vessels. Fenestrations were then created in a standard endovascular graft (Zenith Flex, Cook Medical, Bloomington, IN) using this custom template. The sleeve was slipped over the unsheathed graft and rotated to optimize the positions of the holes relative to the stent struts. The locations of the openings were marked with a pen (Fig 5A) and the fenestrations were cut with an electrocautery device after removing the sleeve (Fig 5B). The graft was re-sheathed and then deployed in the phantom under visual guidance, using alignment of the simulated celiac artery and superior mesenteric artery origins for reference.
Fig 4.
Phantom for fenestration template validation. A, An off-the-shelf model of an AAA that includes the renal arteries. B, Tubes were attached to the AAA phantom to represent the celiac artery and the SMA, and the phantom was embedded in agar in a plastic container. C, The phantom was imaged with a standard CT scanner.
Fig 5.
Graft fenestration and deployment for validation. A, An unsheathed graft is inserted in the fenestration template and the branch vessel origins are marked with a pen. B, Fenestrations are cut with an electrocautery device after removal of the template.
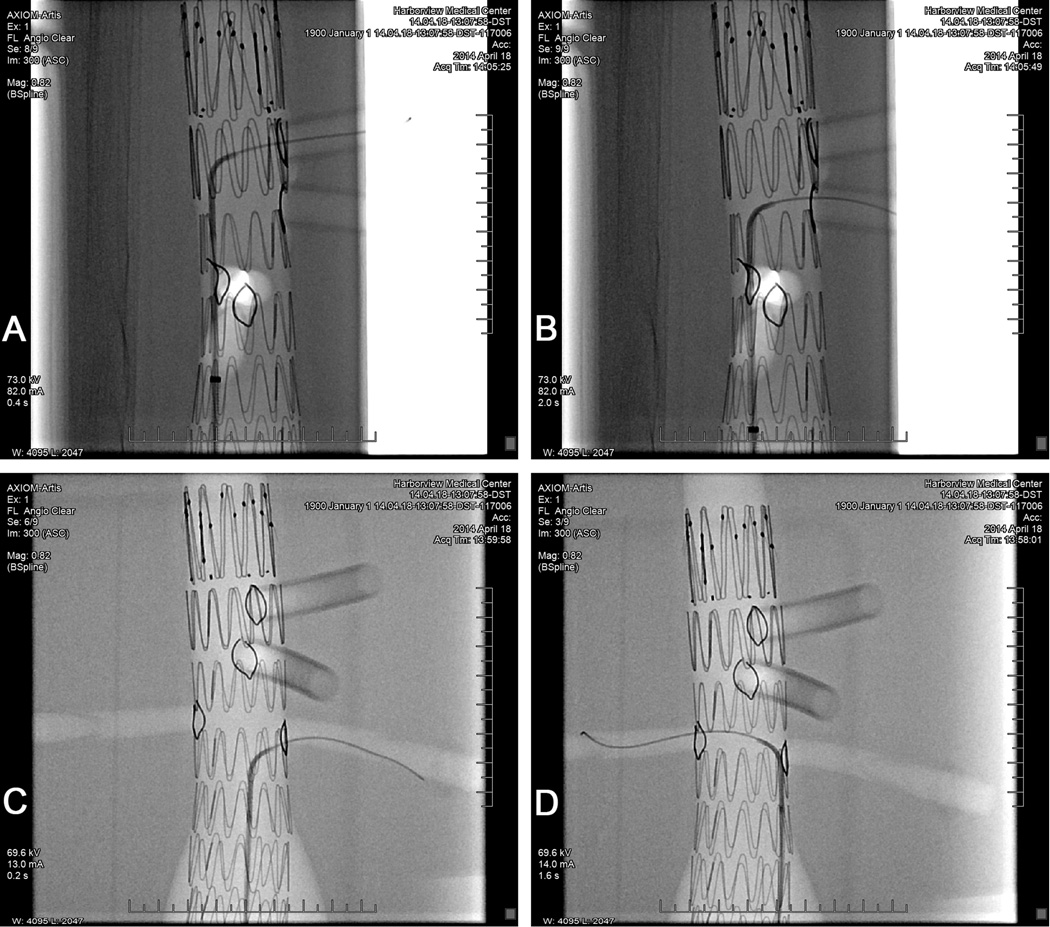
Alignment of the fenestrations was verified by performing continuous fluoroscopy as each branch was targeted with a guidewire introduced through the right iliac branch of the phantom. Guidewires were successfully introduced in each of the four branch vessels (Fig 6), confirming that the graft fenestrations specified by the 3D-printed template were properly aligned with the phantom branch vessel origins.
Fig 6.
Fluoroscopic confirmation of proper alignment of the graft fenestrations with each of the branch vessels in the AAA phantom. A guidewire was successfully deployed through the openings in the fenestrated graft into each of the branch vessels. Gold markers sewn around the graft openings are visible for each fenestration. A, Celiac artery. B, Superior mesenteric artery. C, Left renal artery. D, Right renal artery.
DISCUSSION
We have demonstrated a procedure to create a template based on each patient’s anatomy that provides a simple and accurate guide for on-site placement graft fenestrations. Without the template, fenestration planning time for physician-modified grafts varies with experience and case complexity.7 Early in the experience of one co-author (BWS), the time required for workstation measurements was in the 1–2 hour time frame. After performing over 150 physician-modified endograft procedures, this planning time has decreased to approximately 30 minutes per case. In the operating room, manual measurement of fenestration locations on the graft takes approximately 15 minutes.
The template pre-production time, including manual outlining of the aorta and the subsequent computer processing steps, currently takes approximately 30 minutes. This time could be reduced by incorporating automated image segmentation to generate the lumen outlines. Printing time for the template shown in Fig 3 (6.3 cm long, 2-mm wall thickness) was 9 hours, which would allow production of a template for use the following day. While this current printing time makes the template method impractical for ruptured AAA cases, it would still be applicable to symptomatic cases. Also, it is likely that printing times will decrease in the future as 3D printing technology matures.
In the operating room, use of the template saves time because all of the relative distances of the fenestrations are established by the template; the surgeon is not required to perform any measurements at the time of device deployment. The template transparency allows for rapid assessment of the fenestration locations relative to the stent struts and, most importantly, the potential for measurement errors is eliminated.
Another benefit offered by the 3D-printed template is the potential to shift the pre-operative planning steps from the surgeon to an outside provider. Manufacture at a centralized processing facility, based on electronically-transferred images, would make template guides available to any clinical site performing endovascular repair of AAAs. The raw image data could be sent to the service provider, and the fenestration guide could be shipped back to the surgery site. Clear printing materials are available which are compatible with gas sterilization methods, so templates could either be sterilized before shipment or at the clinical site. Therefore, individual clinical sites would not require expertise with image segmentation, CAD software or 3D printing. This approach could lead to wider use of fenestrated endografts for both elective repairs and treatment of symptomatic AAAs.
CONCLUSIONS
Custom fenestration templates provide for fast and accurate placement of all fenestrations, without the need for manual measurements. The use of the fenestration template with a standard graft provides an “off-the-shelf” solution for endovascular treatment of juxtarenal AAAs. Pending FDA approval, this technique has the potential to save procedural costs and make minimally-invasive AAA repair available to more patients with challenging anatomy.
ACKNOWLEDGEMENT
This work was supported by a grant from NIH/NHLBI (1R21HL113433-01A1).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Western Vascular Society 2014 Annual Meeting, September 20–23, 2014, Coronado, CA, Mini Presentation
Contributor Information
Daniel F. Leotta, University of Washington, Applied Physics Laboratory.
Benjamin W. Starnes, University of Washington, Department of Surgery.
REFERENCES
- 1.United Kingdom EVAR Trial Investigators. Greenhalgh RM, Brown LC, Powell JT, Thompson SG, Epstein D, et al. Endovascular versus open repair of abdominal aortic aneurysm. New Engl J Med. 2010;362:1863–1871. doi: 10.1056/NEJMoa0909305. [DOI] [PubMed] [Google Scholar]
- 2.Schanzer A, Greenberg RK, Hevelone N, Robinson WP, Eslami MH, Goldberg RJ, et al. Predictors of abdominal aortic aneurysm sac enlargement after endovascular repair. Circulation. 2011;123:2848–2255. doi: 10.1161/CIRCULATIONAHA.110.014902. [DOI] [PubMed] [Google Scholar]
- 3.Higashiura W, Nagata T, Tabayashi N, Itoh H, Sakaguchi S, Taniguchi S, et al. Initial experience of branched endovascular graft for abdominal aortic aneurysm with complex anatomy of proximal neck: planning and technical considerations. Jpn J Radiol. 2010;28:66–74. doi: 10.1007/s11604-009-0381-9. [DOI] [PubMed] [Google Scholar]
- 4.Nordon IM, Hinchliffe RJ, Manning B, Ivancev K, Holt PJ, Loftus IM, et al. Toward an "off-the-shelf" fenestrated endograft for management of short-necked abdominal aortic aneurysms: an analysis of current graft morphological diversity. J Endovasc Ther. 2010;17:78–85. doi: 10.1583/09-2895R.1. [DOI] [PubMed] [Google Scholar]
- 5.Resch T, Sonesson B, Malina M. Incidence and management of complications after branched and fenestrated endografting. J Cardiovasc Surg (Torino) 2010;51:105–113. [PubMed] [Google Scholar]
- 6.Starnes BW. Physician-modified endovascular grafts for the treatment of elective, symptomatic, or ruptured juxtarenal aortic aneurysms. J Vasc Surg. 2012;56:601–607. doi: 10.1016/j.jvs.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 7.Starnes BW, Tatum B. Early report from an investigator-initiated investigational device exemption clinical trial on physician-modified endovascular grafts. J Vasc Surg. 2013;58:311–317. doi: 10.1016/j.jvs.2013.01.029. [DOI] [PubMed] [Google Scholar]
- 8.Legget ME, Leotta DF, Bolson EL, McDonald JA, Martin RW, Li XN, et al. System for quantitative three dimensional echocardiography of the left ventricle based on a magnetic field position and orientation sensing system. IEEE Trans Biomed Eng. 1998;45:494–504. doi: 10.1109/10.664205. [DOI] [PubMed] [Google Scholar]
- 9.Causey MW, Jayaraj A, Leotta DF, Paun M, Beach KW, Kohler TR, et al. Three dimensional ultrasound measurements after endovascular aneurysm repair. Ann Vasc Surg. 2013;27:146–153. doi: 10.1016/j.avsg.2012.01.018. [DOI] [PubMed] [Google Scholar]
- 10.Leotta DF, Primozich JF, Beach KW, Bergelin RO, Strandness DE., Jr Serial measurement of cross-sectional area in peripheral vein grafts using three-dimensional ultrasound. Ultrasound Med Biol. 2001;27:61–68. doi: 10.1016/s0301-5629(00)00296-9. [DOI] [PubMed] [Google Scholar]