Abstract
Following infection, naïve CD4 T cells can differentiate into various functionally distinct effector and memory subsets, including T follicular helper (TFH) cells that orchestrate germinal center (GC) reactions necessary for high-affinity, pathogen-specific antibody responses. The origins and function of this cell type have been extensively examined in response to subunit immunization with model antigens. More recently, we are beginning to also appreciate the extent to which microbial infections shape the generation, function and maintenance of TFH cells. Here we review recent advances and highlight additional knowledge gaps in our understanding of how microbial infections influence priming, differentiation, localization and activity of TFH cells following acute and chronic infections.
Introduction
Resolution of infections often depends on the generation of pathogen-specific antibodies. T follicular helper cells (TFH) are key orchestrators of germinal center (GC) reactions, the products of which are plasma cells that secrete high-affinity antibodies that function to resolve primary infection and long-lived memory B cells that afford heightened protection against pathogen re-infection [1*]. Our understanding of the molecular regulation of TFH cell development, function and maintenance is ever expanding and includes well-defined effects of specific cytokines (reviewed in this issue), transcription factors [2], microRNAs [3] and MHCII/TCR interactions [4,5]. By extension, understanding how various microbial infections regulate TFH cell activity remains an important goal. Here, we review recent work that has shaped our current understanding of how TFH responses are regulated during infection. Defining the cellular and molecular processes that govern the activation, function and maintenance of infection-induced TFH cells will ultimately lead to novel strategies to modulate these cells to limit pathogen burden or truncate infection-induced pathologic responses.
Infection-induced modulation of TFH priming and differentiation
Distinct APC may differentially prime TFH responses following infection
Canonical TFH priming is driven by cognate interaction between naïve CD4+ T cells and conventional dendritic cells (cDC) expressing key cytokines (IL-6 in mice and IL-12 in humans) that induce Bcl-6, a transcriptional repressor that promotes expression of CXCR5. CXCR5 endows lymphocytes with the capacity to home to B cell follicles rich in CXCL13. Emerging data highlight how specific infections shape the activation of distinct subsets of APC that may preferentially induce TFH development (Figure 1). During experimental cutaneous Leishmania infection, Langerhans cells facilitate TFH-GC B cell interactions in skin draining lymph nodes, and ablation of Langerin+ cells markedly reduced the number of GC reactions and limited parasite-specific humoral immunity [6]. Recently, targeting antigen to splenic CD169+ marginal zone macrophages triggered long-lived high affinity antibody responses and expanded TFH cells [7], and CD169+ macrophages may be preferentially targeted by some pathogens [8,9]. Notably, in models of systemic LCMV infection, TFH cells are observed by day 2 post-infection, suggesting cDCs are driving this response [10]. In contrast, following IAV infection, a distinct population of CD45+ mononuclear cells undergo CXCR3-dependent migration from the infected lung to the draining lymph nodes with markedly delayed kinetics [11], which coincides with the activation and differentiation of IAV-specific TFH. Adoptive transfer of this APC population was sufficient to accelerate viral clearance, confirming their in vivo relevance to TFH priming. In addition to the initial interactions with DC, or macrophages, new data show that B cells can participate in initial TFH priming [12]. Strikingly, the capacity for B cells to prime TFH differentiation is only apparent after infection, and not protein immunization. Moreover, the requirement of B cells for TFH maintenance may only occur following infection by acute pathogens, because as the infection is resolved antigen becomes limiting. Indeed, when antigen is in excess, B cells can be dispensable for TFH differentiation [13,14]. Finally, the extent to which an infection impacts the biology or activity of antigen presenting cells is also relevant for pathogen re-exposure, as recent work shows that circulating memory TFH cells require interactions with DC in order to potentiate secondary immune responses in vivo [15**]. Thus, modulation of the survival or activity of unique APCs following infection may alter the induction of TFH immunity and pathogen-specific humoral immune responses.
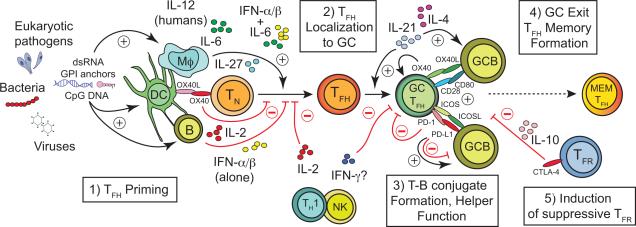
Figure 1.
Acute and chronic infections can impact five key processes that regulate the formation, function and persistence of pathogen-specific T follicular helper (TFH) cells. 1) Infections may induce or limit the activity or survival of unique subsets of antigen presenting cells (APC) bearing capacity to prime TFH responses. TFH priming may also be impacted by specific pathogen-associated molecular patterns (e.g. dsRNA, CpG DNA, glycophosphatidylinositol (GPI) anchors) that may elicit distinct cytokine profiles. APC-secreted cytokines can either promote (IL-12 in humans; IL-27, IL-6 or IL-6 + IFNα/β in mice) or constrain (IL-2 or IFNα/βalone) the differentiation of TFH cells from naïve CD4+ T cell precursors (TN). 2) Many infections cause dysregulation of chemokine expression that coordinates lymphocyte trafficking to or away from follicles (e.g. CXCL13 and CXCL12, CCL19, CCL21, respectively) or controls the architecture of lymphoid tissue (e.g. LTα/β, TNF), thereby limiting the magnitude or quality of TFH-orchestrated germinal center (GC) reactions. The capacity for TFH cells to traffic between GC reactions within a lymph node may also be impacted, although the relative contribution of inter-GC trafficking by TFH cells is less clear. 3) The capacity of TFH to form stable conjugates with antigen-presenting GC B cells (GCB) is necessary to sustain the GC reaction. Infections may alter the secretion of critical cytokines (IL-21, IL-4, IL-2, IFN-γ) or expression of cell surface receptors (ICOS, PD-1, CD80, OX40) and ligands (ICOSL, PD-L1, CD28, OX40L) that mediate these interactions and coordinate the bi-directional communication between TFH and GC B cells. 4) Infections likely impact the formation and stability of memory (MEM) TFH subsets, although the factors that regulate this are not yet clear. 5) Infections also likely trigger the expansion or enhance the suppressive capacity of Foxp3+ follicular regulatory (TFR) cells that impede the development of long-lived memory B cell and secreted antibody responses through specific cytokines (e.g. IL-10) or cell surface receptors and ligands (e.g. CTLA-4). Black arrows and red “T” lines represent factors that either promote or limit TFH and GC B cell responses, respectively.
Infection-induced cytokines can promote and constrain TFH development and activity
Infectious organisms encode and release specific pathogen-associated molecular patterns (PAMPs) that engage pattern recognition receptors (PRRs) on APCs, triggering the release of distinct profiles of cytokines (Figure 1). While PAMPs are widely known to regulate extra-follicular B cell responses following infection or vaccination [16], recent data show that TLR9 signaling in DC and GC B cell numbers and the quantity and quality of secreted antibody [17]. Indeed, the ligand for TLR9 is unmethylated CpG DNA, which is relatively common in bacterial and viral DNA genomes, and TLR9 signaling can lead to the activation and secretion of IL-12 or type I IFN (IFNα/β) which are each known to regulate the priming and activity of TFH cells (discussed below). While this study was limited to an examination of how various PAMPS modulate humoral immunity against model antigens, these data highlight that the nature of the infectious agent may influence priming of TFH responses. Consistent with this, engagement of retinoic acid-inducible gene I (RIG-I), a key PRR for RNA viruses, was recently shown to enhance vaccine-induced humoral immunity [18].
Cytokines play key roles in all phases of TFH cell biology (Figure 1) and several recent studies show specific infections regulate the formation and activity of TFH cells through modulation of cytokine release. IL-6 is key signal for the induction and initial differentiation of TFH, mainly acting through either STAT1 or STAT3 to transactivate Bcl-6 [19,20]. Indeed, STAT3 signaling in T cells is necessary for antiviral humoral immunity and control of chronic LCMV infection [21]. Notably, in that model, STAT3 was dispensable for IFN-γ expressing effector T cell activity, but numbers and frequency of virus-specific Bcl-6+CXCR5+ TFH cells were reduced by 50%. Although IL-6-mediated STAT3 activation and down regulation of CD25 expression (IL-2 signaling) are important for initial TFH differentiation by [19], genetic deficiency of IL-6 does not prevent the eventual development of either TFH or GC responses following acute LCMV infection [22], suggesting that other factors can compensate. IL-21 (or IL-27 discussed below) may serve this compensatory role, as the loss of both IL-6 and IL-21 wholly abrogates TFH and GC B cell responses [23]. SIV infection of macaques is also linked to IL-6 production and expansion of TFH cells [24], although humoral antiviral immunity was not directly examined in those studies. In contrast to the aforementioned studies, IL-6-deficiency in the setting of chronic helminthic infection results in enhanced parasite-specific IgE responses [25], although other aspects of humoral immunity, including TFH responses, were not examined in that study. Collectively, these data highlight the context-specific role of IL-6 in regulating TFH development and activity.
As noted, a related cytokine, IL-27, may also substitute for IL-6 as it can both promote TFH differentiation and trigger STAT3-dependent IL-21 expression by TFH cells during viral infection [26,27]. IL-27 also appears to limit IL-2 expression in effector CD4 T cells [28], which may indirectly promote TFH differentiation because IL-2 and STAT5 signaling are potent negative regulators of TFH development [29,30]. Paradoxically, IL-27 signaling can also activate STAT5. Thus, a critical balance of STAT3 and STAT5 activation likely impacts TFH differentiation. Because IL-2 potently limits TFH development [30], systemic infections associated with relatively high IL-2 expression are therefore likely to sharply dampen TFH responses. Notably, following experimental IAV infection, T regulatory (TREG) cells indirectly promote the formation of GC reactions by consuming excess IL-2 [31**]. It will be of interest to determine whether the ability of TREG to promote TFH differentiation via the consumption of IL-2 is more important for particular types of infection (i.e. localized vs. systemic), or compared to subunit vaccination.
Type I IFN (IFNα/β) are induced by many pathogens and this family of cytokines has varying effects on TFH development. Type I IFN were recently shown to induce Bcl-6, CXCR5 and PD-1, but not IL-21, in CD4 T cells [32], suggesting that type I IFN may promote CD4 T cells to adopt a TFH phenotype. On the other hand, following LCMV infection, IFNα/β signaling directly represses TFH development [33]. In that model, TFH differentiation required STAT3 signaling and in CD4 T cells lacking STAT3, blockade of type I IFN signaling restores the defective TFH response [33]. Adding to the complexity, the timing of either T cell priming or type I IFN signaling following infection may profoundly impact TFH differentiation. CD4 T cells primed during an established persistent infection are less likely to become TH1 cells and almost exclusively develop into TFH cells, a process that requires type I IFN signaling [34*]. Clearly the context of type I IFN signaling determines whether it promotes or constrains TFH development. Type II IFN (IFN-γ) has also been linked to regulating TFH development and activity. Excessive IFN-γ is reported to drive pathologically large TFH responses that contribute to autoimmunity [35]. Conversely, IFN-γ is known to transiently down regulate the expression of CXCL13 and disrupt trafficking of DC and lymphocytes in reactive lymphoid tissue [36]. Moreover, IFN-γ can function in a STAT1-dependent feed-forward loop to activate T-bet [37], which can directly interact with and limit the activity of Bcl-6 [38]. Thus, while promoting TFH development in a genetic model, IFN-γ may restrict the formation or maintenance of TFH during infection. Consistent with the latter, we have observed that IFN-γ can limit TFH and GC B cell responses during blood stage Plasmodium infection (Butler and Zander et al., submitted). Collectively, these reports underscore that distinct APC subsets and specific cytokines shape whether pathogen-specific CD4 T cells adopt a TFH fate and that developing strategies to manipulate these pathways could improve outcomes following infection.
Modulation of TFH trafficking and localization during infection
Following priming by DC, CXCR5-dependent anatomic repositioning of TFH cells into B cell follicles is essential for orchestration of the GC reaction. In addition to CXCL13, TFH motility is regulated by ICOS-ICOSL interactions between TFH and non-cognate B cells at the T-B boarder, which potentiates TFH migration into the follicle [39]. Once in the follicle, TFH activity depends on cognate interactions with B cells, which further reinforces TFH differentiation and function [40,41]. Each step of TFH activation and differentiation critically depends on cell-cell interactions within discreet anatomic structures of lymphoid tissue. Thus, infections that disrupt the organization of lymphoid tissues can negatively impact humoral immunity. Toxoplasma infection dysregulates expression of cytokines that position cells in lymphoid tissue (e.g. LTα and LTβ delays the kinetics of the anti-parasitic antibody response [42]. Experimental malaria models also reveal profound disruption of splenic architecture with impacts on the quality of the parasite-specific antibody response [43]. LPS and associated gram negative bacterial infections also markedly alter cellular organization in lymphoid tissues; infection with Salmonella disrupts lymphoid architecture via dysregulation of chemokine gradients [44]. These observations are notable as trafficking and localization of TFH cells may also determine their relative B cell helping capacity [45], as has been observed following IAV infection [46]. Together, these data underscore that infections that disrupt the organization and homing of cell to lymphoid tissue can directly impact the formation of TFH-regulated antibody responses.
Alteration of TFH-GC B cell conjugates and helper function during infection
TFH engage in bi-directional communication with GC B cells via secreted factors (e.g. IL-21 and IL-4) and cell surface expressed co-stimulatory and co-inhibitory receptors. CD28 is essential for naïve CD4 T cell priming and activation, but new data show that CD28 is also critical for the differentiation and maintenance of TFH cells responding to viral infection [47]. Another costimulatory receptor, OX40, is required for antiviral humoral immunity [48]; however, administration of OX40 agonists early after viral infection halts TFH differentiation [49], suggesting that either the timing or context of OX40 signaling critically regulates TFH differentiation (Figure 1). The co-inhibitory receptor PD-1 is widely used to identify TFH cells, but it also regulates TFH activity. Following vaccination, the absence of PD-1 signaling diminishes the quantity of antigen-specific antibody but enhances the affinity [50]. In contrast, following infection with either helminthes [51] or protozoan parasites [52], disrupting association of PD-1 with its major ligand PD-L1 markedly enhances pathogen-specific antibody responses. Consistent with this, Cubas et al [53**] recently reported higher frequencies of PD-L1 expressing B cells in lymph nodes of HIV-infected individuals and that engagement of PD-1 on TFH suppressed proliferation and expression of ICOS and IL-21. Of note, following vaccinia virus infection, the loss of CD80, but not CD86, on follicular B cells profoundly inhibited TFH and neutralizing antibody responses [54]. It is worth noting that CD80 is an alternative ligand for the PD-L1. Thus, whether the PD-1:PD-L1:CD80 axis differentially regulates TFH function following infection by distinct microbes remains an important question. Finally, inducible deletion of the co-inhibitory receptor CTLA-4 in T cells resulted in TFH expansion and enhancement of antigen-specific B cell and secreted Ab responses [55,56**]. Although this work was restricted to subunit vaccination, these data further support that co-inhibitory molecules can profoundly regulate TFH cell activity in the GC. These data also argue that compared to vaccination, infection may change the relative role of molecules that regulate TFH-GC B cell interactions. This is in line with observations showing Bcl-6−/− mice fail to form sizable and stable CXCR5+ TFH populations following acute Listeria monocytogenes infection [57], but CXCR5+ CD4 T cells develop normally in Bcl-6−/− mice following peptide vaccination [58]. Thus, the contribution of known regulators of TFH activity may depend on the nature of the infection and it will be of particular interest to understand how various infections alter circuits of communication between TFH and GC B cells.
Modulation of TFH plasticity and ‘memory’ formation during infection
A large body of work supports that TFH development is not solely driven by the activity of a single “master” transcription factor (i.e. Bcl-6) and the differentiation of TFH cells is shaped by the composite of cooperative and antagonistic factors (reviewed in [1]). From this perspective, infections may differentially impact both TFH plasticity and the capacity of TFH to form memory subsets. Indeed, TFH cells retain chromatin marks consistent with their ability to revert to TH1, TH2 and TH17 cell differentiation patterns [59] and schistosome-specific TFH cells differentiate from IL-4+GATA-3+ TH2 cells [60], suggesting that TFH cells retain a relatively high degree of plasticity and functional diversity. In contrast, other data show that CD4 T cells “remember” their previous lineage pathway, exhibit evidence of having committed to either TH1 or TFH lineage differentiation and assume their original phenotype and function during secondary immune responses [61**]. Indeed, whether TFH form functional memory populations following infection is an area of intense focus. One of the first reports that show formation of TFH memory cells following infection utilized an IL-21 reporter mouse. In that study, IL-21+ TFH cells formed long-lived populations that could adopt either conventional TH1 effector activity or retain TFH activity during recall responses [62], further supporting the relative plasticity of memory TFH. Circulating memory TFH have been identified and have been shown to be more potent inducers of secondary immune responses compared to primary effector TFH cells [15**]. In some HIV infected individuals, circulating populations of memory-like TFH cells exhibit high functional activity ex vivo and their numbers strongly correlate with broadly neutralizing antibody responses [63]. Of note, cells purported to be TFH precursors, which exhibit a CCR7loPD-1hi phenotype, were recently identified [64**]. Strikingly, these cells appear in the circulation prior to the formation of GC reactions and it was argued these TFH precursors might circulate to non-draining lymph nodes positioning them to rapidly mount humoral immunity should an infection become systemic. The formation, stability and participation of infection-induced circulating memory TFH cells warrant further investigation.
Chronic infections shape TFH development and activity
Chronic HIV, parasitic and bacterial infections significantly impact human health and understanding the extent to which chronic infections regulate TFH cell activity is of interest. In general, data support that persistent infections direct CD4 T cells towards a TFH developmental pathway [65*]. Late expression of IL-6 appears to instruct this developmental redirection during chronic LCMV infection [20]. Moreover, the persistence/density of antigen [41,66,67], DC-T cell dwell time [66] and overall APC-T cell interaction affinity [4] have each been implicated in regulating TFH differentiation or function. Despite data showing that sustained antigenic stimulation promotes TFH development, chronic HIV infection is associated with impaired TFH responses [68]. Moreover, a study in Leishmania-infected macaques showed that as infection transitions from acute to chronic TFH responses undergo contraction and parasite-specific antibody titers wane rapidly [69], arguing that the lack of TFH cell maintenance may underlie inefficient humoral immunity during chronic visceral leishmaniasis. Chronic Litomosoides sigmodontis infection also causes long-term disruption of T-dependent antibody responses linked to reduced frequencies and numbers of TFH cells [70]. Although the exact cellular and molecular mechanisms were not established in the L. sigmodontis model, the induction of regulatory cells was postulated to constrain the induction of humoral immunity. Chronic bacterial infections are also linked to reduced TFH activity. Borrelia bergdorferi infection is associated with dysfunctional GC reactions [71], and recent data show that although B. bergdorferi-specific TFH cells are induced, they only support short-lived antibody responses [72]. While there are conflicting data regarding whether antibody responses are critical for limiting Mycobacterium tuberculosis (Mtb) infection, in murine models, CD4+CXCR5+ T cells accumulate in the Mtb-infected lung and exhibit features of both TFH and TH1 cells [73]. These cells respond to CXCL13, localize within the lung parenchyma and orchestrate the formation of lymphoid follicles within the granuloma to provide optimal control of Mtb. Consistent with this, CXCR5+ B cells and plasma cells secreting MtB-specific antibody are found within granulomas in infected macaques [74]. Finally, emerging evidence suggests that co-infection may also profoundly influence the activity of TFH cells and subsequent pathogen-specific antibody responses [75]. The extent to which medically important chronic infections shape the formation and function of effector and memory TFH cells is only beginning to be understood.
Conclusions
TFH cells are essential for helping B cells produce antibodies that limit microbial infection. APC activity, cytokines, cell trafficking and communication with GC B cells regulate the differentiation, function and formation of effector and memory TFH cells. Recent studies are beginning to reveal how acute and chronic infections impact each facet of TFH development, as well as their plasticity and their capacity to form stable memory populations. However, numerous questions remain. For example, the full extent to which major human pathogens (e.g. Plasmodium and HIV) limit TFH development and function is of significant interest. Indeed, these and other infections that fail to induce long-lived memory B cells and efficacious antibody responses may be linked to direct impacts on TFH biology. Moreover, the relative role and contribution of Foxp3+ T follicular regulatory (TFR) cells [76] during infection warrants investigation. A thorough understanding of the molecular and cellular circuits that regulate TFH activity during infection will help identify opportunities for the treatment of infectious disease.
Highlights.
Infections impact multiple phases of TFH differentiation
Distinct populations of APC may differentially prime pathogen-specific TFH cells
TFH localization and function are influenced by infection
Chronic infections differentially impact TFH -mediated immunity
Acknowledgements
This work was supported by grants from the National Institutes of Health (1K22AI099070 to N.S.B.) and the American Heart Association (13BGIA17140002 to N.S.B.). N.S.B. is also an Oklahoma IDeA Network of Biomedical Research Excellence scholar supported by a grant from the National Institute of General Medical Sciences (8P20GM103447). The authors apologize for not citing all relevant publications due to space limitations.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
* of special interest
** of outstanding interest
- 1*.Crotty S. T follicular helper cell differentiation, function, and roles in disease. Immunity. 2014;41:529–542. doi: 10.1016/j.immuni.2014.10.004. [A thorough and authoritative review on the molecular and cellular factors that govern the biology of TFH cells.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weinmann AS. Regulatory mechanisms that control T-follicular helper and T-helper 1 cell flexibility. Immunol Cell Biol. 2014;92:34–39. doi: 10.1038/icb.2013.49. [DOI] [PubMed] [Google Scholar]
- 3.Baumjohann D, Ansel KM. MicroRNA regulation of the germinal center response. Curr Opin Immunol. 2014;28:6–11. doi: 10.1016/j.coi.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moalli F, Cupovic J, Thelen F, Halbherr P, Fukui Y, Narumiya S, Ludewig B, Stein JV. Thromboxane A2 acts as tonic immunoregulator by preferential disruption of low- avidity CD4+ T cell-dendritic cell interactions. J Exp Med. 2014;211:2507–2517. doi: 10.1084/jem.20140137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keck S, Schmaler M, Ganter S, Wyss L, Oberle S, Huseby ES, Zehn D, King CG. Antigen affinity and antigen dose exert distinct influences on CD4 T-cell differentiation. Proc Natl Acad Sci U S A. 2014;111:14852–14857. doi: 10.1073/pnas.1403271111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zimara N, Florian C, Schmid M, Malissen B, Kissenpfennig A, Mannel DN, Edinger M, Hutchinson JA, Hoffmann P, Ritter U. Langerhans cells promote early germinal center formation in response to Leishmania-derived cutaneous antigens. Eur J Immunol. 2014;44:2955–2967. doi: 10.1002/eji.201344263. [DOI] [PubMed] [Google Scholar]
- 7.Veninga H, Borg EG, Vreeman K, Taylor PR, Kalay H, van Kooyk Y, Kraal G, Martinez- Pomares L, den Haan JM. Antigen targeting reveals splenic CD169 macrophages as promoters of germinal center B-cell responses. Eur J Immunol. 2014 doi: 10.1002/eji.201444983. published online: 14 JAN 2015 DOI: 10.1002/eji.201444983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chtanova T, Han SJ, Schaeffer M, van Dooren GG, Herzmark P, Striepen B, Robey EA. Dynamics of T cell, antigen-presenting cell, and pathogen interactions during recall responses in the lymph node. Immunity. 2009;31:342–355. doi: 10.1016/j.immuni.2009.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Honke N, Shaabani N, Cadeddu G, Sorg UR, Zhang DE, Trilling M, Klingel K, Sauter M, Kandolf R, Gailus N, et al. Enforced viral replication activates adaptive immunity and is essential for the control of a cytopathic virus. Nat Immunol. 2012;13:51–57. doi: 10.1038/ni.2169. [DOI] [PubMed] [Google Scholar]
- 10.Choi YS, Kageyama R, Eto D, Escobar TC, Johnston RJ, Monticelli L, Lao C, Crotty S. ICOS receptor instructs T follicular helper cell versus effector cell differentiation via induction of the transcriptional repressor Bcl6. Immunity. 2011;34:932–946. doi: 10.1016/j.immuni.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoo JK, Fish EN, Braciale TJ. LAPCs promote follicular helper T cell differentiation of Ag-primed CD4+ T cells during respiratory virus infection. J Exp Med. 2012;209:1853–1867. doi: 10.1084/jem.20112256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barnett LG, Simkins HM, Barnett BE, Korn LL, Johnson AL, Wherry EJ, Wu GF, Laufer TM. B cell antigen presentation in the initiation of follicular helper T cell and germinal center differentiation. J Immunol. 2014;192:3607–3617. doi: 10.4049/jimmunol.1301284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goenka R, Barnett LG, Silver JS, O'Neill PJ, Hunter CA, Cancro MP, Laufer TM. Cutting edge: dendritic cell-restricted antigen presentation initiates the follicular helper T cell program but cannot complete ultimate effector differentiation. J Immunol. 2011;187:1091–1095. doi: 10.4049/jimmunol.1100853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deenick EK, Chan A, Ma CS, Gatto D, Schwartzberg PL, Brink R, Tangye SG. Follicular helper T cell differentiation requires continuous antigen presentation that is independent of unique B cell signaling. Immunity. 2010;33:241–253. doi: 10.1016/j.immuni.2010.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15**.Sage PT, Alvarez D, Godec J, von Andrian UH, Sharpe AH. Circulating T follicular regulatory and helper cells have memory-like properties. J Clin Invest. 2014;124:5191–5204. doi: 10.1172/JCI76861. [This study identified that the functional participation of memory TFH cell during secondary responses is linked to additional with interactions with DC. This study also demonstrates that circulating memory-like TFH cells exhibit enhanced B cell helper function compared to effector TFH cells.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.MacLennan IC, Toellner KM, Cunningham AF, Serre K, Sze DM, Zuniga E, Cook MC, Vinuesa CG. Extrafollicular antibody responses. Immunol Rev. 2003;194:8–18. doi: 10.1034/j.1600-065x.2003.00058.x. [DOI] [PubMed] [Google Scholar]
- 17.Rookhuizen DC, DeFranco AL. Toll-like receptor 9 signaling acts on multiple elements of the germinal center to enhance antibody responses. Proc Natl Acad Sci U S A. 2014;111:E3224–3233. doi: 10.1073/pnas.1323985111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kulkarni RR, Rasheed MA, Bhaumik SK, Ranjan P, Cao W, Davis C, Marisetti K, Thomas S, Gangappa S, Sambhara S, et al. Activation of the RIG-I Pathway during Influenza Vaccination Enhances the Germinal Center Reaction, Promotes T Follicular Helper Cell Induction, and Provides a Dose-Sparing Effect and Protective Immunity. J Virol. 2014;88:13990–14001. doi: 10.1128/JVI.02273-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choi YS, Eto D, Yang JA, Lao C, Crotty S. Cutting edge: STAT1 is required for IL-6- mediated Bcl6 induction for early follicular helper cell differentiation. J Immunol. 2013;190:3049–3053. doi: 10.4049/jimmunol.1203032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harker JA, Lewis GM, Mack L, Zuniga EI. Late interleukin-6 escalates T follicular helper cell responses and controls a chronic viral infection. Science. 2011;334:825–829. doi: 10.1126/science.1208421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McIlwain DR, Grusdat M, Pozdeev VI, Xu HC, Shinde P, Reardon C, Hao Z, Beyer M, Bergthaler A, Haussinger D, et al. T-cell STAT3 is required for the maintenance of humoral immunity to LCMV. Eur J Immunol. 2014 doi: 10.1002/eji.201445060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poholek AC, Hansen K, Hernandez SG, Eto D, Chandele A, Weinstein JS, Dong X, Odegard JM, Kaech SM, Dent AL, et al. In vivo regulation of Bcl6 and T follicular helper cell development. J Immunol. 2010;185:313–326. doi: 10.4049/jimmunol.0904023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eto D, Lao C, DiToro D, Barnett B, Escobar TC, Kageyama R, Yusuf I, Crotty S. IL-21 and IL-6 are critical for different aspects of B cell immunity and redundantly induce optimal follicular helper CD4 T cell (Tfh) differentiation. PLoS One. 2011;6:e17739. doi: 10.1371/journal.pone.0017739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petrovas C, Yamamoto T, Gerner MY, Boswell KL, Wloka K, Smith EC, Ambrozak DR, Sandler NG, Timmer KJ, Sun X, et al. CD4 T follicular helper cell dynamics during SIV infection. J Clin Invest. 2012;122:3281–3294. doi: 10.1172/JCI63039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith KA, Maizels RM. IL-6 controls susceptibility to helminth infection by impeding Th2 responsiveness and altering the Treg phenotype in vivo. Eur J Immunol. 2014;44:150–161. doi: 10.1002/eji.201343746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Batten M, Ramamoorthi N, Kljavin NM, Ma CS, Cox JH, Dengler HS, Danilenko DM, Caplazi P, Wong M, Fulcher DA, et al. IL-27 supports germinal center function by enhancing IL-21 production and the function of T follicular helper cells. J Exp Med. 2010;207:2895–2906. doi: 10.1084/jem.20100064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harker JA, Dolgoter A, Zuniga EI. Cell-intrinsic IL-27 and gp130 cytokine receptor signaling regulates virus-specific CD4(+) T cell responses and viral control during chronic infection. Immunity. 2013;39:548–559. doi: 10.1016/j.immuni.2013.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Villarino AV, Stumhofer JS, Saris CJ, Kastelein RA, de Sauvage FJ, Hunter CA. IL-27 limits IL-2 production during Th1 differentiation. J Immunol. 2006;176:237–247. doi: 10.4049/jimmunol.176.1.237. [DOI] [PubMed] [Google Scholar]
- 29.Johnston RJ, Choi YS, Diamond JA, Yang JA, Crotty S. STAT5 is a potent negative regulator of TFH cell differentiation. J Exp Med. 2012;209:243–250. doi: 10.1084/jem.20111174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ballesteros-Tato A, Leon B, Graf BA, Moquin A, Adams PS, Lund FE, Randall TD. Interleukin-2 inhibits germinal center formation by limiting T follicular helper cell differentiation. Immunity. 2012;36:847–856. doi: 10.1016/j.immuni.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31**.Leon B, Bradley JE, Lund FE, Randall TD, Ballesteros-Tato A. FoxP3+ regulatory T cells promote influenza-specific Tfh responses by controlling IL-2 availability. Nat Commun. 2014;5:3495. doi: 10.1038/ncomms4495. [This study demonstrates that T regulatory unexpectedly promote virus-specific TFH responses by consuming IL-2, a potent negative regulator of TFH development.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakayamada S, Poholek AC, Lu KT, Takahashi H, Kato M, Iwata S, Hirahara K, Cannons JL, Schwartzberg PL, Vahedi G, et al. Type I IFN induces binding of STAT1 to Bcl6: divergent roles of STAT family transcription factors in the T follicular helper cell genetic program. J Immunol. 2014;192:2156–2166. doi: 10.4049/jimmunol.1300675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ray JP, Marshall HD, Laidlaw BJ, Staron MM, Kaech SM, Craft J. Transcription factor STAT3 and type I interferons are corepressive insulators for differentiation of follicular helper and T helper 1 cells. Immunity. 2014;40:367–377. doi: 10.1016/j.immuni.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34*.Osokine I, Snell LM, Cunningham CR, Yamada DH, Wilson EB, Elsaesser HJ, de la Torre JC, Brooks D. Type I interferon suppresses de novo virus-specific CD4 Th1 immunity during an established persistent viral infection. Proc Natl Acad Sci U S A. 2014;111:7409–7414. doi: 10.1073/pnas.1401662111. [This study identified that CD4 T cells primed during an ongoing, persistent virus infection preferentially differentiated into TFH cells, a process that was regulated by type I interferon.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee SK, Silva DG, Martin JL, Pratama A, Hu X, Chang PP, Walters G, Vinuesa CG. Interferon-gamma excess leads to pathogenic accumulation of follicular helper T cells and germinal centers. Immunity. 2012;37:880–892. doi: 10.1016/j.immuni.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 36.Mueller SN, Hosiawa-Meagher KA, Konieczny BT, Sullivan BM, Bachmann MF, Locksley RM, Ahmed R, Matloubian M. Regulation of homeostatic chemokine expression and cell trafficking during immune responses. Science. 2007;317:670–674. doi: 10.1126/science.1144830. [DOI] [PubMed] [Google Scholar]
- 37.Djuretic IM, Levanon D, Negreanu V, Groner Y, Rao A, Ansel KM. Transcription factors T- bet and Runx3 cooperate to activate Ifng and silence Il4 in T helper type 1 cells. Nat Immunol. 2007;8:145–153. doi: 10.1038/ni1424. [DOI] [PubMed] [Google Scholar]
- 38.Oestreich KJ, Mohn SE, Weinmann AS. Molecular mechanisms that control the expression and activity of Bcl-6 in TH1 cells to regulate flexibility with a TFH-like gene profile. Nat Immunol. 2012;13:405–411. doi: 10.1038/ni.2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu H, Li X, Liu D, Li J, Zhang X, Chen X, Hou S, Peng L, Xu C, Liu W, et al. Follicular T- helper cell recruitment governed by bystander B cells and ICOS-driven motility. Nature. 2013;496:523–527. doi: 10.1038/nature12058. [DOI] [PubMed] [Google Scholar]
- 40.Johnston RJ, Poholek AC, DiToro D, Yusuf I, Eto D, Barnett B, Dent AL, Craft J, Crotty S. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science. 2009;325:1006–1010. doi: 10.1126/science.1175870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baumjohann D, Preite S, Reboldi A, Ronchi F, Ansel KM, Lanzavecchia A, Sallusto F. Persistent antigen and germinal center B cells sustain T follicular helper cell responses and phenotype. Immunity. 2013;38:596–605. doi: 10.1016/j.immuni.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 42.Glatman Zaretsky A, Silver JS, Siwicki M, Durham A, Ware CF, Hunter CA. Infection with Toxoplasma gondii alters lymphotoxin expression associated with changes in splenic architecture. Infect Immun. 2012;80:3602–3610. doi: 10.1128/IAI.00333-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cadman ET, Abdallah AY, Voisine C, Sponaas AM, Corran P, Lamb T, Brown D, Ndungu F, Langhorne J. Alterations of splenic architecture in malaria are induced independently of Toll-like receptors 2, 4, and 9 or MyD88 and may affect antibody affinity. Infect Immun. 2008;76:3924–3931. doi: 10.1128/IAI.00372-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.St John AL, Abraham SN. Salmonella disrupts lymph node architecture by TLR4- mediated suppression of homeostatic chemokines. Nat Med. 2009;15:1259–1265. doi: 10.1038/nm.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shulman Z, Gitlin AD, Targ S, Jankovic M, Pasqual G, Nussenzweig MC, Victora GD. T follicular helper cell dynamics in germinal centers. Science. 2013;341:673–677. doi: 10.1126/science.1241680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Elsner RA, Ernst DN, Baumgarth N. Single and coexpression of CXCR4 and CXCR5 identifies CD4 T helper cells in distinct lymph node niches during influenza virus infection. J Virol. 2012;86:7146–7157. doi: 10.1128/JVI.06904-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Linterman MA, Denton AE, Divekar DP, Zvetkova I, Kane L, Ferreira C, Veldhoen M, Clare S, Dougan G, Espeli M, et al. CD28 expression is required after T cell priming for helper T cell responses and protective immunity to infection. Elife. 2014;3 doi: 10.7554/eLife.03180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boettler T, Moeckel F, Cheng Y, Heeg M, Salek-Ardakani S, Crotty S, Croft M, von Herrath MG. OX40 facilitates control of a persistent virus infection. PLoS Pathog. 2012;8:e1002913. doi: 10.1371/journal.ppat.1002913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boettler T, Choi YS, Salek-Ardakani S, Cheng Y, Moeckel F, Croft M, Crotty S, von Herrath M. Exogenous OX40 stimulation during lymphocytic choriomeningitis virus infection impairs follicular Th cell differentiation and diverts CD4 T cells into the effector lineage by upregulating Blimp-1. J Immunol. 2013;191:5026–5035. doi: 10.4049/jimmunol.1300013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Good-Jacobson KL, Szumilas CG, Chen L, Sharpe AH, Tomayko MM, Shlomchik MJ. PD-1 regulates germinal center B cell survival and the formation and affinity of long- lived plasma cells. Nat Immunol. 2010;11:535–542. doi: 10.1038/ni.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hams E, McCarron MJ, Amu S, Yagita H, Azuma M, Chen L, Fallon PG. Blockade of B7- H1 (programmed death ligand 1) enhances humoral immunity by positively regulating the generation of T follicular helper cells. J Immunol. 2011;186:5648–5655. doi: 10.4049/jimmunol.1003161. [DOI] [PubMed] [Google Scholar]
- 52.Butler NS, Moebius J, Pewe LL, Traore B, Doumbo OK, Tygrett LT, Waldschmidt TJ, Crompton PD, Harty JT. Therapeutic blockade of PD-L1 and LAG-3 rapidly clears established blood-stage Plasmodium infection. Nat Immunol. 2012;13:188–195. doi: 10.1038/ni.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53**.Cubas RA, Mudd JC, Savoye AL, Perreau M, van Grevenynghe J, Metcalf T, Connick E, Meditz A, Freeman GJ, Abesada-Terk G, Jr., et al. Inadequate T follicular cell help impairs B cell immunity during HIV infection. Nat Med. 2013;19:494–499. doi: 10.1038/nm.3109. [This study identified that HIV infection is associated with functional impairement of TFH cells linked to GC B cells expessing PD-L1.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Salek-Ardakani S, Choi YS, Rafii-El-Idrissi Benhnia M, Flynn R, Arens R, Shoenberger S, Crotty S, Croft M, Salek-Ardakani S. B cell-specific expression of B7-2 is required for follicular Th cell function in response to vaccinia virus. J Immunol. 2011;186:5294–5303. doi: 10.4049/jimmunol.1100406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sage PT, Paterson AM, Lovitch SB, Sharpe AH. The coinhibitory receptor ctla-4 controls B cell responses by modulating T follicular helper, T follicular regulatory, and T regulatory cells. Immunity. 2014;41:1026–1039. doi: 10.1016/j.immuni.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56**.Wing JB, Ise W, Kurosaki T, Sakaguchi S. Regulatory T Cells Control Antigen-Specific Expansion of Tfh Cell Number and Humoral Immune Responses via the Coreceptor CTLA-4. Immunity. 2014;41:1013–1025. doi: 10.1016/j.immuni.2014.12.006. [Together with reference 55, these studies demonstrate that the co-inhibitory receptor CTLA-4 expressed by TFH, TFR and TREG cells modulates the magnitude and quality of GC reactions.] [DOI] [PubMed] [Google Scholar]
- 57.Pepper M, Pagan AJ, Igyarto BZ, Taylor JJ, Jenkins MK. Opposing signals from the Bcl6 transcription factor and the interleukin-2 receptor generate T helper 1 central and effector memory cells. Immunity. 2011;35:583–595. doi: 10.1016/j.immuni.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu X, Yan X, Zhong B, Nurieva RI, Wang A, Wang X, Martin-Orozco N, Wang Y, Chang SH, Esplugues E, et al. Bcl6 expression specifies the T follicular helper cell program in vivo. J Exp Med. 2012209:1841–1852. S1841–1824. doi: 10.1084/jem.20120219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lu KT, Kanno Y, Cannons JL, Handon R, Bible P, Elkahloun AG, Anderson SM, Wei L, Sun H, O'Shea JJ, et al. Functional and epigenetic studies reveal multistep differentiation and plasticity of in vitro-generated and in vivo-derived follicular T helper cells. Immunity. 2011;35:622–632. doi: 10.1016/j.immuni.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Glatman Zaretsky A, Taylor JJ, King IL, Marshall FA, Mohrs M, Pearce EJ. T follicular helper cells differentiate from Th2 cells in response to helminth antigens. J Exp Med. 2009;206:991–999. doi: 10.1084/jem.20090303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61**.Hale JS, Youngblood B, Latner DR, Mohammed AU, Ye L, Akondy RS, Wu T, Iyer SS, Ahmed R. Distinct memory CD4+ T cells with commitment to T follicular helper- and T helper 1-cell lineages are generated after acute viral infection. Immunity. 2013;38:805–817. doi: 10.1016/j.immuni.2013.02.020. [This study demonstrates that memory CD4 T cells, including TFH cells, may be fate- committed and therefore poised to re-acquire lineage-specific effector function following antigen re-encounter.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Luthje K, Kallies A, Shimohakamada Y, Belz GT, Light A, Tarlinton DM, Nutt SL. The development and fate of follicular helper T cells defined by an IL-21 reporter mouse. Nat Immunol. 2012;13:491–498. doi: 10.1038/ni.2261. [DOI] [PubMed] [Google Scholar]
- 63.Locci M, Havenar-Daughton C, Landais E, Wu J, Kroenke MA, Arlehamn CL, Su LF, Cubas R, Davis MM, Sette A, et al. Human circulating PD-1+CXCR3-CXCR5+ memory Tfh cells are highly functional and correlate with broadly neutralizing HIV antibody responses. Immunity. 2013;39:758–769. doi: 10.1016/j.immuni.2013.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64**.He J, Tsai LM, Leong YA, Hu X, Ma CS, Chevalier N, Sun X, Vandenberg K, Rockman S, Ding Y, et al. Circulating precursor CCR7(lo)PD-1(hi) CXCR5(+) CD4(+) T cells indicate Tfh cell activity and promote antibody responses upon antigen reexposure. Immunity. 2013;39:770–781. doi: 10.1016/j.immuni.2013.09.007. [This study describes the appearance of putaive TFH precursor cells in the circulation prior to the formation of GC reactions, a process that may enhance the ability to more rapidly and effectively control systemic infeciton.] [DOI] [PubMed] [Google Scholar]
- 65*.Fahey LM, Wilson EB, Elsaesser H, Fistonich CD, McGavern DB, Brooks DG. Viral persistence redirects CD4 T cell differentiation toward T follicular helper cells. J Exp Med. 2011;208:987–999. doi: 10.1084/jem.20101773. [This study identified that CD4 T cells primed during an established persistent infection are directed toward a program of TFH differentiation.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tubo NJ, Pagan AJ, Taylor JJ, Nelson RW, Linehan JL, Ertelt JM, Huseby ES, Way SS, Jenkins MK. Single naive CD4+ T cells from a diverse repertoire produce different effector cell types during infection. Cell. 2013;153:785–796. doi: 10.1016/j.cell.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fazilleau N, McHeyzer-Williams LJ, Rosen H, McHeyzer-Williams MG. The function of follicular helper T cells is regulated by the strength of T cell antigen receptor binding. Nat Immunol. 2009;10:375–384. doi: 10.1038/ni.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pallikkuth S, Parmigiani A, Silva SY, George VK, Fischl M, Pahwa R, Pahwa S. Impaired peripheral blood T-follicular helper cell function in HIV-infected nonresponders to the 2009 H1N1/09 vaccine. Blood. 2012120:985–993. doi: 10.1182/blood-2011-12-396648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rodrigues V, Laforge M, Campillo-Gimenez L, Soundaramourty C, Correia-de-Oliveira A, Dinis-Oliveira RJ, Ouaissi A, Cordeiro-da-Silva A, Silvestre R, Estaquier J. Abortive T follicular helper development is associated with a defective humoral response in Leishmania infantum-infected macaques. PLoS Pathog. 2014;10:e1004096. doi: 10.1371/journal.ppat.1004096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Haben I, Hartmann W, Breloer M. Nematode-induced interference with vaccination efficacy targets follicular T helper cell induction and is preserved after termination of infection. PLoS Negl Trop Dis. 2014;8:e3170. doi: 10.1371/journal.pntd.0003170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hastey CJ, Elsner RA, Barthold SW, Baumgarth N. Delays and diversions mark the development of B cell responses to Borrelia burgdorferi infection. J Immunol. 2012;188:5612–5622. doi: 10.4049/jimmunol.1103735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Elsner RA, Hastey CJ, Baumgarth N. CD4+ T Cells Promote Antibody Production but Not Sustained Affinity Maturation during Borrelia burgdorferi Infection. Infect Immun. 2015;83:48–56. doi: 10.1128/IAI.02471-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Slight SR, Rangel-Moreno J, Gopal R, Lin Y, Fallert Junecko BA, Mehra S, Selman M, Becerril-Villanueva E, Baquera-Heredia J, Pavon L, et al. CXCR5(+) T helper cells mediate protective immunity against tuberculosis. J Clin Invest. 2013;123:712–726. doi: 10.1172/JCI65728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Phuah JY, Mattila JT, Lin PL, Flynn JL. Activated B cells in the granulomas of nonhuman primates infected with Mycobacterium tuberculosis. Am J Pathol. 2012;181:508–514. doi: 10.1016/j.ajpath.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wolf AI, Strauman MC, Mozdzanowska K, Whittle JR, Williams KL, Sharpe AH, Weiser JN, Caton AJ, Hensley SE, Erikson J. Coinfection with Streptococcus pneumoniae modulates the B cell response to influenza virus. J Virol. 2014;88:11995–12005. doi: 10.1128/JVI.01833-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Linterman MA, Pierson W, Lee SK, Kallies A, Kawamoto S, Rayner TF, Srivastava M, Divekar DP, Beaton L, Hogan JJ, et al. Foxp3+ follicular regulatory T cells control the germinal center response. Nat Med. 2011;17:975–982. doi: 10.1038/nm.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]