Abstract
Age-induced bone loss is associated with greater bone resorption and decreased bone formation resulting in osteoporosis and osteoporosis-related fractures. The etiology of this age-induced bone loss is not clear but has been associated with increased generation of reactive oxygen species (ROS) from leaky mitochondria. ROS are known to oxidize/damage the surrounding proteins/amino acids/enzymes and thus impair their normal function. Among the amino acids, the aromatic amino acids are particularly prone to modification by oxidation. Since impaired osteoblastic differentiation from bone marrow mesenchymal stem cells (BMMSCs) plays a role in age-related bone loss, we wished to examine whether oxidized amino acids (in particular the aromatic amino acids) modulated BMMSC function. Using mouse BMMSCs, we examined the effects of the oxidized amino acids di-tyrosine and kynurenine on proliferation, differentiation and Mitogen-Activated Protein Kinase (MAPK) pathway. Our data demonstrate that amino acid oxides (in particular kynurenine) inhibited BMMSC proliferation, alkaline phosphatase expression and activity and the expression of osteogenic markers (Osteocalcin and Runx2). Taken together, our data are consistent with a potential pathogenic role for oxidized amino acids in age-induced bone loss.
Keywords: Kynurenine, Proliferation, Differentiation, Amino acids, Runx2, Oxidized nutrients, BMMSCs
1. Introduction
Dietary modifications have been shown to impact the aging process. In particular caloric restriction in rodents of between 30 and 40% extends lifespan by over 30% (Mattson, 2005). Dietary protein restriction has also been shown to extend lifespan, and it has been proposed that the beneficial effect of caloric restriction is due in part to the decrease in dietary protein intake (Rizza et al., 2014; Solon-Biet et al., 2014). Depending on the model system examined, reductions in the sulfur-containing amino acids (in particular methionine), branched chain amino acids (leucine, isoleucine and valine) and serine, threonine and valine have all been associated with increase in longevity (Mirzaei et al., 2014). It is now well recognized that amino acids are signaling molecules in their own right and contribute to the regulation of whole body metabolism. Direct amino acid effects on islet B cells, hepatocytes and skeletal muscle cells appear to be mediated by calcium-binding receptors (Conigrave and Hampson, 2006). The calcium-sensing receptor (CaSR) is stereo-specific for the L-isomer, indicating the existence of a specific amino acid binding site on the receptor (Conigrave et al., 2000).
Previous human studies that measured the post absorptive plasma concentrations of the large neutral amino acids in healthy subjects showed that Tryptophan (Trp) was the only amino acid to exhibit a significant response to age in males, consisting of a 14% decline in elderly subjects (Caballero et al., 1991). Trp is one of nine essential amino acids and plays a major role in several metabolic pathways including general protein synthesis, serotonin synthesis and kynurenine (Kyn) production (Jones et al., 2013). Kyn is an oxidation product for tryptophan and its levels are known to increase with aging and associated with a drop in tryptophan levels (Braidy et al., 2011). Elevated levels of Kyn pathway metabolites, such as quinolinic acid and others, have been observed (Beal et al., 1991) in several age-associated neuro-pathological conditions in rats as well as diseases involving immune activation in humans (Guillemin et al., 2005). Previous in vivo studies in young, middle-aged and old female Wistar rats showed that Trp levels and tryptophan 2, 3-dioxygenase (TDO) activity decreased in all tissues with age. Peak Trp concentrations were observed in the brain, liver and kidney of young adult rats (3 mo.) and subsequently decreased with advancing age. Brain Kyn content increased significantly with age while the Kyn content in the liver and kidney was significantly lower in middle-aged rats (12 mo.) than in young rats (3 mo.) but increased with advancing age to 24 months (Braidy et al., 2011).
Oxidative stress has been proposed to play a role in the aging process (Wang et al., 2014). The toxic effect of these free radicals can be ameliorated by cellular defense mechanisms such as antioxidants like ascorbic acid, glutathione and ubiquinol as well as reactive oxygen species (ROS)-detoxifying enzymes such as superoxide dismutase (SOD), which plays a role in eliminating O2- to produce H2O2 (Valentine et al., 2005). A cell is able to overcome small amounts of free radicals and maintain its original state; moderate oxidative stress can trigger apoptosis and high levels of ROS may cause necrosis (Valko et al., 2005). Age-related loss of bone mass and strength has been linked to increased ROS release from leaky mitochondria and their effects on bone cells (Manolagas, 2010). Indeed, studies have shown a decrease in osteoblast number and bone formation when mice were treated with a glutathione inhibitor (Jagger et al., 2005). Furthermore, studies using murine models of premature aging and signs of oxidative stress showed osteoporotic features (de Boer et al., 2002; Tyner et al., 2002). In vivo studies on mice showed an association between oxidative stress and a decrease in bone mineral density (BMD) (Basu et al., 2001; Maggio et al., 2003; Oh et al., 2007). Human clinical studies have also shown an effect of antioxidants on bone resorption (Pasco et al., 2006).
We have previously shown that aromatic amino acids (tyrosine, tryptophan and phenylalanine) activate distinct anabolic signaling pathways in BMMSCs (El Refaey et al., 2014). In the present study, we examined whether the effects of the aromatic amino acid oxides on BMMSCs were similar or different to those of the unmodified aromatic amino acid. We report that the oxidized amino acids (in particular Kyn) block BMMSC proliferation and osteogenic differentiation, and our findings suggest that age-related accumulation of these oxidized metabolites could play a pathogenic role in the decreases in bone mass seen with aging.
2. Material and methods
2.1. Isolation and culture of BMMSCs
All experiments were approved by the Institutional Animal Care and Use Committee at Georgia Regents University (Protocol #: BR09-11-265; Augusta, GA). The mice were housed in AAALAC-accredited facilities under the supervision of a veterinarian. Georgia Regents University complies with the NIH policy on animal welfare, the Animal Welfare Act, and all other applicable federal, state and local laws. Male C57BL/6 mice were purchased from the National Institute on Aging (Bethesda, MD, USA) aged rodent colony. BMMSCs were isolated from 18-month-old male C57BL/6 mice at the Georgia Regents University Stem Cell Core Facility as previously described (Zhang et al., 2008). In brief, six mice were euthanized by CO2 overdose followed by thoracotomy. The femora and tibiae were dissected free of soft tissues and kept in cold phosphate-buffered saline (PBS) on ice. BMMSCs were isolated from the bone marrow of these long bones using a modified protocol (Peister et al., 2004; Tropel et al., 2004) by negative immune-depletion followed by positive immuno-selection. Enriched BMMSCs were grown in Dulbecco's modified Eagle medium (cat#10-013; DMEM; Cellgro, Mediatech, Manassas, VA, USA) supplemented with 10% heat-inactivated fetal bovine serum (cat#S11150; FBS; Atlanta Biologicals, Lawrenceville, GA, USA) and 1% penicillin-streptomycin (cat#SV30010; Hyclone Laboratories, Inc.) (Zhang et al., 2008). For experiments under low oxygen tension, cells were grown and maintained in a hypoxic chamber (BioSpherix Ltd, Lacona, NY) under 3% oxygen tension (physiologic hypoxia) that more closely resembles the normal physiologic conditions these cells are exposed to in their niches as previously described (El Refaey et al., 2014). BMMSCs were split and maintained under hypoxic conditions in C-chambers that fit into the tissue culture incubator. The tissue culture medium was also maintained under hypoxic conditions prior to use on cells.
2.2. Generation of oxidized amino acids
Four oxidized amino acids were synthesized; one product of tyrosine oxidation, 3′, 3′-dityrosine (di-Tyr) and three products of tryptophan oxidation: oxindolylalanine (Oia), kynurenine (Kyn) and N-formylkynurenine (NFK). di-Tyr was synthetized as previously described (Tilley et al., 2004). Briefly, L-tyrosine (Tyr) (100 mg, 552 mmol) was dissolved in 20 mL deionized water followed by the addition of 1 mL 1.6 M HCl and divided over 30 glass 13 × 100-mm culture tubes. Next, 2 mL of a 2.4 mM KBrO3 aqueous solution (5 μmol) was added per tube. The tubes were covered in foil, heated to 150 °C for 25 min then cooled and lyophilized. The product was dissolved in deionized water, centrifuged through 0.22 μm nylon filters and purified by high-pressure liquid chromatography (HPLC). The product was confirmed by NMR and mass spectrometry (expected mass = 360.4; actual mass = 360.4).
Oia was synthesized through oxidation of L-Trp as previously described (Labroo and Cohen, 1990). Briefly, 9 mL DMSO (125 mmol) was added to a 50 mL 12 N HCl, followed by the addition of 1 g phenol (10 mmol). A solution of L-Trp (10 g, 50 mmol) in 300 mL glacial acetic acid was added to the acidic solution. After 5 hours, solvents were removed by rotary evaporation and the product was crystallized overnight in glacial acetic acid. The crystalline product was collected by filtration. The product was confirmed by NMR and mass spectrometry (expected mass = 220.2; actual mass = 221.1) and was purified by reversed-phase HPLC.
Kyn was synthesized through oxidation of Oia as previously described (Todorovski et al., 2011) The oxidation was performed by dissolving 1 g Oia (4.5 mmol) in 100 mL 0.15 M NaOH, pH 12.75 containing 50 mM KCl (0.37 g, 5 mmol). Air was bubbled through the solution for approximately 2 hrs. Product formation was monitored by mass spectrometry. Upon completion, the reaction was neutralized to pH 7 by the addition of HCl. Excess solvent was removed by rotary evaporation and purified by solid phase extraction. The product was confirmed by NMR and mass spectrometry (expected mass = 208.2; actual mass = 209.1).
NFK was synthesized by formylation of Kyn as described (Todorovski et al., 2011). Kyn (1 g, 5 mmol) was dissolved in 2.2 mL formic acid. A mixture of formic acid and acetic anhydride (0.97 mL and 0.48 mL respectively) was added and the reaction was stirred for 2 hours. This was then poured into diethyl ether and the precipitate was isolated and crystallized in ethanol. The product was confirmed by NMR and mass spectrometry (expected mass = 236.2; actual mass = 236.2).
2.3. Preparation of cell lysate for reverse-phase protein array
Two sets of 18-month-old BMMSCs were seeded under two different conditions. One set was grown under normoxic conditions (21% O2/5% CO2) and the other set under low O2 tension (3% O2/5% CO2; physiologic hypoxia). Treatment (initiated when cells were approximately 80% confluent) not only consisted of normoxia versus hypoxia but also compared the effects of oxidized aromatic amino acids at 100 μM (Kyn and di-Tyr) under normoxia and hypoxia. The cells were treated in serum-free media [Krebs Ringer Buffer (KRB)] for 3 hours. RPPA samples were run and analyzed as previously described (El Refaey et al., 2014).
2.4. Western blotting
Whole cell lysates of treated BMMSCs were prepared in complete Lysis-M EDTA-free buffer containing protease inhibitors (cat#04719964001; Roche Diagnostics, Indianapolis, IN, USA). Equal amounts (50 μg) of protein lysates were subjected to SDS–PAGE and transferred to 0.45 μm PVDF membranes (cat#IPFL00010; Millipore, Billerica, MA, USA). Membranes were blocked with blocking buffer (cat#927-40000; Licor Biosciences). Membranes were probed with specific primary antibodies at 4 °C overnight. Primary antibodies used were phospho-ERK (cat#4370), ERK (cat#4696), phospho-c-Raf (cat#9427), β-actin (cat#3700) and alkaline phosphatase (cat#15065) followed by secondary antibody incubation for 1 h at room temperature. Secondary antibodies used were goat anti-rabbit IgG antibody IRDye® 800 Conjugated (cat # 611-132-122), Alexa Fluor® 680 goat anti-Mouse IgG (H + L), highly cross-adsorbed (cat#A21058), donkey anti-mouse IgG (H&L) antibody IRDye® 700DX conjugated (cat#610-730-124) and donkey anti-goat IgG (H&L) antibody IRDye® 800 Conjugated (605-732-002) (Table 1). Bound antibodies were visualized using a LiCor scanner and quantified using LiCor image analysis software.
Table 1.
Antibodies for Western blotting.
| Antibodies | Dilution | Supplier |
|---|---|---|
| Primary antibodies | ||
| Signaling | ||
| Ms anti-ERK | 1:1000 | Cell Signaling |
| Rb anti-phospho-ERK | 1:400 | Cell Signaling |
| Rb phospho-c-Raf | 1:400 | Cell Signaling |
| Osteogenic | ||
| D anti-alkaline phosphatase | 1:100 | Santa Cruz Biotechnology |
| Housekeeping | ||
| Ms anti-B-actin | 1:2500 | Cell Signaling |
| Secondary antibodies | ||
| G anti-Rb IgG conjugated | 1:10,000 | Rockland |
| G anti-Ms IgG conjugated | 1:10,000 | Invitrogen |
| D anti-Ms IgG conjugated | 1:10,000 | Rockland |
| D anti-Rb IgG conjugated | 1:10,000 | Rockland |
Ms, mouse; Rb, rabbit; D, donkey; G, goat.
2.5. Assessment of the effects of oxidized amino acids on signaling pathways
BMMSCs were cultured under normoxic conditions (21% O2/5% CO2), and when cells were 80% confluent, they were starved for at least 3 h in serum-free media (KRB) and then treated with L-Trp and its oxides (Oia, Kyn and NFK) or L-Tyr and its oxide (100 μM) for 5 min based on previous findings showing that ERK phosphorylation peaked between 5 and 10 min and declined at 30 min (Ding et al., 2011). Protein was collected and measured and phosphorylation levels were assessed using western analysis as previously described.
2.6. In vitro proliferation assay
Proliferation was measured 24 h post-treatment using the bromodeoxyuridine (BrdU) incorporation assay as previously described (Jung et al., 2011) (Cell Proliferation ELISA, BrdU; colorimetric) (cat#11 647 229 001; Roche Diagnostics, Indianapolis, IN, USA) to determine the effect of the oxidized nutrients versus aromatic amino acids on BMMSC proliferation. Untreated cells and D-amino acids were used as negative controls (as only L-type amino acids bind to the stereospecific extracellular Ca2+ receptor) (Conigrave et al., 2000). Cell background with anti-BrdU and without BrdU was also used as an experimental control.
2.7. Osteogenic differentiation and determination of alkaline phosphatase protein expression
BMMSCs were cultured in DMEM (cat#10-013; DMEM; Cellgro, Mediatech, Manassas, VA, USA) supplemented with 10% FBS (cat#S11150; FBS; Atlanta Biologicals, Lawrenceville, GA, USA) and 1% penicillin–streptomycin (cat#SV30010; Hyclone Laboratories, Inc.), and when 80% confluent cells were treated with 100 μM L-Trp, Oia, Kyn or NFK for 24 h in osteogenic induction media previously as described by our group (Herberg et al., 2013) and consisting of 50 μM L-ascorbic acid (cat#A5960; Sigma-Aldrich Co.), 10 mM β-glycerophosphate (cat#G9422; Sigma-Aldrich Co.), and 100 nM dexamethasone (cat#D4902; Sigma-Aldrich Co.) in addition to 5% FBS and 1% penicillin-streptomycin in DMEM. Untreated cells and D-Trp were used as negative controls. Osteogenic induction media were changed every other day for 10 days (day 0 was when cells were confluent). ALP protein expression was determined using western blotting. Protein extraction was performed after 10 days as previously described by others (Fedarko et al., 1990) and cell lysates were analyzed by western blotting using β-actin for protein normalization. In another set of experiments, cells were treated with 100 μM L-Tyr and di-Tyr for 24 h in osteogenic induction media. Untreated cells and D-Tyr were used as negative controls. A positive control (JAR Cell Lysate; sc-2276; Santa Cruz Biotechnology, Inc., Dallas, Texas) was also used. ALP protein expression was compared between the different treatment groups.
2.8. Alkaline phosphatase activity
BMMSCs were plated in DMEM supplemented with 10% FBS and 1% penicillin–streptomycin at a density of 4000 cells/well. When cells were 80% confluent, they were treated with L-Trp, D-Trp or their oxides (100 μM) for 24 hours in the osteo-inductive media previously described (Li and Wang, 2013). In another set of experiments, cells were treated with D-Tyr, L-Tyr and di-Tyr (100 μM). Untreated cells were used as a negative control. Cells were grown in osteo-inductive media for 10 days (day 0 was when cells were confluent). Cells then were washed with phosphate-buffered saline (PBS). One hundred microliters of 0.05% Triton was added to each well. Cells were frozen for 30 min at −80 °C and then thawed at 37 °C for 30 min; this procedure was repeated three times. The ALP reaction consisted of 20 μL cell lysate and 80 μL amino methyl propanol (AMP) (Li and Wang, 2013). The reaction was read at 405 nm using a multi-well spectrophotometer (ELISA reader). The protein content was later assayed using 20 μL cell lysate and 180 μL diluted protein assay reagent for normalization. The reaction was read at 600 nm using the multi-well spectrophotometer (ELISA reader).
2.9. RNA extraction and quantitative RT-PCR
Total cellular RNA was isolated from cells treated with D-Trp, L-Trp, its oxides (100 μM) or untreated cells using RNeasy Mini Kits (cat#74104; Qiagen). In another set of experiments, RNA was isolated from untreated cells and cells treated with 100 μM L-Tyr, D-Tyr and di-Tyr. Equal amounts of total RNA (1 μg) were reverse transcribed using iScript cDNA synthesis kits (cat#170-8891; Bio-Rad Laboratories, Inc.) by incubating complete reaction mix for 5 min at 25 °C, 30 min at 42 °C and 5 min at 85 °C. The cDNA was amplified using Platinum PCR SuperMix (cat#11306016; Invitrogen) at an annealing temperature of 57 °C. The specificity was confirmed by agarose gel electrophoresis of PCR products. The cDNA (1 μL) was used as template for real-time RT-PCR analysis using SYBR Green Master Mix (Applied Biosystems) and qRT-PCR primers listed in Table 2. PCR reactions were performed in triplicate for 40 cycles, and the levels of expression of osteogenic markers were calculated by the ΔΔCt method using GAPDH as an internal control.
Table 2.
Oligonucleotide primer sequences for qRT-PCR.
2.10. Statistical analysis
Experiments were performed at least three independent times. For RPPA data, all data are expressed as means ± SEM. Two-sided one-sample t-tests were performed for the hypothesis that the ratio was not equal to 100% (ratio is treatment/control). For western blot analyses, alkaline phosphatase activity and proliferation assays, data are expressed as means ± SEM. Upper tail one-sample t-tests were performed for the hypothesis that the expression relative to control was greater than 100. A log transformation was used to stabilize the variance. For real-time PCR data analyses, data are expressed as mean fold change relative to control ± SEM. One-sample t-tests were performed for the hypothesis that the expression relative to control was different by 1. Null hypotheses were rejected at the 0.05 level. No multiple testing adjustments were made. Data were analyzed using SAS© 9.3 (SAS Institute, Inc., Cary, NC).
3. Results
We have previously shown that signaling pathways activated by aromatic amino acids vary depending on whether cells were stimulated under normoxic (21%) or hypoxic (3%) conditions (El Refaey et al., 2014). However, we had not previously examined the effect of the oxidized aromatic amino acids on signaling pathways under varying oxygen concentration. We have proposed that the experiments done under the 21% oxygen tension conditions more closely resemble those of an aging environment because of the higher concentration of reactive oxygen species compared to those under a 3% oxygen environment. Aging has a dual effect on nutrient sensing by the stem cell both by depleting the levels of essential amino acids like Trp and Tyr (though oxidation) and also by increasing the levels of the amino acid oxides which might have different signaling properties than the unmodified amino acids. Experiments in Sections 3.1 and 3.2 were done as screening experiments to survey multiple signaling pathways using the reverse phase protein arrays in order to determine if in fact just varying oxygen tension impacted the results for the AA oxides (Kyn and di-Tyr). As demonstrated in the results to be discussed later, under a 3% oxygen environment the oxidized amino acids modulated signaling pathways that affected proliferation and cellular energetics. In contrast, under a 21% oxygen environment these oxides modulated pathways responding to oxidative stress and stem cell commitment. Subsequent experiments (Figs. 3–8) were done under 21% oxygen since they were felt to more closely resemble the aging environment and focused on proliferation and differentiation pathways in BMMSCs.
Fig. 3.
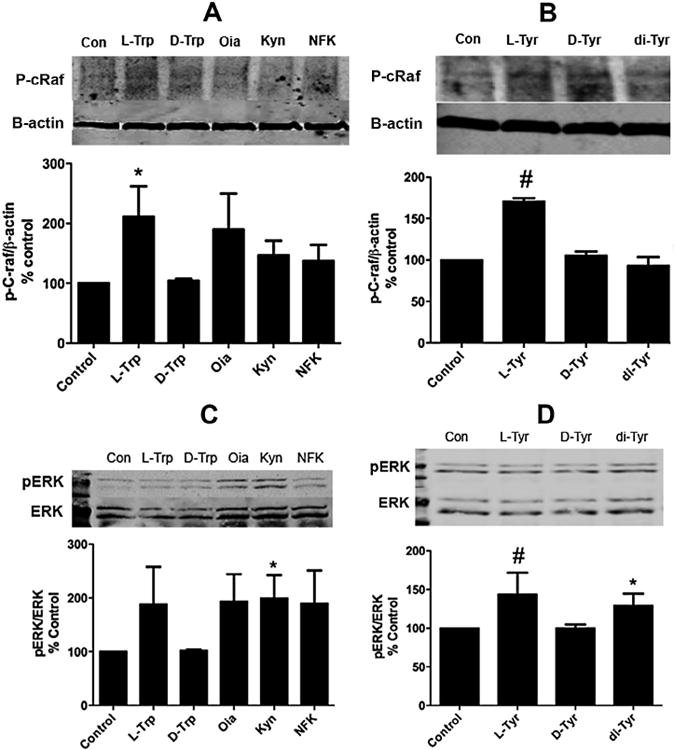
Effects of aromatic amino acids versus their oxides on c-Raf and ERK phosphorylation. (A) Western blot analysis of BMMSCs treated with 100 μM L-Trp, D-Trp or its three oxides, oxindolylalanine (Oia), kynurenine (Kyn) and N-formylkynurenine (NFK), with p-c-Raf normalized to β-actin. (B) Western blot analysis of BMMSCs treated with 100 μM L-Trp, D-Trp or its three oxides: Oia, Kyn and NFK with p-ERK normalized to total ERK. (C) Western blot analysis of BMMSCs treated with 100 μM L-Tyr, D-Tyr and its oxide, di-Tyr, with p-c-Raf normalized to β-actin. (D) Western blot analysis of BMMSCs treated with 100 μM L-Tyr, D-Tyr and di-Tyr with p-ERK normalized to total ERK. Results are expressed as means ±SEM for at least three independent experiments. *p ≤ 0.05 and #p ≤ 0.01.
Fig. 8.

Effects of L-Tyr and its oxide on osteogenic gene expression. Cells were treated with 100 μM L-Tyr and its oxide, di-Tyrfor 24 h in osteogenic induction media. Untreated cells and D-Tyr were used as controls. Total cellular RNA was isolated from treated and untreated cells. Real-time PCR were performed to determine the effect of the treatment on OCN and Runx2 gene expression. Effect of L-Tyr versus untreated cells and di-Tyr on (A) OCN expression and (B) Runx2 expression. Results are expressed as means ± SEM for at least three independent experiments. *p ≤ 0.05 and #p ≤ .01.
3.1. Effects of oxidized nutrients on cell signaling pathways under 3% oxygen
Under hypoxic conditions oxidized nutrients increased the levels of phospho-protein enriched in astrocytes 15 (PEA15) (only Kyn showed a statistical significance), which controls autophagy. Our data also showed that oxidized nutrients up-regulated both Ribosomal Protein S6 (RPS6) and Ribosomal Protein S6 Kinase Beta-1 (RPS6KB1) (both oxides showed a statistical significance for RPS5KB1) that play a role in proliferation. Levels of mTOR or FK506-binding protein 12-rapamycin-associated protein 1 (FRAP1) were also upregulated by oxides (only di-Tyr showing a statistical significance). Kyn showed a non statistical higher level of phospho-Protein Kinase B or AKT that regulates cell renewal and proliferation (Fig. 1).
Fig. 1.

Effects of oxidized amino acids versus control under hypoxic conditions. Reverse-phase protein array analysis of BMMSCs grown under hypoxic conditions (3% O2/5% CO2). When cells were 80% confluent, they were treated with either (A) Kyn or (B) di-Tyr (100 μM) in serum-free media for 3 h and compared to the untreated control (100%). Results are expressed as means ±SEM forthree independent experiments. *p ≤ 0.05 and #p ≤ 0.01. RPS6: Ribosomal protein s6 (S6_pS240_S244-R-V). RPS6KB1: Ribosomal protein S6 kinase beta-1 (p70S6K_pT389-R-V). AKT1 AKT2 AKT3: protein kinase B (Akt_pT308-R-V). FRAP1: mTOR or FK506-binding protein 12-rapamycin-associated protein 1 (mTOR_pS2448-R). MAPK14: Mitogen-activated protein kinase 14 or p38 (p38_pT180_Y182-R-V). SCD1: Stearoyl-CoAdesaturase-1 (SCD1-M-V). PEA15: Phospho-protein enriched in astrocytes 15 (PEA15_pS116-R-V). MAP2K1: Mitogen-activated protein kinase 1 (MEK1-R-V). MAPK1 MAPK3: Mitogen-activated protein kinase (MAPK_pT202_Y204-R-V). ERBB2: Receptor tyrosine-protein kinase erbB-2 (HER2-M-V). IRS1: Insulin receptor substrate 1 (IRS1-R-V). PRKCD: PKC-delta_pS664-R-V (PKC-delta_pS664-R-V). ERBB3: Receptor tyrosine-protein kinase erbB-3 (HER3_pY1298-R). EIF4E: Eukaryotic Initiation Factor-4 (eIF4E-R-V). MAPK8: c-Jun N-terminal kinases (JNK_pT183_pT185-R-V).
3.2. Effects of oxidized nutrients on cell signaling pathways under 21% oxygen
Under normoxic conditions, both oxides statistically increased the expression of FOXM1 and FOXO 3 (Fig. 2). FOX (Forkhead box) proteins are a family of transcription factors important in the regulation of proliferation and cell survival genes. They are thought to be important in protecting the cell from oxidative stress (Iyer et al., 2013). Our previous studies would suggest that a high oxygen environment (the so-called normoxic or 21% oxygen) is what would normally exist in an aged animal bone marrow microenvironment. Both oxides also up-regulated RPS6 (Fig. 2).
Fig. 2.

Effects of oxidized amino acids versus control under normoxic conditions. Reverse-phase protein array analysis of BMMSCs grown under normoxic conditions (21% O2/5% CO2). When cells were 80% confluent, they were treated with either (A) Kyn or (B) di-Tyr (100 μM) in serum-free media for 3 hand compared to the untreated control (100%). The cells were washed twice with PBS and lysis buffer was added to the plates. Results are expressed as means ±SEM for three independent experiments. *p ≤0.05 and #p ≤ 0.01. FOXM1: Fork-head box protein M1 (FoxM1-R-V). MAP2K1: Mitogen-activated protein kinase (MEK1-R-V). RPS6: Ribosomal protein s6 (S6_pS240_S244-R-V). CDKN1B: Cyclin-dependent kinase inhibitor 1B (p27-R-V). PRKCD: PKC-delta_pS664-R-V(PKC-delta_pS664-R-V). BCl2L11: Bcl-2-like protein 11(Bim-R-V). FOXO3: FOX03a-R-C(FOX03a-R-C). MAPK14: Mitogen-activated protein kinase 14 or p38 (p38_pT180_Y182-R-V). FRAP1: mTOR or FK506-binding protein 12-rapamycin-associated protein 1 (mTOR_pS2448-R). PEA15: Phospho-protein enriched in astrocytes 15 (PEA15_pS116-R-V). ANXA7: Annexin_VII-M-V (Annexin_VII-M-V). BECN1: Beclin-1 (Beclin-G).
3.3. Effects of the oxidized amino acids on c-Raf and ERK phosphorylation
We began by comparing the effects of L-aromatic amino acid versus its oxides and the D-form of the AAs, which was used as a negative control (Conigrave et al., 2000) on c-Raf phosphorylation. As can be seen (Fig. 3 panels A and B) both Trp and Tyr significantly increased c-Raf phosphorylation while neither the AA oxides or its D stereoisomers had any effect.
In contrast when ERK phosphorylation was examined (Fig. 3 panels C and D), both the Tyr and Trp oxides (di-Tyr and Kyn) significantly increased ERK phosphorylation. While both L-Tyr and Trp also increased ERK phosphorylation it was only statistically significant for Tyr.
3.4. In vitro proliferation
Next, we investigated the effects of several oxidized amino acids on BMMSC proliferation (Fig. 4). BMMSCs were seeded at a density of 4000 cells/well in 96-well plates and subsequently, treatments (L-Trp and its oxides or L-Tyr and its oxide) (100 μM) and BrdU were added to the cells in low serum media for 24 hours. Our data showed a statistically significant twofold increase in the number of proliferating cells in response to L-Trp versus the untreated control (Fig. 4A), whereas Tyr and its oxidized analog had no significant effect on proliferation (Fig. 4B). Kyn used at higher concentration induced fewer proliferating cells (Fig. 4C). BMMSCs were then stimulated by placing in complete culture medium containing 10% fetal calf serum. As shown in Fig. 4D, Kyn was able to significantly inhibit complete medium-stimulated proliferation at all doses tested.
Fig. 4.
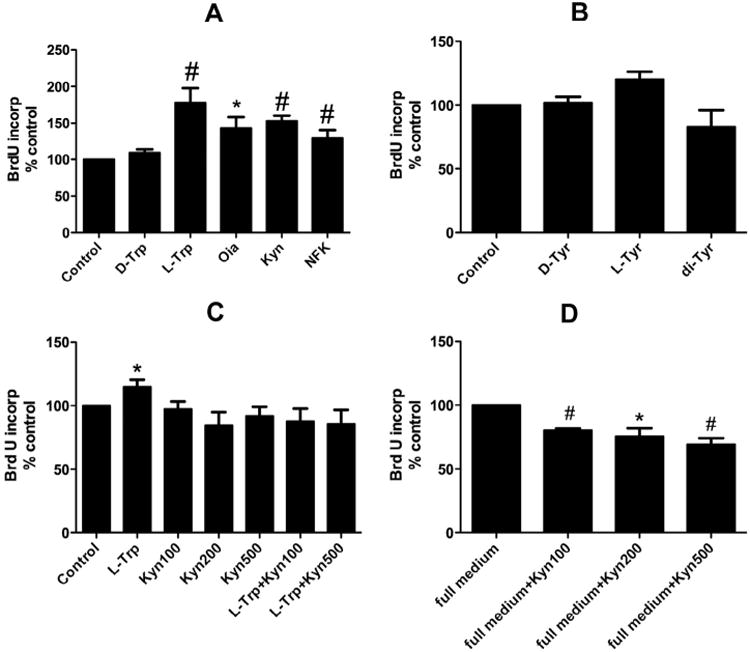
In vitro proliferation assay. BMMSCs were seeded at a density of 4000 cells/well in 96-well plates. Subsequently, treatments (L-Trp and its oxides or L-Tyr and its oxide) (100 μM) and BrdU were added to the cells in low serum media for 24 hours. In some groups, Kyn was used also at 200 and 500 μM concentrations. The cells were fixed and BrdU incorporation was determined. (A) Treatment with L-Trp, Oia, Kyn or NFK or no treatment (control). (B) Treatment with L-Tyr versus di-Tyr treatment or no treatment (control). (C) Treatment with Kyn (200 μM or 500 μM) alone and in combination with Trp (100 μM). (D) Treatment with Kyn at increasing concentrations in the presence of complete serum-containing medium. Results are expressed as means ±SEM for at least three independent experiments. *p ≤ 0.05 and #p ≤ 0.01.
3.5. Alkaline phosphatase protein expression
In order to study the effects of the oxidized amino acids versus the non-oxidized counterparts on BMMSC differentiation, ALP protein expression as a marker of differentiation was evaluated (Fig. 5). When BMMSCs were cultured in osteogenic induction media for 14 days, L-Trp elicited a statistically significant increase in ALP protein expression relative to the oxides (Fig. 5A). Similarly, western blot analysis showed greater ALP protein expression in the Tyr-treated group versus oxides (Fig. 5B).
Fig. 5.

Effects of aromatic amino acids and their oxides on alkaline phosphatase protein expression. (A) Western blot analysis of BMMSCs treated with 100 μM L-Trp, D-Trp or its three oxides, Oia, Kyn and NFK, showing ALP protein expression normalized to β-actin after culture of BMMSCs in osteogenic induction media for 14 days. (B) Western blot analysis of BMMSCs treated with 100 μM L-Tyr, D-Tyr or its oxide, di-Tyr, illustrating ALP protein expression normalized to β-actin. Results are expressed as means ±SEM for at least three independent experiments. *p ≤ 0.05 and #p ≤ 0.01.
3.6. Alkaline phosphatase activity
Next, we studied the effects of oxidized versus unoxidized aromatic amino acids on ALP activity. BMMSCs were treated with L-Trp, D-Trp and its oxides for 24 h in osteoinductive media or with D-Tyr, L-Tyr and di-Tyr (100 μM). As shown in Fig. 6 L-Trp increased ALP activity to a greater extent than its oxides or the untreated control cells, inducing a statistically significant increase in ALP activity as shown in (Fig. 6A). L-Tyr tended also to stimulate ALP activity although the difference did not achieve statistical significance (Fig. 6B).
Fig. 6.

Effects of aromatic amino acids and their oxides on alkaline phosphatase activity. BMMSCs were plated at a density of 4000 cells/well. When cells were 80% confluent, they were treated with (A) L-Trp, D-Trp or its oxides or (B) D-Tyr, L-Tyr or di-Tyr (all at 100 μM) for 24 h in osteo-inductive media. Untreated cells were used as a negative control. The alkaline phosphatase reaction consisted of 20 μL cell lysate and 80 μL amino methyl propanol (AMP) and values were normalized to the protein content. Results are expressed as means ±SEM for at least three independent experiments. *p ≤ 0.05 and #p ≤ 0.01.
3.7. Effects of oxidized nutrients on osteogenic markers
We further compared the effects of Trp and its oxides on osteogenic marker gene expression. We monitored the expression of OCN as a late differentiation marker and Runx2 as a regulator of osteogenic differentiation. Cells were treated with 100 μM L-Trp, Oia, Kyn or NFK for 24 h in osteogenic induction media. Untreated cells and D-Trp were used as negative controls. Real-time PCR data indicated that L-Trp and Oia tended to increase the expression of OCN versus the untreated cells, whereas Kyn had no effect. Only Oia induced a statistically significant change. However, L-Trp significantly increased Runx2 gene expression (about twofold) versus the untreated control and the oxides (Fig. 7).
Fig. 7.

Effects of L-Trp and its oxides on osteogenic gene expression. Cells were treated with 100 μM L-Trp, Oia, Kyn or NFK for 24 h in osteogenic induction media. Untreated cells and D-Trp were used as controls. Total cellular RNA was isolated from treated and untreated cells. Real-time PCR were performed to determine the effect of the treatments on OCN and Runx2 gene expression. (A) OCN expression upon treatment with L-Trp versus untreated control cells and the oxides and (B) Runx2 expression in cells treated with L-Trp versus the untreated control or the oxides. Results are expressed as means ±SEM for at least three independent experiments. #p ≤ 0.01.
Lastly, we investigated the effects of Tyr and its oxide on osteogenic marker gene expression. Cells were treated with 100 μM L-Tyr or its oxide for 24 h in osteogenic induction media. Real-time PCR data demonstrated that L-Tyr significantly increased the expression of OCN versus the untreated cells around twofold as well as Runx2 gene expression with a more than twofold increase versus the untreated control cells. As can be seen in Fig. 8, di-Tyr slightly but significantly increased the expression of OCN; however, Runx2 gene expression was not affected.
4. Discussion
The present study examined how oxidation of nutritional signals altered their signaling properties. We report that oxidized proteins (amino acids), generated in the stem cell niche (Urao and Ushio-Fukai, 2013), exert different effects than their unoxidized counterparts on stem cell proliferation and differentiation down the osteoblastic pathway. In fact, for certain cellular responses (like BMMSC proliferation) the oxidized counterparts can block the normal anabolic signals generated by nutritional signals.
It has been hypothesized that part of the benefits of caloric restriction on life span relate to a drop in the mitochondrial generation of reactive oxygen species. However in the case of dietary protein restriction this cannot be the only factor since restriction of certain amino acids such as methionine, branched chain amino acids and serine, threonine and valine also have beneficial effects (Rizza et al., 2014). These data would suggest that oxidation of some amino acids have additional harmful effects on stem cell function.
L-type amino acids are known to activate several members of the class 3 G-protein-coupled receptor superfamily including the extracellular Ca2+-sensing receptor (CaSR), which is modulated by aromatic, aliphatic and polar amino acids and member 6A of the G-protein coupled receptor family C (GPRC6A). Ca2+-binding to CaSR has been found to activate multiple signaling pathways; amino acid binding to CaSR acts more selectively on the ERK1/2 signaling pathway (Davies et al., 2006).
Western blot data showed an increase in c-Raf and ERK phosphorylation by L-Trp. ERK is important in cell survival and proliferation and, together with other mitogen-activated protein kinases (MAPKs), activates important intracellular signaling pathways for various pro-proliferative molecules in many cells including mesenchymal stem cells (Rodrigues et al., 2010). In vitro proliferation data showed a statistically significant increase in the number of proliferating cells in response to L-Trp. In contrast, Kyn significantly inhibited BMMSC proliferation stimulated by complete serum-containing media. These data suggest that L-Trp increases the proliferation of BMMSCs but that when it is oxidized it becomes a potent inhibitory signal for proliferation. The observed Trp effects on BMMSCs are consistent with studies performed by others that demonstrated that Trp was more effective than other essential amino acids in increasing the growth of osteoblasts isolated from calvariae of newborn Sprague-Dawley rats (Conconi et al., 2001).
When BMMSCs were cultured in osteogenic induction media for 14 d, both L-Trp and L-Tyr showed a statistically significant increase in alkaline phosphatase protein expression (a marker of differentiation) versus the oxides. Moreover, L-Trp and L-Tyr increased ALP activity to a greater extent than their oxides. These data suggest a role of L-Trp and L-Tyr in osteoblastic differentiation and are consistent with previous studies that showed alkaline phosphatase activity was significantly enhanced in calvarial osteoblasts treated with lysine (Lys), threonine (Thr), methionine (Met), Trp or arginine (Arg) at doses equivalent to 0.1-, 1- and 10-fold their plasma concentrations (Conconi et al., 2001). With respect to the osteogenic markers OCN and Runx2, L-Trp increased osteocalcin expression while its oxide had no effect. Taken together these data suggest a role for aromatic amino acids in osteoblastic differentiation. Whether the amino acid oxidation products are binding to the calcium sensing receptor in a competitive manner or activating a separate signaling pathway which blocks the normal anabolic pathway activated by amino acids is not clear.
5. Conclusions
Our data suggest a role for L-Trp in BMMSC proliferation and differentiation down the osteoblastic pathway. In contrast, oxidized derivatives of Trp (e.g., Kyn) appear to block the anabolic signals associated with the unoxidized amino acid counterparts, suggesting that with aging and the accompanying increase in oxidative stress, nutrient signals that normally promote osteoblastic proliferation and differentiation are converted into factors that inhibit these processes. Thus, some oxidized amino acids may perform a pathogenic role in age-induced bone loss. Aging then has a negative impact on BMMSCs both by depleting the normally anabolic tryptophan and tyrosine signals (by oxidation) and increasing the levels of the anti-anabolic oxidized tryptophan and tyrosine metabolites.
Acknowledgments
Funding for this research was provided by the National Institute on Aging (P01 AG036675). WBB is supported by a VA Research Career Scientist Award. The contents of this article do not represent the views of the Department of Veterans Affairs or the United States Government.
References
- Basu S, Michaelsson K, Olofsson H, Johansson S, Melhus H. Association between oxidative stress and bone mineral density. Biochem Biophys Res Commun. 2001;288:275–279. doi: 10.1006/bbrc.2001.5747. [DOI] [PubMed] [Google Scholar]
- Beal MF, Ferrante RJ, Swartz KJ, Kowall NW. Chronic quinolinic acid lesions in rats closely resemble Huntington's disease. J Neurosci. 1991;11:1649–1659. doi: 10.1523/JNEUROSCI.11-06-01649.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braidy N, Guillemin GJ, Mansour H, Chan-Ling T, Grant R. Changes in kynurenine pathway metabolism in the brain, liver and kidney of aged female Wistar rats. FEBS J. 2011;278:4425–4434. doi: 10.1111/j.1742-4658.2011.08366.x. [DOI] [PubMed] [Google Scholar]
- Caballero B, Gleason RE, Wurtman RJ. Plasma amino acid concentrations in healthy elderly men and women. Am J Clin Nutr. 1991;53:1249–1252. doi: 10.1093/ajcn/53.5.1249. [DOI] [PubMed] [Google Scholar]
- Conconi MT, Tommasini M, Muratori E, Parnigotto PP. Essential amino acids increase the growth and alkaline phosphatase activity in osteoblasts cultured in vitro. Farmaco. 2001;56:755–761. doi: 10.1016/s0014-827x(01)01126-0. [DOI] [PubMed] [Google Scholar]
- Conigrave AD, Hampson DR. Broad-spectrum L-amino acid sensing by class 3 G-protein-coupled receptors. Trends Endocrinol Metab. 2006;17:398–407. doi: 10.1016/j.tem.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Conigrave AD, Quinn SJ, Brown EM. L-amino acid sensing by the extracellular Ca2+-sensing receptor. Proc Natl Acad Sci U S A. 2000;97:4814–4819. doi: 10.1073/pnas.97.9.4814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer J, Andressoo JO, de Wit J, Huijmans J, Beems RB, et al. Premature aging in mice deficient in DNA repair and transcription. Science. 2002;296:1276–1279. doi: 10.1126/science.1070174. [DOI] [PubMed] [Google Scholar]
- Davies SL, Gibbons CE, Vizard T, Ward DT. Ca2+-sensing receptor induces Rho kinase-mediated actin stress fiber assembly and altered cell morphology, but not in response to aromatic amino acids. Am J Physiol Cell Physiol. 2006;290:C1543–C1551. doi: 10.1152/ajpcell.00482.2005. [DOI] [PubMed] [Google Scholar]
- Ding K, Zhong Q, Shi X, Bollag W, Cain M, et al. Dietary Amino Acids Activate the MAPK Pathway in Bone Marrow Stromal Cells. American Society for Bone and Mineral Research; San Diego, CA: 2011. [Google Scholar]
- El Refaey M, Zhong Q, Hill WD, Shi XM, Hamrick MW, et al. Aromatic amino acid activation of signaling pathways in bone marrow mesenchymal stem cells depends on oxygen tension. PLoS ONE. 2014;9:e91108. doi: 10.1371/journal.pone.0091108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedarko NS, Bianco P, Vetter U, Robey PG. Human bone cell enzyme expression and cellular heterogeneity: correlation of alkaline phosphatase enzyme activity with cell cycle. J Cell Physiol. 1990;144:115–121. doi: 10.1002/jcp.1041440115. [DOI] [PubMed] [Google Scholar]
- Guillemin GJ, Kerr SJ, Brew BJ. Involvement of quinolinic acid in AIDS dementia complex. Neurotox Res. 2005;7:103–123. doi: 10.1007/BF03033781. [DOI] [PubMed] [Google Scholar]
- Herberg S, Fulzele S, Yang N, Shi X, Hess M, et al. Stromal cell-derived factor-1beta potentiates bone morphogenetic protein-2-stimulated osteoinduction of genetically engineered bone marrow-derived mesenchymal stem cells in vitro. Tissue Eng Part A. 2013;19:1–13. doi: 10.1089/ten.tea.2012.0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer S, Ambrogini E, Bartell SM, Han L, Roberson PK, et al. FOXOs attenuate bone formation by suppressing Wnt signaling. J Clin Invest. 2013;123:3409–3419. doi: 10.1172/JCI68049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagger CJ, Lean JM, Davies JT, Chambers TJ. Tumor necrosis factor-alpha mediates osteopenia caused by depletion of antioxidants. Endocrinology. 2005;146:113–118. doi: 10.1210/en.2004-1058. [DOI] [PubMed] [Google Scholar]
- Jones SP, Guillemin GJ, Brew BJ. The kynurenine pathway in stem cell biology. Int J Tryptophan Res. 2013;6:57–66. doi: 10.4137/IJTR.S12626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung HJ, Park JY, Jeon HS, Kwon TH. Aquaporin-5: a marker protein for proliferation and migration of human breast cancer cells. PLoS ONE. 2011;6:e28492. doi: 10.1371/journal.pone.0028492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labroo RB, Cohen LA. Preparative separation of the diastereoisomers of dioxindolyl-L-alanine and assignment of stereochemistry at C-3. J Org Chem. 1990;55:4901–4904. [Google Scholar]
- Li Q, Wang Z. Influence of mesenchymal stem cells with endothelial progenitor cells in co-culture on osteogenesis and angiogenesis: an in vitro study. Arch Med Res. 2013;44:504–513. doi: 10.1016/j.arcmed.2013.09.009. [DOI] [PubMed] [Google Scholar]
- Maggio D, Barabani M, Pierandrei M, Polidori MC, Catani M, et al. Marked decrease in plasma antioxidants in aged osteoporotic women: results of a cross-sectional study. J Clin Endocrinol Metab. 2003;88:1523–1527. doi: 10.1210/jc.2002-021496. [DOI] [PubMed] [Google Scholar]
- Manolagas SC. From estrogen-centric to aging and oxidative stress: a revised perspective of the pathogenesis of osteoporosis. Endocr Rev. 2010;31:266–300. doi: 10.1210/er.2009-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP. Energy intake, meal frequency, and health: a neurobiological perspective. Annu Rev Nutr. 2005;25:237–260. doi: 10.1146/annurev.nutr.25.050304.092526. [DOI] [PubMed] [Google Scholar]
- Mirzaei H, Suarez JA, Longo VD. Protein and amino acid restriction, aging and disease: from yeast to humans. Trends Endocrinol Metab. 2014;25:558–566. doi: 10.1016/j.tem.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh B, Kim SY, Kim DJ, Lee JY, Lee JK, et al. Associations of catalase gene polymorphisms with bone mineral density and bone turnover markers in postmenopausal women. J Med Genet. 2007;44:e62. doi: 10.1136/jmg.2006.042259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasco JA, Henry MJ, Wilkinson LK, Nicholson GC, Schneider HG, et al. Antioxidant vitamin supplements and markers of bone turnover in a community sample of nonsmoking women. J Womens Health (Larchmt) 2006;15:295–300. doi: 10.1089/jwh.2006.15.295. [DOI] [PubMed] [Google Scholar]
- Peister A, Mellad JA, Larson BL, Hall BM, Gibson LF, et al. Adult stem cells from bone marrow (MSCs) isolated from different strains of inbred mice vary in surface epitopes, rates of proliferation, and differentiation potential. Blood. 2004;103:1662–1668. doi: 10.1182/blood-2003-09-3070. [DOI] [PubMed] [Google Scholar]
- Rizza W, Veronese N, Fontana L. What are the roles of calorie restriction and diet quality in promoting healthy longevity? Ageing Res Rev. 2014;13:38–45. doi: 10.1016/j.arr.2013.11.002. [DOI] [PubMed] [Google Scholar]
- Rodrigues M, Griffith LG, Wells A. Growth factor regulation of proliferation and survival of multipotential stromal cells. Stem Cell Res Ther. 2010;1:32. doi: 10.1186/scrt32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solon-Biet SM, McMahon AC, Ballard JW, Ruohonen K, Wu LE, et al. The ratio of macronutrients, not caloric intake, dictates cardiometabolic health, aging, and longevity in ad libitum-fed mice. Cell Metab. 2014;19:418–430. doi: 10.1016/j.cmet.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilley M, Benjamin RE, Srivarin P, Tilley KA. Nonenzymatic preparative-scale synthesis of dityrosine and 3-bromotyrosine. Anal Biochem. 2004;334:193–195. doi: 10.1016/j.ab.2004.07.014. [DOI] [PubMed] [Google Scholar]
- Todorovski T, Fedorova M, Hennig L, Hoffmann R. Synthesis of peptides containing 5-hydroxytryptophan, oxindolylalanine, N-formylkynurenine and kynurenine. J Pept Sci. 2011;17:256–262. doi: 10.1002/psc.1322. [DOI] [PubMed] [Google Scholar]
- Tropel P, Noel D, Platet N, Legrand P, Benabid AL, et al. Isolation and characterisation of mesenchymal stem cells from adult mouse bone marrow. Exp Cell Res. 2004;295:395–406. doi: 10.1016/j.yexcr.2003.12.030. [DOI] [PubMed] [Google Scholar]
- Tyner SD, Venkatachalam S, Choi J, Jones S, Ghebranious N, et al. p53 mutant mice that display early ageing-associated phenotypes. Nature. 2002;415:45–53. doi: 10.1038/415045a. [DOI] [PubMed] [Google Scholar]
- Urao N, Ushio-Fukai M. Redox regulation of stem/progenitor cells and bone marrow niche. Free Radic Biol Med. 2013;54:26–39. doi: 10.1016/j.freeradbiomed.2012.10.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentine JS, Doucette PA, Zittin Potter S. Copper-zinc superoxide dismutase and amyotrophic lateral sclerosis. Annu Rev Biochem. 2005;74:563–593. doi: 10.1146/annurev.biochem.72.121801.161647. [DOI] [PubMed] [Google Scholar]
- Valko M, Morris H, Cronin MT. Metals, toxicity and oxidative stress. Curr Med Chem. 2005;12:1161–1208. doi: 10.2174/0929867053764635. [DOI] [PubMed] [Google Scholar]
- Wang D, Wang C, Wu X, Zheng W, Sandberg K, et al. Endothelial dysfunction and enhanced contractility in microvessels from ovariectomized rats: roles of oxidative stress and perivascular adipose tissue. Hypertension. 2014 doi: 10.1161/HYPERTENSIONAHA.113.02284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Ou G, Hamrick M, Hill W, Borke J, et al. Age-related changes in the osteogenic differentiation potential of mouse bone marrow stromal cells. J Bone Miner Res. 2008;23:1118–1128. doi: 10.1359/JBMR.080304. [DOI] [PMC free article] [PubMed] [Google Scholar]


