Abstract
The term immunodominance was originally defined as a restricted T cell response to a short peptide sequence derived from a given protein [1]. The question of what determines immunodominance has been a longstanding battle for the past two decades. Hundreds of papers have been written on different aspects of epitope selection during antigen processing documenting the complexity of the process. Antigen processing machinery involves several accessory molecules and chaperons coevolved with proteins of Major Histocompatibility Complex (MHC) molecules that each plays its part in epitope selection. These molecules are targeted to specialized vesicular compartments that also accommodate antigen processing enzymes called cathepsins. Within the antigen processing compartments, highly regulated pH gradient and reducing conditions and enzymes necessary for denaturation of the antigens are available and function to optimize processing of antigen and selection of the fittest for transport to the cell membrane and presentation to T cells. Despite the complexity, a cell free reductionist antigen processing system was recently reported that included only few purified proteins, but was shown to process and select physiologically relevant epitopes from full length protein antigens [2]. Because of its minimalist nature the system has been quite helpful in dissecting the factors that contribute to epitope selection during antigen processing. In this review, we would summarize and highlight models that may explain how the dominant epitope may be selected for presentation to CD4+ helper T cells.
Introduction
Antigen presentation to CD4+ T cells by APCs begins by the uptake of exogenous antigens and their processing that involves transfer through a series of endosomal compartments containing suitable denaturing environment, accessory molecules and molecular chaperons, as well as proteolytic enzymes cathepsins [3]. Newly synthesized MHC class II (MHC II) molecule associates with the class II invariant chain (Ii), which targets it to specialized endosomal compartments called MIIC where the Ii is proteolysed until only a fragment known as the class II-associated invariant chain peptide (CLIP) remains bound in the MHC II peptide-binding groove. Efficient displacement of CLIP from the MHC groove requires the accessory molecule HLA-DM in human or H2-M in mice (DM) [4]. DM functions by inducing conformational changes in pMHC II complexes resulting in the release of the bound peptide inducing a peptide-receptive MHC II [5]. A peptide-receptive MHC II can quickly sample a large pool of peptides derived from exogenously acquired proteins. Hence, in addition to removal of CLIP, DM helps in shaping epitope selection (more details to follow). Cathepsins present in processing compartments contribute by cutting and trimming of the protein antigens. Somehow during these processes few peptides from many peptides that can bind to MHC II are selected to represent the antigen to T cells. In this review, we would focus of the factors that influence this selection process.
Why should there be a selection for immunodominant epitopes?
To recognize many potential pathogenic antigens, naive T cells are evolved to express a large variety of unique receptors and be contained in the limited space of lymph nodes. Because of space limitations, despite continuous output of native T cells from the thymus and the exponential increase in number of specific T cell during infections, the T cell number in the periphery remains relatively stable throughout life [6]. Cell death during the contraction phase of the immune response is a major contributor to the maintenance of cell numbers, but some memory T cells and B cells remain in our lymphatic system for future pathogen attacks, causing a slight change in lymph node size after each infection [7]. Having to keep memory T cells specific for each antigen for life necessitates that their overall numbers to remain within numbers that can be accommodated by the limited space of lymph nodes. Thus, too many memory T cells specific for the same antigen might create accommodation issues, hence there is a need for the immune system for restricting the total number of the T cells specific for each antigen.
Epitope accessibility
Multiple factors can contribute to immunodominance and one of which is ‘epitope accessibility’ [8], meaning that the stretch of epitope selected as immunodominant must be accessible to the groove of MHC molecule, and/or the cathepsins that cut and trim the epitopes. Evidence in support of accessibility notion comes from localization of many known immunodominant epitopes to the more exposed C- or N-termini of antigens [9–16], or to the flexible strands of proteins [8]. Alternatively, to make epitopes accessible, in addition to denaturing environment in the antigen processing compartments, a specialized enzyme, gamma-interferon-inducible lysosomal thiol reductase (GILT) releases disulphide bonds in proteins. A strong support for the role of GILT in release of dominant epitopes was shown in GILT deficient mice, where presentation of some buried Hen Egg Lysozyme (HEL) determinants was inhibited [17]. Two paths for the capture of the selected epitopes by the MHC molecules have been described [18]. Epitopes can first be captured by the MHC groove followed by fragmentation by the cathepsins (bind first, trim later), or protein can be first be cut by the cathepsins and then be sorted out for optimal binding for the MHC II groove (cut first, bind later). While evidence for both models exist in literature, a recent report applying the reductionist, cell free antigen processing system, discussed earlier, to several antigens derived from pathogens or autoantigens, and provided convincing data that antigen processing of pathogen-derived proteins or autoantigens follow distinct paths. Autoantigen-derived immunodominant epitopes were shown to be resistant to digestion by cathepsins, whereas pathogen-derived epitopes were sensitive [19]. Accompanied by direct evidence for epitope capture to precede cathepsin digestion for pathogen derived epitopes, authors favored the epitope capture model; and by showing that autoantigen-derived core dominant epitopes were resistant to further proteolysis by the cathepsins they strengthened the ‘cut first, bind later’ model [19].
Cathepsins in antigen processing
Of the most significant contributors to processing antigens are endocytic proteases or cathepsins (Cat). Cysteine proteases, aspartyl proteases and serine proteases are the three types of cathepsins studied for their roles in antigen processing [20,21]. The significance of cathepsins in antigen processing and the selection of immunodominant epitopes lie in their regulated expression levels and activity in different cell types and activation state, as well as occurrence of specific inhibitors of cathepsin activities in antigen presenting cells [22–24]. Two main roles attributed to cathepsins in antigen processing are to cleave off invariant chain, and to process antigens. Among the most extensively studied cathepsins are CatB, CatD, CatL, and CatS [25–27]. CatS was reported to be involved in Ii cleavage and antigen processing [28–31]. Mice deficient in CatL and CatS showed impairment of late stage invariant chain degradation in thymus and periphery respectively [32,33]. Asparagine endopeptidase (AEP) has been shown to have some role in the initial invariant chain cleavage [34], and it can either generate or destroy antigenic epitopes [35]. CatB and CatD knockout mice showed some but not complete processing defect, hence their role in antigen processing has been considered as dispensable [36].
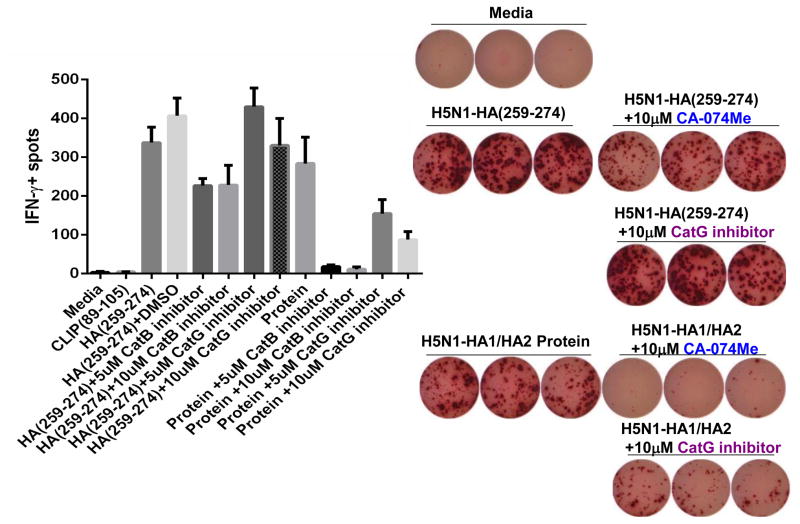
Recent studies by Kim et al [19] using their cell free processing system showed that inclusion of only three cathepsins, CatB, CatH, and CatS was sufficient to mimic the processing conditions necessary to produce the immunodominant epitopes from several antigens. While CatB and CatH are mainly exoproteases, they also have endopeptidase activities, although the pH requirement might be different [27]. The team also evaluated the need for CatB in processing of two antigens in cells and observed a complete blockage of processing in the presence of a cell-permeable CatB inhibitor, CA-074ME. Importantly, authors showed that having only CatB and CatH was sufficient to select for the dominant epitope of influenza HA1 epitopes, but CatS failed to produce the dominant epitope in the absence of CatB and CatH (Table 1). Besides the cathepsins mentioned above, it has been suggested that other groups of cathepsins such as cat G and E might also play some role in regulating antigen processing [37,38]. However, when serine protease inhibitor that inhibits Cat G, or aspartic protease inhibitors, pepstatin A and pepstatin A-penetratin, were used during the processing and presentation of type II collagen and H5N1-HA proteins results indicated that CatB has a major role in epitope selections whereas CatG, CatD, and CatE influence the generation of epitopes, but may not be critically important as CatB and CatS (Figure 1) [19]. However, for a more comprehensive cell free system it would be desirable to include CatG and CatD in addition to CatS and CatB and CatH.
Table 1. CatB and CatH are necessary and sometimes sufficient for the selection of the immunodominant epitope of HA1.
Summary of rHA1 derived peptides identified in the cell-free reductionist antigen processing system [2]. DR1 and DM were incubated with rHA1 protein followed by addition of CatB and H, CatB, CatH and CatS, or CatS alone. Peptide/DR1 complexes were isolated and peptides were eluted from DR1. Immunodominant epitope from influenza strain A/Texas/1/77 HA(306-318) is shown in Red color. Recombinant chimeric HA1 protein used here is a fusion product of influenza Puerto Rico strain A/PR/8/34 and the HA(306-318) from A/Texas/1/77 attached at C-terminal end. Blue and magenta fonts show other nondominant epitopes.
| Recombinant Influenza hemagglutinin
(rHA1) MRGSHHHHHHTDPSSRSADADTICIGYHANNSTDTVDTVLEKNVTVTHSVNLLEDSHNGKLCRLKGIAPLQLKCNIAGWLLGNPECDPLLPVRSWSYIVETPNSENGICYPGDFIDYEELREQLSSVSSFERFEIFPKESSWPNHNTNGVTAACSHEGKSSFYRNLLWLTEKEGSYPKLKNSYVNKK GKEVLVLWGIHHPPNSKEQQNLYQNENAYVSVVTSNYNRRFTPEIAERPKVRDQAGRMNYYWTLLKPGDTIIFEANGNLIAPMYAFALSRGFGSGIITSNASMHECNTKCQTPLGAINSSLPYQNIHPVTIGECPKYVRSAKLRMVTGLRNIPSIQSRGACPKYVKQNTLKLATGMRKLHHHHHH | ||
|---|---|---|
| Cathepsins included in cell-free system |
rHA1 derived peptides | Identified Peptide Sequences |
| CatB and CatH Or | HA306-318 from A/Texas/1/77 | GACcamPKYVKQNTLKLATGMoxR GACcamPKYVKQNTLKLATGMoxRK GACcamPKYVKQNTLKLATGMoxR SRGACcamPKYVKQNTLKLATGMoxRK |
| CatB, CatH, and CatS | HA298-317 from A/PR/8/34 | INSSLPYQNIHPVITIGECcamPK |
| AINSSLPYQNIHPVITIGECcamPK | ||
| CatS only | HA from A/PR/8/34 | NSYVNKKGKEVLVL |
| HA from A/PR/8/34 | HEGKSSFYRNL | |
Figure 1. CatB, but not CatG is a critical endosomal protease for generating H5N1-HA(259-274) immunodominant epitope.
ELISPOT assay measuring IFN-g production of T cells isolated from DR1-transgenic mice immunized with H5N1-HA(259-274) peptide in CFA. Cells from draining lymph nodes were stimulated with HA(259-274) or proteins in vitro for 48h in the presence, or absence of CA-074ME, which is a cell-permeable CatB inhibitor, or a CatG specific inhibitor. Error bars represent SD of spots from triplicate cultures shown. Peptide stimulation in vitro serves as controls to assess the toxicity of the enzyme inhibitors. Processing of HA1 protein by APC in vitro yields the dominant epitopes identified by the cell free processing system. Roles of CatB, CatD, and CatE in generation of the H5N1-HA(259-274) immunodominant epitope upon processing of HA1 protein by the APC in 48 h culture in ELISPOT assay using specific inhibitors has been described in ref 19.
Not to dismiss the role for pH, cathepsins involved in antigen processing generally are activated at acidic pH in APC, which itself is highly regulated. Trombetta et al demonstrated that efficient formation of peptide-MHC class II complexes is enhanced by activation of the vacuolar proton pump upon DC maturation that enhanced lysosomal acidification and antigen proteolysis [22]. These findings emphasize the critical role that cathepsins play in antigen presentation by establishing that strict regulatory mechanisms are in place to allow for the generation of antigenic epitopes when needed.
Role of HLA-DM in immunodominance
‘Kinetic stability’, or affinity model for MHC class II/peptide has been a popular model as determinant of immunodominance [39]. According to this model, immunodominant determinants form highly stable complexes with MHC class II. Hence much emphasis has been placed on screening of antigens for epitopes that bind MHC molecules stably. The model was in part built on studies reporting that the affinity of pMHC II complex correlated with susceptibility to DM, i.e., fast dissociating pMHC II complexes were susceptible to DM-mediated peptide exchange, but slow dissociating pMHC II were relatively resistant to DM [40–42]. This viewpoint changed by the demonstration of structural properties of pMHC II complexes. It was reported that empty or partially filled P1 pocket of the MHC II molecule, HLA-DR1 (DR1) generated a favorable substrate for interaction with DM, whereas compact conformation induced by a filled P1 pocket was an unfavorable substrate [43–45]. An important contribution to the DM mechanism was provided by Pos et al [46], who solved a high-resolution crystal structure of DM/DR1 complex. The structure revealed that the P1 pocket was the primary target for interaction with DM and that by filling the P1 pocket the interaction of DM with DR was disrupted [47]. Another interesting finding was recently reported by Yin et al who demonstrated that in the absence of poor P1 anchor, peptides that had strong pocket 9 binding residues were resistant to HLA-DM-mediated peptide exchange [48]. A substantial study by Yin et al compared multiple methods commonly used for the determination of immunodominance, such as intrinsic dissociation t(1/2), IC(50), DM-mediated dissociation t(1/2), and two epitope prediction algorithms for a large set of peptides from vaccinia virus and compared these properties to peptide-specific CD4(+) T cell responses. They found that MHC II-peptide complex kinetic stability in the presence of DM distinguished T cell epitopes from nonrecognized peptides. Taken together, these analyses demonstrated that DM-mediated dissociation t(1/2) is the major factor governing peptide immunogenicity [21]. All these reports document the importance of DM in screening for immunodominant epitope selection to peptide selection. It is noteworthy however, that majority of our understanding of DM effects in epitope selection comes from the studies that utilize DR1 molecules while some MHC class II alleles are rather refractory to DM effects as they do not have a conformation recognized by DM [5,43,49,50]. For those alleles, perhaps kinetic stability may be the determinant of epitope selection.
A fresh understanding for dominant epitope selection was recently provided by Kim et al, who showed that peptides derived from pathogens, or autoantigens, behaved differently in response to DM[19]. Authors showed that for autoantigens DM susceptibility was not a required criterion, whereas for pathogen-derived dominant epitopes, DM resistance was a crucial factor. Immunodominance emerged as a result of the combined effects of DM and the antigen processing cathepsins. Autoantigen-derived immunodominant epitopes were resistant to digestion by cathepsins, whereas pathogen-derived epitopes were sensitive. As such, sensitivity to cathepsins enforced capture of pathogen-derived epitopes by MHC II before processing, and resistance to DM-mediated-dissociation preserved the longevity of those epitopes. The overall findings established that immunodominance is established by higher relative abundance of the selected epitopes that survive cathepsin digestion, either by binding to MHC II and resisting DM-mediated-dissociation, or by being chemically resistant to cathepsins degradation. Non-dominant epitopes were found to be sensitive to both DM and cathepsins [19] (Figure 2).
Figure 2. Two processing pathways for antigens derived from pathogens or self.
Pathogen-derived protein antigens may be captured by MHC II first, and then processed by cathepsins. Pathogen-derived dominant epitopes are highly sensitive to destruction by the cathepsins, are generally DM-insensitive, and are protected by the groove of MHC II. On the contrary, auto antigen-derived epitopes are resistant to cathepsins, and may, or may not be sensitive to DM-mediated peptide exchange (from Ref 19). Cathepsins are shown as scissors, peptides and epitopes are depicted as part of the denatured proteins, or in short stretches of sequences that carry a MHC II P1 fitting anchor or no anchor. The selected pMHC complexes are transported to the APC cell surface waiting for T cell stimulation. Small dots represent degraded peptides.
Role of HLA-DO in immunodominance
In addition to DM, another non-classical MHC class II accessory molecule, HLA-DO (H2-O in mice), is known to play a role in peptide exchange [51–53]. Unlike DM, the expression of HLA-DO or H2-O (DO) is restricted to B cells, thymic medulla and certain subsets of DCs, and its cellular trafficking depends on DM. Understanding the role of DO in antigen processing has been a challenge for decades. Recently, the crystal structure of DM in complex with DO was solved, which showed that DO binds to HLA-DM at the same sites implicated in MHCII interaction [54]. Hence, the findings were interpreted as DO competing with DM in interaction with DR leading to the inhibition of DM function. While this model has some support in literature [53], kinetic peptide binding and dissociation experiments performed in the presence of DM and/or DO suggested a different mechanism [51,52]. It was reported that DO reduced binding of DR1 to some peptides, and enhanced the binding of some other peptides to DR1. Peptides that were negatively affected by DO were DM-sensitive, whereas peptides that were enhanced by DO were DM-resistant. The positive and negative effects of DO could only be measured on binding kinetics as peptide dissociation kinetics were not affected by DO. Direct binding of DO to a peptide-receptive, but not a closed conformation of DR1 was a strong indication of direct role of DO in epitope selection rather than through inhibition of DM. Thus, DO and DM work in synergy for optimizing peptide exchange and the selection of DM-resistant peptides. In light of these findings, inclusion of DO in our cell free system can better assess the dominant epitope selection.
What about T cells?
We have discussed different factors that contribute to the dominant epitope selection by the MHC II molecules. Once the immunodominant epitope is selected, another layer of complexity to consider is the availability of the specific T cells that would recognize the selected epitopes. Not only the presence of the right T cells is important, T cells bearing TCR with different affinity and/or avidity might recognize and respond to the same pMHC pair differently [55–57]. In addition, Unanue and colleagues have long studied an interesting phenomenon that reveals different clonal T cells recognize the same pMHC pair differently; Type A and Type B T cells recognize two conformations of pMHC that differ by their sensitivity to DM-mediated dissociation [58]. Another variable that was recently introduced by Jenkins and colleagues was differences in precursor frequency for naïve T cells recognizing different pMHC specificities. Authors used pMHC II tetramer based enrichment assays to show that there were up to ten folds variation in precursor populations. The study further correlated the magnitude of the T cell responses to their initial frequency [59]. Therefore, while T cells may have the final say in determination of the immunodominance, they would fail to respond if their specific pMHC is missing.
Concluding remarks
Here we have discussed a variety of contributing factors to immunodominance for CD4 helper T cells. Our own studies combined with our colleagues have led to the conclusions that it is necessary for the immune system to select for few dominant epitopes. The process involves many factors, but the success of our cell free reductionist system in identifying immunodominant epitopes from many different protein antigens has shed some light in the most critical contributors [2,19]. In combination with DM and cathepsins, immunodominant epitopes are carefully selected and appear as the most abundant peptides bound to the MHC II molecules. This gain in abundance is due to the combined effects of resistance to DM and/or cathepsins in the system as well as the elimination of the nondominant epitopes, which are susceptible to both DM and cathepsins. Once the dominant epitopes are selected, they are recognized by their cognate T cells, which hopefully do exist and hence initiate an adaptive immune response.
Highlights.
Immunodominant epitope processing and selection requires DM, MHC II, and cathepsins
Immunodominance is determined by epitope abundance
Antigens from different source might be processed differently
Pathogen derived epitopes are captured on intact antigens and are insensitive to DM
Autoantigens are captured as peptides and are insensitive to cathepsins
Acknowledgments
Authors thank Dr. Isamu Z. Hartman who contributed to some of the work discussed here. This work was supported by grants from NIAID R01AI063764 and R21 AI101987 to SS-N.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sercarz EE, Lehmann PV, Ametani A, Benichou G, Miller A, Moudgil K. Dominance and crypticity of T cell antigenic determinants. Annu Rev Immunol. 1993;11:729–766. doi: 10.1146/annurev.iy.11.040193.003501. [DOI] [PubMed] [Google Scholar]
- 2**.Hartman IZ, Kim A, Cotter RJ, Walter K, Dalai SK, Boronina T, Griffith W, Lanar DE, Schwenk R, Krzych U, et al. A reductionist cell-free major histocompatibility complex class II antigen processing system identifies immunodominant epitopes. Nat Med. 2010;16:1333–1340. doi: 10.1038/nm.2248. A reductionist cell-free antigen processing system was established that included MHC II, DM and three cathepsins. The system successfully identified immunodominant epitopes from protein antigens and closely mimics antigen processing in vivo. Thus, it provides a new tool for the identification of physiologically relevant helper T cell epitopes from antigens. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blum JS, Wearsch PA, Cresswell P. Pathways of antigen processing. Annual review of immunology. 2013;31:443–473. doi: 10.1146/annurev-immunol-032712-095910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morris P, Shaman J, Attaya M, Amaya M, Goodman S, Bergman C, Monaco JJ, Mellins E. An essential role for HLA-DM in antigen presentation by class II major histocompatibility molecules. Nature. 1994;368:551–554. doi: 10.1038/368551a0. [DOI] [PubMed] [Google Scholar]
- 5.Narayan K, Chou CL, Kim A, Hartman IZ, Dalai S, Khoruzhenko S, Sadegh-Nasseri S. HLA-DM targets the hydrogen bond between the histidine at position beta81 and peptide to dissociate HLA-DR-peptide complexes. Nat Immunol. 2007;8:92–100. doi: 10.1038/ni1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29:848–862. doi: 10.1016/j.immuni.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 7*.Vezys V, Yates A, Casey KA, Lanier G, Ahmed R, Antia R, Masopust D. Memory CD8 T-cell compartment grows in size with immunological experience. Nature. 2009;457:196–199. doi: 10.1038/nature07486. In this paper author show that the size of the memory CD8 T-cell compartment doubled in response to vaccination to accommodate new effector memory CD8 T cells. Therefore, developing vaccines that abundantly introduce new memory CD8 T cells should not necessarily ablate pre-existing immunity to other infections because of the increase in lymph node size. [DOI] [PubMed] [Google Scholar]
- 8.Dai G, Steede NK, Landry SJ. Allocation of helper T-cell epitope immunodominance according to three-dimensional structure in the human immunodeficiency virus type I envelope glycoprotein gp120. J Biol Chem. 2001;276:41913–41920. doi: 10.1074/jbc.M106018200. [DOI] [PubMed] [Google Scholar]
- 9.Lee P, Matsueda GR, Allen PM. T cell recognition of fibrinogen. A determinant on the A alpha-chain does not require processing. J Immunol. 1988;140:1063–1068. [PubMed] [Google Scholar]
- 10.Thomas JW, Danho W, Bullesbach E, Fohles J, Rosenthal AS. Immune response gene control of determinant selection. III. Polypeptide fragments of insulin are differentially recognized by T but not by B cells in insulin immune guinea pigs. J Immunol. 1981;126:1095–1100. [PubMed] [Google Scholar]
- 11.Zamvil SS, Mitchell DJ, Moore AC, Kitamura K, Steinman L, Rothbard JB. T-cell epitope of the autoantigen myelin basic protein that induces encephalomyelitis. Nature. 1986;324:258–260. doi: 10.1038/324258a0. [DOI] [PubMed] [Google Scholar]
- 12.Ultee ME, Margoliash E, Lipkowski A, Flouret G, Solinger AM, Lebwohl D, Matis LA, Chen C, Schwartz RH. The T lymphocyte response to cytochrome c--II. Molecular characterization of a pigeon cytochrome c determinant recognized by proliferating T lymphocytes of the B10. A mouse. Mol Immunol. 1980;17:809–822. doi: 10.1016/0161-5890(80)90030-9. [DOI] [PubMed] [Google Scholar]
- 13.Nepom GT, Lippolis JD, White FM, Masewicz S, Marto JA, Herman A, Luckey CJ, Falk B, Shabanowitz J, Hunt DF, et al. Identification and modulation of a naturally processed T cell epitope from the diabetes-associated autoantigen human glutamic acid decarboxylase 65 (hGAD65) Proceedings of the National Academy of Sciences of the United States of America. 2001;98:1763–1768. doi: 10.1073/pnas.98.4.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guillet JG, Lai MZ, Briner TJ, Smith JA, Gefter ML. Interaction of peptide antigens and class II major histocompatibility complex antigens. Nature. 1986;324:260–262. doi: 10.1038/324260a0. [DOI] [PubMed] [Google Scholar]
- 15.Thomas DW, Hsieh KH, Schauster JL, Mudd MS, Wilner GD. Nature of T lymphocyte recognition of macrophage-associated antigens. V. Contribution of individual peptide residues of human fibrinopeptide B to T lymphocyte responses. J Exp Med. 1980;152:620–632. doi: 10.1084/jem.152.3.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Solinger AM, Ultee ME, Margoliash E, Schwartz RH. T-lymphocyte response to cytochrome c. I. Demonstration of a T-cell heteroclitic proliferative response and identification of a topographic antigenic determinant on pigeon cytochrome c whose immune recognition requires two complementing major histocompatibility complex-linked immune response genes. J Exp Med. 1979;150:830–848. doi: 10.1084/jem.150.4.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maric M, Arunachalam B, Phan UT, Dong C, Garrett WS, Cannon KS, Alfonso C, Karlsson L, Flavell RA, Cresswell P. Defective antigen processing in GILT-free mice. Science. 2001;294:1361–1365. doi: 10.1126/science.1065500. [DOI] [PubMed] [Google Scholar]
- 18.Trombetta ES, Mellman I. Cell biology of antigen processing in vitro and in vivo. Annu Rev Immunol. 2005;23:975–1028. doi: 10.1146/annurev.immunol.22.012703.104538. [DOI] [PubMed] [Google Scholar]
- 19**.Kim A, Hartman IZ, Poore B, Boronina T, Cole RN, Song N, Ciudad MT, Caspi RR, Jaraquemada D, Sadegh-Nasseri S. Divergent paths for the selection of immunodominant epitopes from distinct antigenic sources. Nature communications. 2014;5:5369. doi: 10.1038/ncomms6369. Using a reductionist cell free processing system authors describe two paths for the capture of immunodominant epitopes from pathogens versus autoantigens. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van den Hoorn T, Paul P, Jongsma ML, Neefjes J. Routes to manipulate MHC class II antigen presentation. Current opinion in immunology. 2011;23:88–95. doi: 10.1016/j.coi.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 21**.Yin L, Calvo-Calle JM, Dominguez-Amorocho O, Stern LJ. HLA-DM constrains epitope selection in the human CD4 T cell response to vaccinia virus by favoring the presentation of peptides with longer HLA-DM-mediated half-lives. Journal of immunology. 2012;189:3983–3994. doi: 10.4049/jimmunol.1200626. The study compared multiple methods commonly used for the determination of immunodominance for a large set of peptides from vaccinia virus for peptide-specific CD4(+) T cell responses. They found that that resistance to DM-mediated dissociation was the major factor governing peptide immunogenicity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCurley N, Mellman I. Monocyte-derived dendritic cells exhibit increased levels of lysosomal proteolysis as compared to other human dendritic cell populations. PloS one. 2010;5:e11949. doi: 10.1371/journal.pone.0011949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Delamarre L, Pack M, Chang H, Mellman I, Trombetta ES. Differential lysosomal proteolysis in antigen-presenting cells determines antigen fate. Science. 2005;307:1630–1634. doi: 10.1126/science.1108003. [DOI] [PubMed] [Google Scholar]
- 24.Trombetta ES, Ebersold M, Garrett W, Pypaert M, Mellman I. Activation of lysosomal function during dendritic cell maturation. Science. 2003;299:1400–1403. doi: 10.1126/science.1080106. [DOI] [PubMed] [Google Scholar]
- 25.Manoury B. Proteases: essential actors in processing antigens and intracellular toll-like receptors. Frontiers in immunology. 2013;4:299. doi: 10.3389/fimmu.2013.00299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hsing LC, Rudensky AY. The lysosomal cysteine proteases in MHC class II antigen presentation. Immunol Rev. 2005;207:229–241. doi: 10.1111/j.0105-2896.2005.00310.x. [DOI] [PubMed] [Google Scholar]
- 27.Chapman HA. Endosomal proteases in antigen presentation. Curr Opin Immunol. 2006;18:78–84. doi: 10.1016/j.coi.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 28.Villadangos JA, Ploegh HL. Proteolysis in MHC class II antigen presentation: who’s in charge? Immunity. 2000;12:233–239. doi: 10.1016/s1074-7613(00)80176-4. [DOI] [PubMed] [Google Scholar]
- 29.Honey K, Rudensky AY. Lysosomal cysteine proteases regulate antigen presentation. Nat Rev Immunol. 2003;3:472–482. doi: 10.1038/nri1110. [DOI] [PubMed] [Google Scholar]
- 30.Pluger EB, Boes M, Alfonso C, Schroter CJ, Kalbacher H, Ploegh HL, Driessen C. Specific role for cathepsin S in the generation of antigenic peptides in vivo. Eur J Immunol. 2002;32:467–476. doi: 10.1002/1521-4141(200202)32:2<467::AID-IMMU467>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 31.Riese RJ, Wolf PR, Bromme D, Natkin LR, Villadangos JA, Ploegh HL, Chapman HA. Essential role for cathepsin S in MHC class II-associated invariant chain processing and peptide loading. Immunity. 1996;4:357–366. doi: 10.1016/s1074-7613(00)80249-6. [DOI] [PubMed] [Google Scholar]
- 32.Nakagawa TY, Brissette WH, Lira PD, Griffiths RJ, Petrushova N, Stock J, McNeish JD, Eastman SE, Howard ED, Clarke SR, et al. Impaired invariant chain degradation and antigen presentation and diminished collagen-induced arthritis in cathepsin S null mice. Immunity. 1999;10:207–217. doi: 10.1016/s1074-7613(00)80021-7. [DOI] [PubMed] [Google Scholar]
- 33.Nakagawa T, Roth W, Wong P, Nelson A, Farr A, Deussing J, Villadangos JA, Ploegh H, Peters C, Rudensky AY. Cathepsin L, critical role in Ii degradation and CD4 T cell selection in the thymus. Science. 1998;280:450–453. doi: 10.1126/science.280.5362.450. [DOI] [PubMed] [Google Scholar]
- 34.Manoury B, Mazzeo D, Fugger L, Viner N, Ponsford M, Streeter H, Mazza G, Wraith DC, Watts C. Destructive processing by asparagine endopeptidase limits presentation of a dominant T cell epitope in MBP. Nat Immunol. 2002;3:169–174. doi: 10.1038/ni754. [DOI] [PubMed] [Google Scholar]
- 35.Watts C, Moss CX, Mazzeo D, West MA, Matthews SP, Li DN, Manoury B. Creation versus Destruction of T Cell Epitopes in the Class II MHC Pathway. Ann N Y Acad Sci. 2003;987:9–14. doi: 10.1111/j.1749-6632.2003.tb06028.x. [DOI] [PubMed] [Google Scholar]
- 36.Deussing J, Roth W, Saftig P, Peters C, Ploegh HL, Villadangos JA. Cathepsins B and D are dispensable for major histocompatibility complex class II-mediated antigen presentation. Proc Natl Acad Sci U S A. 1998;95:4516–4521. doi: 10.1073/pnas.95.8.4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burster T, Reich M, Zaidi N, Voelter W, Boehm BO, Kalbacher H. Cathepsin E regulates the presentation of tetanus toxin C-fragment in PMA activated primary human B cells. Biochemical and biophysical research communications. 2008;377:1299–1303. doi: 10.1016/j.bbrc.2008.10.162. [DOI] [PubMed] [Google Scholar]
- 38.Burster T, Macmillan H, Hou T, Boehm BO, Mellins ED. Cathepsin G, roles in antigen presentation and beyond. Molecular immunology. 2010;47:658–665. doi: 10.1016/j.molimm.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lazarski CA, Chaves FA, Jenks SA, Wu S, Richards KA, Weaver JM, Sant AJ. The kinetic stability of MHC class II:peptide complexes is a key parameter that dictates immunodominance. Immunity. 2005;23:29–40. doi: 10.1016/j.immuni.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 40.Sloan VS, Cameron P, Porter G, Gammon M, Amaya M, Mellins E, Zaller DM. Mediation by HLA-DM of dissociation of peptides from HLA-DR. Nature. 1995;375:802–806. doi: 10.1038/375802a0. [DOI] [PubMed] [Google Scholar]
- 41.Weber DA, Evavold BD, Jensen PE. Enhanced dissociation of HLA-DR-bound peptides in the presence of HLA-DM. Science. 1996;274:618–620. doi: 10.1126/science.274.5287.618. [DOI] [PubMed] [Google Scholar]
- 42.Kropshofer H, Vogt AB, Moldenhauer G, Hammer J, Blum JS, Hammerling GJ. Editing of the HLA-DR-peptide repertoire by HLA-DM. Embo J. 1996;15:6144–6154. [PMC free article] [PubMed] [Google Scholar]
- 43.Chou CL, Sadegh-Nasseri S. HLA-DM recognizes the flexible conformation of major histocompatibility complex class II. J Exp Med. 2000;192:1697–1706. doi: 10.1084/jem.192.12.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sadegh-Nasseri S, Chou CL, Hartman IZ, Kim A, Narayan K. How HLA-DM works: recognition of MHC II conformational heterogeneity. Frontiers in bioscience. 2012;4:1325–1332. doi: 10.2741/s334. [DOI] [PubMed] [Google Scholar]
- 45.Pu Z, Lovitch SB, Bikoff EK, Unanue ER. T cells distinguish MHC-peptide complexes formed in separate vesicles and edited by H2-DM. Immunity. 2004;20:467–476. doi: 10.1016/s1074-7613(04)00073-1. [DOI] [PubMed] [Google Scholar]
- 46**.Pos W, Sethi DK, Call MJ, Schulze MS, Anders AK, Pyrdol J, Wucherpfennig KW. Crystal structure of the HLA-DM-HLA-DR1 complex defines mechanisms for rapid peptide selection. Cell. 2012;151:1557–1568. doi: 10.1016/j.cell.2012.11.025. The X-ray structure of the HLA-DM-HLA-DR complex showed major conformational changes in HLA-DR peptide-binding groove and P1 sites reverses the conformational changes, terminating selection through DM dissociation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Anders AK, Call MJ, Schulze MS, Fowler KD, Schubert DA, Seth NP, Sundberg EJ, Wucherpfennig KW. HLA-DM captures partially empty HLA-DR molecules for catalyzed removal of peptide. Nat Immunol. 2011;12:54–61. doi: 10.1038/ni.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48**.Yin L, Trenh P, Guce A, Wieczorek M, Lange S, Sticht J, Jiang W, Bylsma M, Mellins ED, Freund C, et al. Susceptibility to HLA-DM is determined by a dynamic conformation of major histocompatibility complex class II molecule bound with peptide. The Journal of biological chemistry. 2014 doi: 10.1074/jbc.M114.585539. Peptides interacting non-optimally in P1 pocket exhibited low MHCII binding affinity and kinetic instability and were highly susceptible to HLA-DM-mediated peptide exchange. These changes were accompanied by conformational alterations, which could be reversed by substitution of the P9 pocket anchor residue. Thus a dynamic MHCII conformational determinant rather than P1 pocket occupancy is the key factor determining susceptibility to HLA-DM-mediated peptide exchange. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wolf PR, Tourne S, Miyazaki T, Benoist C, Mathis D, Ploegh HL. The phenotype of H-2M-deficient mice is dependent on the MHC class II molecules expressed. Eur J Immunol. 1998;28:2605–2618. doi: 10.1002/(SICI)1521-4141(199809)28:09<2605::AID-IMMU2605>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 50.Bikoff EK, Wutz G, Kenty GA, Koonce CH, Robertson EJ. Relaxed DM Requirements During Class II Peptide Loading and CD4+ T Cell Maturation in BALB/c Mice. J Immunol. 2001;166:5087–5098. doi: 10.4049/jimmunol.166.8.5087. [DOI] [PubMed] [Google Scholar]
- 51.Poluektov YO, Kim A, Sadegh-Nasseri S. HLA-DO and Its Role in MHC Class II Antigen Presentation. Frontiers in immunology. 2013;4:260. doi: 10.3389/fimmu.2013.00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52**.Poluektov YO, Kim A, Hartman IZ, Sadegh-Nasseri S. HLA-DO as the optimizer of epitope selection for MHC class II antigen presentation. PloS One. 2013;8:e71228. doi: 10.1371/journal.pone.0071228. Using recombinant HLA-DO and kinetic binding and dissociation studies authors showed that HLA-DO in cooperation with DM optimizes epitope exchange for the best fitting peptides. Thus, DO in not an inhibitor of DM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Denzin LK, Cresswell P. Sibling rivalry: competition between MHC class II family members inhibits immunity. Nature structural & molecular biology. 2013;20:7–10. doi: 10.1038/nsmb.2484. [DOI] [PubMed] [Google Scholar]
- 54.Guce AI, Mortimer SE, Yoon T, Painter CA, Jiang W, Mellins ED, Stern LJ. HLA-DO acts as a substrate mimic to inhibit HLA-DM by a competitive mechanism. Nature structural & molecular biology. 2013;20:90–98. doi: 10.1038/nsmb.2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Campos-Lima PO, Levitsky V, Imreh MP, Gavioli R, Masucci MG. Epitope-dependent selection of highly restricted or diverse T cell receptor repertoires in response to persistent infection by Epstein-Barr virus. J Exp Med. 1997;186:83–89. doi: 10.1084/jem.186.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Perkins DL, Wang YS, Fruman D, Seidman JG, Rimm IJ. Immunodominance is altered in T cell receptor (beta-chain) transgenic mice without the generation of a hole in the repertoire. J Immunol. 1991;146:2960–2964. [PubMed] [Google Scholar]
- 57.Kedl RM, Kappler JW, Marrack P. Epitope dominance, competition and T cell affinity maturation. Curr Opin Immunol. 2003;15:120–127. doi: 10.1016/s0952-7915(02)00009-2. [DOI] [PubMed] [Google Scholar]
- 58.Lovitch SB, Unanue ER. Conformational isomers of a peptide-class II major histocompatibility complex. Immunol Rev. 2005;207:293–313. doi: 10.1111/j.0105-2896.2005.00298.x. [DOI] [PubMed] [Google Scholar]
- 59.Moon JJ, Chu HH, Pepper M, McSorley SJ, Jameson SC, Kedl RM, Jenkins MK. Naive CD4(+) T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity. 2007;27:203–213. doi: 10.1016/j.immuni.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]