Abstract
During adulthood electroencephalographic (EEG) recordings are used to distinguish wake, non rapid eye movement (NREM) sleep, and rapid eye movement (REM) sleep. However, the close association between behavioral states and EEG rhythms is reached only late during development, after birth in humans and by the end of the second postnatal week in rats and mice. This critical time is also when cortical activity switches from a discontinuous to a continuous pattern, and we will review the major cellular and network changes that can account for this transition. After this close link is established, new evidence suggests that the slow waves of NREM sleep may function as markers to track cortical development. Before the EEG can be used to identify behavioral states, however, two distinct sleep phases – quiet sleep and active sleep – are identified based on behavioral criteria and muscle activity. During this early phase of development cortical activity is far from being disorganized, despite the presence of long periods of neuronal silence and the poor modulation by behavioral states. Specific EEG patterns such as spindle bursts and gamma oscillations have been identified very early on, and are believed to play a significant role in the refinement of brain circuits. Since most early EEG patterns do not map to a specific behavioral state, their contribution to the presumptive role of sleep in brain maturation remains to be established, and should be a major focus for future research.
Keywords: active sleep, REM sleep, quiet sleep, NREM sleep, gamma activity, spindle bursts, theta activity
Dissociation between cortical rhythms and sleep/wake behavior in early development
In the adult mammalian brain specific cortical EEG patterns are used to define behavioral states. The wake EEG is dominated by low-voltage fast-activity in the beta (16–30 Hz) and gamma (>30 Hz) range (“activated” EEG). During NREM sleep instead, the EEG mainly shows waxing and waning oscillations at around 12–15 Hz called sleep spindles, and slow (delta) waves of large amplitude. Slow wave activity (SWA) - the EEG power in the 0.5–4.5 Hz - is a convenient way to assess number and amplitude of slow waves and is a marker of NREM sleep intensity, i.e. arousal thresholds during sleep are higher when SWA is higher (1). SWA is also an established marker of sleep pressure, since its value reflects the duration of prior wake, peaking at sleep onset, further increasing after acute sleep deprivation, and declining in the course of sleep (2). Thus, the number and amplitude of slow waves in the adult brain reflect both the need for sleep and its depth. REM sleep is characterized by a wake-like, tonically activated EEG, but can be distinguished from wake due to the presence of phasic events (e.g. rapid eye movements and twitches of the limbs) and tonic phenomena (e.g. loss of tone in antigravitary muscles).
The close association between specific cortical EEG patterns and behavioral states starts only after the cerebral cortex has completed most of its anatomical development. In rodents, this occurs by the end of the second week after birth, at around postnatal days P11–P12. Between P0 and P10, a period during which the rat cortex shows explosive growth, cortical oscillations are instead only weakly modulated by behavioral states and EEG activity is discontinuous, with long periods of neuronal silence interrupted by spindle bursts, evoked gamma oscillations and other short-lasting EEG patterns (Fig. 1). Since rodents are altricial - born in a far less mature condition than humans - the cortical maturity of the rat during the first postnatal week corresponds to that of the young premature human brain (3) (4, 5). Rodents are therefore good models to study the development of the sleep/wake cycle and its EEG rhythms, because more immature stages of these processes can be studied in postnatal life when they are more experimentally accessible. Here we will focus on studies in rats and mice because the development of the sleep/wake cycle, the maturation of EEG rhythms, and their underlying cellular mechanisms are best characterized in these species.
Figure 1.
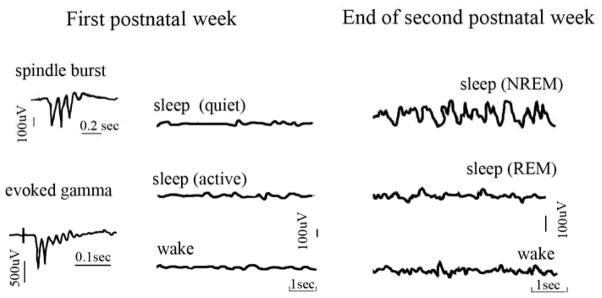
Schematic representations of cortical EEG recordings in a rat pup. During the first postnatal week transient events such as spindle bursts and evoked gamma oscillations can be observed, but EEG activity is mainly independent of behavioral states (see text for details). Traces are redrawn from (46) (spindle bursts), (64) (evoked gamma), and (13) (sleep/wake traces). Behavioral states during the first postnatal week were defined based on behavioral observation (respiration pattern, body, vibrissae, and tail movements, eyes open/closed).
Development of the rodent cortex
The development of the rat brain has been subdivided into four stages (6). Briefly, in the fetal period cell division produces 94–97% of all brain cells. The first postnatal phase (P0–P10) is characterized by explosive growth: at P0 the brain is only 15% of its final size, but by P10 most growth of cells, and especially of axons and dendrites, has been completed (7–9). In the third phase (P11–P20) the rate of growth is much reduced, the extracellular space decreases significantly, blood vessels grow, mature astrocytes and oligodendrocytes are easily found, and myelinization starts (10, 11). After weaning (usually at P21 in mice and rats) growth is very slow (fourth phase). As described in detail in the Supplemental Discussion, the second week represents a pivotal point in the transition from immature to mature cortex: number of synapses and overall connectivity increase, silent synapses almost disappear, GABA A signaling becomes hyperpolarizing, and the rules of synaptic plasticity change.
Development of the sleep/wake cycle in rodents
Several studies in rodent pups have described how specific behaviors emerge and evolve during the first postnatal weeks, concurrent with the profound anatomical changes occurring in cortex. For instance in an early study, Bolles and Woods provide a detailed behavioral observation of wake and sleep activities in rat litters and their mothers from birth to P26 (12). They describe sleep as “very fitful” in the first few days, constantly interrupted by “convulsion-like spasms”, attempts to find the preferred sleeping position, and movements from siblings. At P1 movements during sleep are described as gentle fluttering often involving the whole body, while twitching becomes more localized to the limbs and tail starting at P4, increases in frequency and strength until P9, and becomes more “peaceful” in the next 10 days. Pups were estimated to spend >80% of total time “sleeping or resting”. According to the authors, however, this was probably an overestimation due to two reasons: 1) the inability to clearly see the pups when lying under the mother, and 2) the use of visual observation as the only criterion to define sleep.
More recent studies have identified sleep by combining visual observation with measurements of muscle activity and EEG recordings (e.g. (13–15)). Using this approach some authors have suggested that sleep starts as a disorganized mixed state (16, 17). However, recent evidence is more consistent with the presence from birth of two distinct sleep phases, quiet sleep and active sleep, which represent early forms of what later, by the end of the second postnatal week, are recognizable as NREM sleep and REM sleep, respectively (14, 18, 19). Quiet sleep is a state of behavioral immobility and reduced muscle tone sometimes interrupted by startles. Startles are among the first behavioral events recorded in human fetuses and neonatal rats and consist of sudden, spontaneous and simultaneous contractions of muscles throughout the body. Although rare (1–2/min) startles occur in all behavioral states, and thus are not a unique feature of quiet sleep (20). Active sleep, by contrast, is uniquely characterized by frequent myoclonic twitches of isolated muscles that occur on a background of muscle atonia. Several pontine and medullary areas that mediate muscle atonia and twitches during REM sleep in adults are also involved in the generation of these REM sleep components in infants, consistent with the view that infant sleep is qualitatively similar to adult sleep (21, 22).
During the second postnatal week EEG activity becomes continuous and strongly modulated by behavioral states. Specifically, the total power in the cortical EEG signal in all behavioral states increases after P9 (13), consistent with P10 being a crucial step in cortical maturation and synaptogenesis (23) (24). Neuronal firing in the rat visual cortex also increases sharply in both quiet and active sleep at P11–P12 (25). After P12 the total power in the EEG signal during quiet sleep is consistently higher than in wake and active sleep, due to the fact that slow waves start to be detected from the scalp (13–15). After the appearance of slow waves, which map into quiet sleep exclusively, this state can be unambiguously identified as NREM sleep. Spindles start appearing during NREM sleep at P14, and by P18 the EEG is adult-like (13). At the same time, during the second postnatal week the frequency of twitches during active sleep declines sharply, the EEG becomes tonically activated, and active sleep can be unambiguously identified with REM sleep (14, 18) (26). It is important to point out that there isn’t a single discrete event occurring after P11–P12 that dramatically changes the structure of sleep and wake. Rather, by the end of the second postnatal week the distinction across the three behavioral states as defined in adults becomes obvious, due to the appearance of slow waves in the scalp EEG and the progressive evolution of EEG activity from discontinuous to continuous.
It was generally assumed that in newborn pups most if not all sleep is active sleep. In fact, the estimates for time spent in each sleep phase differ widely in early studies, perhaps in part because of differences in the duration of the recordings and their precise timing during the day. According to one report (27) quiet sleep is not present during the first postnatal week, and pups are either awake or, more often, in active sleep (60–70% of the time). By contrast, two other studies reported that at least 30% of total sleep is quiet sleep (28, 29). The discrepancy may be due to the fact that in the study that failed to identify quiet sleep in the first postnatal week this behavioral state was defined as a period of at least 30 sec of behavioral quiescence (27). This is a very conservative criterion, since quiescent periods in rats are often less than 2 sec long in the first postnatal week, and the mean duration of quiet sleep bouts exceeds 30 sec only by P13 (14, 22).
Still, the amount of quiet sleep in early development remains unclear. At P11 and P13 active and quiet sleep each account for ~35% of total recording time when sleep scoring is based only on muscle activity and absence/presence of twitches (14). However, when the EEG is used as an additional scoring criterion the percentages change to 50% for quiet sleep and 20% for active sleep (14). This suggests that even before P11 the time spent in quiet sleep may have been underestimated.
The single most important feature that distinguishes sleep from quiet wake is the presence of an increased arousal threshold, i.e. a reduced ability to respond to stimuli. Assessing arousal thresholds is difficult in infant rodents, because sleep bouts are very short and most sensory systems are immature at birth. Thus, there is still little direct evidence that the state of hypotonia/atonia that we call infant sleep is indeed not just rest. One seminal study, however, found that at P8 the threshold to exhibit polypnea in response to an olfactory stimulus is higher in active sleep relative to wake, consistent with an increased sensory threshold during sleep (30). To our knowledge there are no data about arousal thresholds in sleep during the first postnatal week, or in quiet sleep specifically.
Another important feature of sleep is the presence of homeostatic mechanisms that trigger changes in sleep duration and intensity after sleep loss. Infant sleep is already homeostatically regulated during the first two postnatal weeks. Specifically, in both P2 and P8 rat pups, sleep deprivation for 30 min increases sleep pressure, indicated by the progressive increase in the number of arousal stimuli needed to keep the animals awake, and is followed by an increase in sleep duration (31). Between the third and forth postnatal week young rats and mice also start showing changes in sleep intensity after extended wake, i.e. SWA at sleep onset is higher after sleep deprivation relative to baseline. Of note, the SWA rebound is not immediately apparent around the end of second week, when slow waves first appear in the sleep EEG, most likely because of a ceiling effect due to their very high amplitude (32). Indeed, in rats (33), mice (34) and humans (35–37) SWA during development follows an inverted U-shape that is believed to reflect cortical synaptic changes, first increasing and peaking due to synaptogenesis, and then declining through adolescence into adulthood due to refinement and/or pruning of synaptic connections (33, 34, 38).
General features of early patterned activity
Cortical activity during early development is already very structured, but has unique features relative to the one seen in the adult brain. The presence of long periods of electric silence is one of the main differences between neonatal and adult activity. With maturation, these periods of inactivity become progressively shorter (from many seconds to 200–500 msecs), confined to one behavioral state (OFF periods of NREM sleep), and tend to occur synchronously in many areas, although they are rarely global (39). Many factors may account for the discontinuity of early cortical activity, including the paucity of synaptic connections, the presence of silent NMDA synapses, the scarcity of thalamocortical connections and long-range intracortical connections, and the intrinsic neuronal properties (40, 41). Immature neurons, for instance, are very sensitive to synaptic inputs due to their high input resistance but fire few action potentials, probably because they express few voltage-gated channels.
Another main difference between immature and mature cortical activity is that the former is not strongly modulated even by the most obvious changes in behavior, i.e. the cycling between sleep and wake. As we have seen, in the infant pup sleep/wake behaviors are defined by changes in muscle activity, which are driven by brainstem and spinal cord circuits. Early on, therefore, cortex and subcortical areas may function independent of each other. One reason may be the weak effects of ascending projections from the arousal systems to the cortex. The adult pattern of noradrenergic innervation in rat visual and motor cortex is reached only by P14, and by the end of the third week the density of noradrenergic fibers is at adult-like levels (42). Many noradrenergic neurons in the rat locus coeruleus are not spontaneously active during the first postnatal week, and adult-like rates of tonic firing are only reached at ~P20 (43). Similarly, cholinergic fibers grow significantly in the supragranular layers only during the second postnatal week (44), and a mature cholinergic innervation is reached only between the third and fourth week (45). These findings suggest that it is only when subcortical areas “take full control” of the maturing cortex that behavior (sleep/wake) and cortical activity no longer dissociate.
Early cortical patterns in vivo
Early cortical activity is also heterogeneous, with different patterns occurring in different cortical areas. Due to space limitations we will focus on spindle bursts, the best characterized early oscillations in vivo, and gamma activity, but a discussion of other patterns, including in the hippocampus, can be found in the Supplemental Discussion.
During the first postnatal week spindle bursts represent the dominant pattern of spontaneous organized activity in rat primary sensory (46, 47) and motor (48, 49) areas. They consist of spindle-shaped bursts of network activity in the alpha-beta frequency range (8–25 Hz) associated with rhythmic unit discharges (46) (Fig. 1). Their name reflects the many similarities they share with the spindles recorded in the adult brain during NREM sleep, with three important differences: spindle bursts tend to be spatially confined within a few hundred micron wide “column” and do not often spread from one cortical area to another, occur also in waking, and are in most cases triggered by movements or sensory stimulation (46). Despite the name, spindle bursts are actually nested in an envelope of delta waves (1–4 Hz) that becomes evident using full-band depth EEG (46) (50, 51). For this reason they closely resemble delta brushes, spindle-like activity superimposed on slow/delta waves (0.3–1.5 Hz) that are detected in humans starting from the seventh month of gestation (52). Spindle bursts in rodents decline during the second postnatal week, when they can still be observed but are obscured by the continuous ongoing activity. Similarly, delta brushes in humans are replaced by more continuous EEG activity around the time of normal birth (40).
Spindle bursts in rat somatosensory cortex (S1)
In S1 spindle bursts can be detected at P1, when they last ~ 1–2 sec and occur every 20–30 sec, interrupting long periods of silence. They become more frequent (one every ~10 sec) and larger during the first postnatal week (53), peak at P8 and then decline over the next week (26). Roughly 25% of spindle bursts persist after sensory deafferentation, indicating that they are self-organized endogenous patterns, probably of thalamocortical or cortical origin (46). In the intact brain, however, their frequency is strongly modulated by the high rate of spontaneous movements triggered by stochastic bursts in the spinal cord network (46). Video recordings in freely moving pups show that these movements include not only the isolated twitches observed during active sleep, but also wake-related movements such as whole-body startles, crawling, and sucking (46). Thus, although quantitative data for all behavioral states are missing, it seems that spindle bursts in S1 occur independent of sleep and wake states. Delta brushes in the somatosensory cortex of premature human neonates also occur in all behavioral states, can be triggered by sensory feedback from spontaneous movements (in ~30% of cases during sleep), but in most cases occur in the absence of overt movements, consistent with the idea that like spindle bursts, they are endogenous network patterns (54).
Studies in barrel cortex demonstrate that spindle bursts are driven by activation of glutamatergic synapses. The fast spindle-like activity is mainly dependent on AMPA receptor activation, while associated delta waves are driven by both NMDA and AMPA receptors (50, 51). GABAergic synapses are not crucial for the generation of spindle bursts, but are essential for their confinement to restricted cortical domains (50). Glutamatergic synapses on cortical neurons are provided by intracortical connections, and/or by thalamocortical inputs. Thus, during the first postnatal week spindle bursts have been ascribed a crucial role in shaping thalamocortical connections and cortical maps (46). Consistent with this idea, blockade of glutamate receptors during the first postnatal weeks disrupts the refinement of thalamocortical as well as intracortical connectivity (55, 56). For instance, blocking both NMDA and non-NMDA receptors in S1 during the first two postnatal weeks does not prevent the clustering of thalamocortical axons in a vibrissae-related pattern, but impairs the elimination of inappropriate connections between thalamus and layer IV neurons, and between layer IV neurons and neurons in supragranular layers of neighboring barrels, resulting in inappropriate cortical responses to stimulation of the principal vibrissa (55, 56). More direct evidence for the crucial role of spindle bursts in rat cortical development was revealed by the selective ablation of S1 subplate neurons at P0. This intervention not only causes the almost complete disappearance of spindle bursts, but also prevents the strengthening of thalamocortical connections and the formation of a normal barrel pattern (57).
Calcium imaging of large ensemble of neurons in layers II/III of mouse barrel cortex also shows highly correlated bursts of firing among neurons located within 100um of each other during the first postnatal week. This highly synchronous bursting activity, reminiscent of spindle bursts, is substituted by mainly decorrelated activity after P12, despite no major changes in average firing rates (58). Whole-cell recordings suggest that the decorrelation in the activity of pyramidal neurons may be due to a decrease in intrinsic excitability and an increase in inhibitory inputs, both of which occur during the second postnatal week; decorrelation instead does not depend on sensory experience, since it still occurs after whisker plucking (58).
Spindle bursts, short bursts, and SATs in rat primary visual cortex (V1)
In pups anesthetized with urethane or deep ice-cooling anesthesia spindle bursts in V1 appear at P2 and increase in frequency in the first postnatal week (47). They share many similarities with spindle bursts recorded in S1 in non-anesthetized pups (46): 1) they have similar dominant frequency, shape and duration; 2) they are mainly local events, associated with cortical multi-unit activity in deep cortical layers; 3) they are endogenous, i.e. they persist after block of action potential propagation in the optic nerve or removal of the retina, although they cannot be triggered by cortical stimulation (59); 4) they are often triggered by peripheral stimuli and specifically, in V1, by spontaneous retinal waves that propagate from the retina to the lateral geniculate nucleus and then to cortex. These similarities suggest that the fundamental features of these early oscillations are not significantly affected either by the presence/absence of anesthesia, or by the specific type of anesthetic agent.
A recent study in non-anesthetized pups described the relationship between spindle bursts and visual evoked responses in V1 (60). During the first postnatal week spindle bursts cannot be evoked by light flashes because rod- and cone-mediated visual signaling is not functional (negative phototaxis mediated by melanopsin-expressing retinal ganglion cells is instead already present (61)). Starting at P8 light flashes produce an early visual evoked response followed by an evoked spindle burst. During the first part of the second week, however, the spontaneous EEG still includes long periods of neuronal silence, and spontaneous activity is poorly modulated by sleep and wake (“bursting period”). By contrast, starting around P12 light flashes result in adult-like visual evoked responses that are no longer followed by evoked spindle bursts, and the EEG is strongly modulated by behavioral state and more continuous, presumably because cortical horizontal connections develop by the end of the second week (“acuity period”). The transition from the bursting to the acuity period is rapid, occurring within 12 hours in each individual pup, and depends at least in part on the activity of arousal systems such as the noradrenergic neurons of the locus coeruleus (60). This switch precedes eye opening by 2 days and does not depend on experience, since it happens also with dark rearing or with forced eye opening at P7. The switch is associated with changes in intracolumnar processing: during the bursting period light stimulation induces a multilayer burst with no lag, and even minimal stimulation produces large, all-or-none responses. By contrast, during the acuity period the visual evoked response follows the typical columnar flow, with a sink in layer IV followed by spreading to layers II/III. Thus, until P12 cortical activity in V1 is dominated by spindle bursts and mostly reflects the maturation of cortical circuits, while after P12 it relies more heavily on experience triggered by exposure to the external world.
Slow activity transients (SATs) were originally identified as a primary feature of the EEG in premature human neonates (62). In a recent study that used direct-coupled (DC) EEG recordings SATs were also detected in rat V1, where they appear as infra-slow negative potentials (8–16 secs long) that include multiple spindle bursts separated by quiet periods (63). Consistent with the results summarized above (47), DC recordings found that during the first postnatal week spindle bursts/SATs represent the main form of correlated spontaneous activity. It was also found that SATs disappear after enucleation, strongly suggesting that they represent the cortical response to a retinal input (retinal waves) (63). Crucially, however, starting at P9 DC recordings also found additional simple field negative shifts that occur independent of spindle bursts/SATs. These short bursts (<1 sec) are associated with relatively slow activity (<10 Hz) and not, like spindle bursts, with alpha/beta oscillations. They are linked to strong cortical multi-unit activity and by P13 they represent most of cortical spontaneous events, resembling the slow waves of NREM sleep, while SATs become less consistent and are difficult to separate from the ongoing cortical activity (63). The opposite developmental profile and the fact that the occurrence of short bursts increases, rather than decreases, after enucleation, suggest that spindle bursts and short bursts represent different forms of cortical activity, the former driven from the periphery, the latter intrinsically generated in the cortex.
Gamma activity in S1
During the first postnatal week two other patterns of spontaneous oscillatory activity exist in S1, fast and frequent gamma oscillations (~ 38Hz, every 10sec, lasting 0.2sec) that occur only in the barrel field, and rare, long oscillations (~ 13Hz, every 20min, lasting >1min) that can spread across the entire cortex (53). Like spindle bursts, both types of oscillations are spontaneous but can be triggered by sensory stimulation (53). Indeed, starting as early as P0–P1 the deflection of the principal whisker triggers gamma activity in the ventral posterior medial nucleus of the thalamus, followed by gamma bursts and then spindle bursts in the granular layer of the corresponding barrel cortex (64, 65) (Fig. 1). This sensory-evoked “vertical” gamma activity results from excitatory inputs to granular cells with little involvement of inhibitory postsynaptic currents, and abruptly declines by P8. During the second postnatal week, when horizontal connections start forming, vertical gamma oscillations are gradually replaced by “horizontal” ones that are maximal in supragranular layers and spread across neighboring columns (64). It is unknown whether gamma oscillations are simply a marker of the ongoing maturation of cortical circuits or have any specific function. Since in vitro stimulation mimicking the early sensory-evoked gamma activity results in the potentiation of thalamocortical EPSPs (while spindle bursts cause depression of the same synapses), it has been suggested that gamma oscillations play a crucial role in the early refinement of thalamocortical circuits (64). Of note, similarly to spindle bursts, this early gamma activity is not modulated by behavioral state, i.e. it can occur during both sleep and wake. Thus, its putative role in promoting growth and synaptic potentiation of thalamocortical circuits may not be confined to sleep.
Spindle bursts in rat motor cortex (M1)
Simultaneous recordings of forepaw movements and cortical activity in S1 and M1 in P3–P5 pups under urethane anesthesia show that tactile forepaw stimulation triggers spindle bursts in S1, followed 5–20 msec later by gamma and spindle bursts in M1 (48). Analysis of spontaneous activity also shows that at this age most (~2/3) spontaneous gamma and spindle bursts in M1 are motor-related, either following or, less frequently, preceding forepaw movements; they are also temporally correlated with spindle bursts in S1, consistent with the idea that gamma and spindle bursts in M1 are important for development and maintenance of sensorimotor maps (48). The remaining 30% of spontaneous spindle bursts in M1, smaller and of shorter duration, are uncorrelated to movements. Local injection of lidocaine in the forepaw abolishes all cortical responses normally evoked in S1 and M1 by mechanical stimulation of the forepaw, as well as all spontaneous forepaw movements. By contrast, spontaneous gamma and spindle activity in M1 is reduced (by ~50%) but not abolished, indicating that a considerable fraction of burst activity in M1 is generated within the cortex itself, as it is the case in S1 and V1 (48). Another study suggests that the ability of spontaneous movements to trigger spindle bursts in M1 depends on behavioral state (49). Thus, recordings in M1 of P8 to P10 non-anesthetized pups found that manual flexion of the hindlimb triggers an increase in M1 unit activity and spindle bursts during both wake and sleep. Spontaneous hindlimb movements, instead, do not trigger M1 activity during wake, but do so during active sleep, especially in association with twitches, which are followed by spindle bursts with a latency of at least 100msecs (49). Overall, these results are consistent with the idea that via proprioceptive feedback fetal movements during the third trimester of gestation, like spontaneous movements in premature neonates, may play an important role in the maturation of cortical sensorimotor maps (54).
Oscillations in prefrontal cortex
Under urethane anesthesia, discontinuous spindle-like activity is present in prefrontal cortex starting at P3. It resembles spindle bursts, but its frequency is lower, in the theta range (66). Starting at P5, some spindle bursts, mainly in the prelimbic cortex, have nested gamma oscillations, while “simple” spindle bursts are more frequent in the cingulate cortex. Occurrence, duration, amplitude, and dominant frequency of both spindle bursts and nested gamma spindle bursts increase with age, until oscillatory activity rapidly switches to continuous activity at P10–P11. At that time the sparse unit activity (~0.7 Hz) observed in prefrontal cortex during the first postnatal week also switches to much higher levels. Thus, the emergence of discontinuous spindle bursts is delayed in prefrontal cortex, but the switch to continuous activity occurs at the same time as in primary sensory areas.
Conclusions
Starting immediately after birth distinct oscillatory patterns have been identified in different cortical areas in rodents. It has been suggested that until P7–P8 spindle bursts/SATs and gamma bursts play an important role in promoting the refinement of thalamocortical connectivity in primary sensory areas. Specifically, spindle bursts in somatosensory and motor cortex have been proposed to be important for the formation of the body map (40, 46, 48, 49). However, spindle bursts/SATs and gamma bursts are not confined to sleep during early development, and thus there is no strong evidence that sleep is required for their putative functions. Hippocampal theta bursts, on the other hand, have been involved in the refinement of prefrontal cortex connectivity (66) and there is a strong link between hippocampal theta activity and active sleep (67) (68), although even in this case theta bouts can occur outside of this state (see Supplemental Discussion). More causal experiments are needed to determine whether interfering with specific cortical or hippocampal patterns affects the maturation of brain circuits, whether the effects are irreversible, and to what extent they depend on behavioral state.
S1 and V1 are the cortical areas that have been best characterized. In these regions a similar developmental switch in the pattern of neuronal activity seems to occur, from the discontinuous EEG dominated by synchronous bursts triggered by movements or retinal waves during the first postnatal week, to the continuous and more decorrelated EEG activity present by the end of the second postnatal week. In both areas the switch occurs quite abruptly starting at P8, once cortical synapses “explode” in number and cortical horizontal connections start forming. In both areas early bursting activity and late decorrelated activity have a different origin, the former mainly reflecting activation coming from the periphery via the thalamus, the second representing intrinsic activity generated in cortex itself. It is after this switch that distinct cortical EEG patterns can be used to identify sleep and wake states. Thus, it is only after the cortex becomes able to generate its own oscillatory rhythms, independent of sensory stimulation, that distinct patterns of activity become associated with different behavioral states. The switch also roughly corresponds to the time when the pups start exploring and walking and open their eyes. At the same time the cortical rules of plasticity change, switching from a strong bias towards synaptic potentiation to a finer balance between potentiation and depression; this coincides with the time when plasticity switches from being mainly driven by spontaneous activity to being strongly dependent on experience caused by direct exposure to the external world. Thus, one could speculate that during the first week the goal of neural activity is to refine cortical circuits, by pruning and synaptic potentiation, and this process seems to occur at all times, in wake and sleep. By the end of the second week, instead, the cortex is mature enough to sustain its activity independent of peripheral events. Cortical activity also becomes strongly modulated by behavioral states: during wake the subject is poised to adapt to an ever-changing environment, while during sleep he is disconnected from the external world.
Supplementary Material
Footnotes
Financial Disclosures: This work was supported by grant 1R01MH091326 to CC and GT. Dr. Cirelli reports no biomedical financial interests or potential conflicts of interest. Dr. Tononi has consulted for Philips Respironics and is involved in a research study in humans supported by Philips Respironics. This study is not related to the work presented in the current manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Neckelmann D, Ursin R. Sleep stages and EEG power spectrum in relation to acoustical stimulus arousal threshold in the rat. Sleep. 1993;16(5):467–477. [PubMed] [Google Scholar]
- 2.Achermann P, Borbely AA. Mathematical models of sleep regulation. Front Biosci. 2003;8:s683–693. doi: 10.2741/1064. [DOI] [PubMed] [Google Scholar]
- 3.Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early human development. 1979;3(1):79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- 4.Gottlieb A, Keydar I, Epstein HT. Rodent brain growth stages: an analytical review. Biology of the neonate. 1977;32(3–4):166–176. doi: 10.1159/000241012. [DOI] [PubMed] [Google Scholar]
- 5.Clancy B, et al. Web-based method for translating neurodevelopment from laboratory species to humans. Neuroinformatics. 2007;5(1):79–94. doi: 10.1385/ni:5:1:79. [DOI] [PubMed] [Google Scholar]
- 6.McIlwain H. Biochemistry and the Central Nervous System. Churchill; London: 1966. Chemical and enzymic make-up of the brain during development; pp. 270–299. [Google Scholar]
- 7.Caley DW, Maxwell DS. An electron microscopic study of neurons during postnatal development of the rat cerebral cortex. J Comp Neurol. 1968;133(1):17–44. doi: 10.1002/cne.901330103. [DOI] [PubMed] [Google Scholar]
- 8.Caley DW, Maxwell DS. Development of the blood vessels and extracellular spaces during postnatal maturation of rat cerebral cortex. J Comp Neurol. 1970;138(1):31–47. doi: 10.1002/cne.901380104. [DOI] [PubMed] [Google Scholar]
- 9.Caley DW, Maxwell DS. An electron microscopic study of the neuroglia during postnatal development of the rat cerebrum. J Comp Neurol. 1968;133(1):45–70. doi: 10.1002/cne.901330104. [DOI] [PubMed] [Google Scholar]
- 10.Parnavelas JG, Luder R, Pollard SG, Sullivan K, Lieberman AR. A qualitative and quantitative ultrastructural study of glial cells in the developing visual cortex of the rat. Philos Trans R Soc Lond B Biol Sci. 1983;301(1103):55–84. doi: 10.1098/rstb.1983.0022. [DOI] [PubMed] [Google Scholar]
- 11.Bushong EA, Martone ME, Ellisman MH. Maturation of astrocyte morphology and the establishment of astrocyte domains during postnatal hippocampal development. International journal of developmental neuroscience: the official journal of the International Society for Developmental Neuroscience. 2004;22(2):73–86. doi: 10.1016/j.ijdevneu.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 12.Bolles RC, Woods PJ. The ontogeny of behaviour in the albino rat. Animal Behaviour. 1964;12:427–441. [Google Scholar]
- 13.Gramsbergen A. The development of the EEG in the rat. Dev Psychobiol. 1976;9(6):501–515. doi: 10.1002/dev.420090604. [DOI] [PubMed] [Google Scholar]
- 14.Seelke AM, Blumberg MS. The microstructure of active and quiet sleep as cortical delta activity emerges in infant rats. Sleep. 2008;31(5):691–699. doi: 10.1093/sleep/31.5.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frank MG, Heller HC. Development of diurnal organization of EEG slow-wave activity and slow- wave sleep in the rat. Am J Physiol. 1997;273(2 Pt 2):R472–478. doi: 10.1152/ajpregu.1997.273.2.R472. [DOI] [PubMed] [Google Scholar]
- 16.Frank MG, Heller HC. Development of REM and slow wave sleep in the rat. Am J Physiol. 1997;272(6 Pt 2):R1792–1799. doi: 10.1152/ajpregu.1997.272.6.R1792. [DOI] [PubMed] [Google Scholar]
- 17.Frank MG, Heller HC. The ontogeny of mammalian sleep: a reappraisal of alternative hypotheses. J Sleep Res. 2003;12(1):25–34. doi: 10.1046/j.1365-2869.2003.00339.x. [DOI] [PubMed] [Google Scholar]
- 18.Seelke AM, Karlsson KA, Gall AJ, Blumberg MS. Extraocular muscle activity, rapid eye movements and the development of active and quiet sleep. Eur J Neurosci. 2005;22(4):911–920. doi: 10.1111/j.1460-9568.2005.04322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blumberg MS, Gall AJ, Todd WD. The development of sleep-wake rhythms and the search for elemental circuits in the infant brain. Behav Neurosci. 2014;128(3):250–263. doi: 10.1037/a0035891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karlsson KA, Mohns EJ, di Prisco GV, Blumberg MS. On the co-occurrence of startles and hippocampal sharp waves in newborn rats. Hippocampus. 2006;16(11):959–965. doi: 10.1002/hipo.20224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karlsson KA, Gall AJ, Mohns EJ, Seelke AM, Blumberg MS. The neural substrates of infant sleep in rats. PLoS Biol. 2005;3(5):e143. doi: 10.1371/journal.pbio.0030143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blumberg MS, Seelke AM. The form and function of infant sleep: from muscle to neocortex. In: Blumberg MS, Freeman JH, Robinson SR, editors. Oxford Handbook of Developmental Behavioral Neuroscience. Oxford University Press; 2010. pp. 391–423. [Google Scholar]
- 23.Aghajanian GK, Bloom FE. The formation of synaptic junctions in developing rat brain: a quantitative electron microscopic study. Brain Res. 1967;6(4):716–727. doi: 10.1016/0006-8993(67)90128-x. [DOI] [PubMed] [Google Scholar]
- 24.Ashby MC, Isaac JT. Maturation of a recurrent excitatory neocortical circuit by experience-dependent unsilencing of newly formed dendritic spines. Neuron. 2011;70(3):510–521. doi: 10.1016/j.neuron.2011.02.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mirmiran M, Corner M. Neuronal discharge patterns in the occipital cortex of developing rats during active and quiet sleep. Brain Res. 1982;255(1):37–48. doi: 10.1016/0165-3806(82)90074-8. [DOI] [PubMed] [Google Scholar]
- 26.Marcano-Reik AJ, Prasad T, Weiner JA, Blumberg MS. An abrupt developmental shift in callosal modulation of sleep-related spindle bursts coincides with the emergence of excitatory-inhibitory balance and a reduction of somatosensory cortical plasticity. Behav Neurosci. 2010;124(5):600–611. doi: 10.1037/a0020774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jouvet-Mounier D, Astic L, Lacote D. Ontogenesis of the states of sleep in rat, cat, and guinea pig during the first postnatal month. Dev Psychobiol. 1970;2(4):216–239. doi: 10.1002/dev.420020407. [DOI] [PubMed] [Google Scholar]
- 28.Gramsbergen A, Schwartze P, Prechtl HF. The postnatal development of behavioral states in the rat. Dev Psychobiol. 1970;3(4):267–280. doi: 10.1002/dev.420030407. [DOI] [PubMed] [Google Scholar]
- 29.Hilakivi LA, Hilakivi IT. Sleep-wake behavior of newborn rats recorded with movement sensitive method. Behav Brain Res. 1986;19(3):241–248. doi: 10.1016/0166-4328(86)90024-0. [DOI] [PubMed] [Google Scholar]
- 30.Seelke AM, Blumberg MS. Sniffing in infant rats during sleep and wakefulness. Behav Neurosci. 2004;118(2):267–273. doi: 10.1037/0735-7044.118.2.267. [DOI] [PubMed] [Google Scholar]
- 31.Todd WD, Gibson JL, Shaw CS, Blumberg MS. Brainstem and hypothalamic regulation of sleep pressure and rebound in newborn rats. Behav Neurosci. 2010;124(1):69–78. doi: 10.1037/a0018100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nelson AB, Faraguna U, Zoltan JT, Tononi G, Cirelli C. Sleep Patterns and Homeostatic Mechanisms in Adolescent Mice. Brain Sciences. 2013 doi: 10.3390/brainsci3010318. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Olini N, Kurth S, Huber R. The effects of caffeine on sleep and maturational markers in the rat. PLoS One. 2013;8(9):e72539. doi: 10.1371/journal.pone.0072539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Vivo L, et al. Developmental patterns of sleep slow wave activity and synaptic density in adolescent mice. Sleep. 2013 doi: 10.5665/sleep.3570. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Campbell IG, Feinberg I. Longitudinal trajectories of non-rapid eye movement delta and theta EEG as indicators of adolescent brain maturation. Proc Natl Acad Sci U S A. 2009;106(13):5177–5180. doi: 10.1073/pnas.0812947106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kurth S, et al. Mapping of cortical activity in the first two decades of life: a high-density sleep electroencephalogram study. J Neurosci. 2010;30(40):13211–13219. doi: 10.1523/JNEUROSCI.2532-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buchmann A, et al. EEG sleep slow-wave activity as a mirror of cortical maturation. Cereb Cortex. 2011;21(3):607–615. doi: 10.1093/cercor/bhq129. [DOI] [PubMed] [Google Scholar]
- 38.Ringli M, Huber R. Developmental aspects of sleep slow waves: linking sleep, brain maturation and behavior. Prog Brain Res. 2011;193:63–82. doi: 10.1016/B978-0-444-53839-0.00005-3. [DOI] [PubMed] [Google Scholar]
- 39.Nir Y, et al. Regional slow waves and spindles in human sleep. Neuron. 2011;70(1):153–169. doi: 10.1016/j.neuron.2011.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khazipov R, Buzsaki G. Early patterns of electrical activity in the developing cortex. In: Blumberg MS, Freeman JH, Robinson SR, editors. Oxford Handbook of Developmental Behavioral Neuroscience. Oxford University Press; 2010. pp. 161–177. [Google Scholar]
- 41.Dehorter N, Vinay L, Hammond C, Ben-Ari Y. Timing of developmental sequences in different brain structures: physiological and pathological implications. Eur J Neurosci. 2012;35(12):1846–1856. doi: 10.1111/j.1460-9568.2012.08152.x. [DOI] [PubMed] [Google Scholar]
- 42.Latsari M, Dori I, Antonopoulos J, Chiotelli M, Dinopoulos A. Noradrenergic innervation of the developing and mature visual and motor cortex of the rat brain: a light and electron microscopic immunocytochemical analysis. J Comp Neurol. 2002;445(2):145–158. doi: 10.1002/cne.10156. [DOI] [PubMed] [Google Scholar]
- 43.Nakamura S, Kimura F, Sakaguchi T. Postnatal development of electrical activity in the locus ceruleus. J Neurophysiol. 1987;58(3):510–524. doi: 10.1152/jn.1987.58.3.510. [DOI] [PubMed] [Google Scholar]
- 44.Janiesch PC, Kruger HS, Poschel B, Hanganu-Opatz IL. Cholinergic control in developing prefrontal-hippocampal networks. J Neurosci. 2011;31(49):17955–17970. doi: 10.1523/JNEUROSCI.2644-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reboreda A, Raouf R, Alonso A, Seguela P. Development of cholinergic modulation and graded persistent activity in layer v of medial entorhinal cortex. J Neurophysiol. 2007;97(6):3937–3947. doi: 10.1152/jn.01233.2006. [DOI] [PubMed] [Google Scholar]
- 46.Khazipov R, et al. Early motor activity drives spindle bursts in the developing somatosensory cortex. Nature. 2004;432(7018):758–761. doi: 10.1038/nature03132. [DOI] [PubMed] [Google Scholar]
- 47.Hanganu IL, Ben-Ari Y, Khazipov R. Retinal waves trigger spindle bursts in the neonatal rat visual cortex. J Neurosci. 2006;26(25):6728–6736. doi: 10.1523/JNEUROSCI.0752-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.An S, Kilb W, Luhmann HJ. Sensory-evoked and spontaneous gamma and spindle bursts in neonatal rat motor cortex. J Neurosci. 2014;34(33):10870–10883. doi: 10.1523/JNEUROSCI.4539-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tiriac A, Del Rio-Bermudez C, Blumberg MS. Self-Generated Movements with “Unexpected” Sensory Consequences. Curr Biol. 2014 doi: 10.1016/j.cub.2014.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Minlebaev M, Ben-Ari Y, Khazipov R. Network mechanisms of spindle-burst oscillations in the neonatal rat barrel cortex in vivo. J Neurophysiol. 2007;97(1):692–700. doi: 10.1152/jn.00759.2006. [DOI] [PubMed] [Google Scholar]
- 51.Minlebaev M, Ben-Ari Y, Khazipov R. NMDA receptors pattern early activity in the developing barrel cortex in vivo. Cereb Cortex. 2009;19(3):688–696. doi: 10.1093/cercor/bhn115. [DOI] [PubMed] [Google Scholar]
- 52.Khazipov R, Luhmann HJ. Early patterns of electrical activity in the developing cerebral cortex of humans and rodents. Trends Neurosci. 2006;29(7):414–418. doi: 10.1016/j.tins.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 53.Yang JW, Hanganu-Opatz IL, Sun JJ, Luhmann HJ. Three patterns of oscillatory activity differentially synchronize developing neocortical networks in vivo. J Neurosci. 2009;29(28):9011–9025. doi: 10.1523/JNEUROSCI.5646-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Milh M, et al. Rapid cortical oscillations and early motor activity in premature human neonate. Cereb Cortex. 2007;17(7):1582–1594. doi: 10.1093/cercor/bhl069. [DOI] [PubMed] [Google Scholar]
- 55.Fox K, Schlaggar BL, Glazewski S, O’Leary DD. Glutamate receptor blockade at cortical synapses disrupts development of thalamocortical and columnar organization in somatosensory cortex. Proc Natl Acad Sci U S A. 1996;93(11):5584–5589. doi: 10.1073/pnas.93.11.5584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dagnew E, et al. Glutamate receptor blockade alters the development of intracortical connections in rat barrel cortex. Somatosensory & motor research. 2003;20(1):77–84. doi: 10.1080/0899022031000083852. [DOI] [PubMed] [Google Scholar]
- 57.Tolner EA, Sheikh A, Yukin AY, Kaila K, Kanold PO. Subplate neurons promote spindle bursts and thalamocortical patterning in the neonatal rat somatosensory cortex. J Neurosci. 2012;32(2):692–702. doi: 10.1523/JNEUROSCI.1538-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Golshani P, et al. Internally mediated developmental desynchronization of neocortical network activity. J Neurosci. 2009;29(35):10890–10899. doi: 10.1523/JNEUROSCI.2012-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hanganu IL, Staiger JF, Ben-Ari Y, Khazipov R. Cholinergic modulation of spindle bursts in the neonatal rat visual cortex in vivo. J Neurosci. 2007;27(21):5694–5705. doi: 10.1523/JNEUROSCI.5233-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Colonnese MT, et al. A conserved switch in sensory processing prepares developing neocortex for vision. Neuron. 2010;67(3):480–498. doi: 10.1016/j.neuron.2010.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Johnson J, et al. Melanopsin-dependent light avoidance in neonatal mice. Proc Natl Acad Sci U S A. 2010;107(40):17374–17378. doi: 10.1073/pnas.1008533107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vanhatalo S, et al. Slow endogenous activity transients and developmental expression of K+-Cl− cotransporter 2 in the immature human cortex. Eur J Neurosci. 2005;22(11):2799–2804. doi: 10.1111/j.1460-9568.2005.04459.x. [DOI] [PubMed] [Google Scholar]
- 63.Colonnese MT, Khazipov R. “Slow activity transients” in infant rat visual cortex: a spreading synchronous oscillation patterned by retinal waves. J Neurosci. 2010;30(12):4325–4337. doi: 10.1523/JNEUROSCI.4995-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Minlebaev M, Colonnese M, Tsintsadze T, Sirota A, Khazipov R. Early gamma oscillations synchronize developing thalamus and cortex. Science. 2011;334(6053):226–229. doi: 10.1126/science.1210574. [DOI] [PubMed] [Google Scholar]
- 65.Yang JW, et al. Thalamic network oscillations synchronize ontogenetic columns in the newborn rat barrel cortex. Cereb Cortex. 2013;23(6):1299–1316. doi: 10.1093/cercor/bhs103. [DOI] [PubMed] [Google Scholar]
- 66.Brockmann MD, Poschel B, Cichon N, Hanganu-Opatz IL. Coupled Oscillations Mediate Directed Interactions between Prefrontal Cortex and Hippocampus of the Neonatal Rat. Neuron. 2011;71(2):332–347. doi: 10.1016/j.neuron.2011.05.041. [DOI] [PubMed] [Google Scholar]
- 67.Leblanc MO, Bland BH. Developmental aspects of hippocampal electrical activity and motor behavior in the rat. Exp Neurol. 1979;66(2):220–237. doi: 10.1016/0014-4886(79)90076-1. [DOI] [PubMed] [Google Scholar]
- 68.Mohns EJ, Blumberg MS. Synchronous bursts of neuronal activity in the developing hippocampus: modulation by active sleep and association with emerging gamma and theta rhythms. J Neurosci. 2008;28(40):10134–10144. doi: 10.1523/JNEUROSCI.1967-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


