Abstract
Objective
The purpose of this study was 1) to establish a gender- and BMI-specific reference database of cartilage T2 values, and 2) to assess the associations between cartilage T2 values and gender, age, and BMI in knees without radiographic osteoarthritis or MRI-based (WORMS 0/1) evidence of cartilage degeneration.
Design
481 subjects between the ages of 45-65 years with Kellgren-Lawrence Scores 0/1 in the study knee were selected from the Osteoarthritis Initiative database. Baseline morphologic cartilage 3T MRI readings (WORMS scoring) and T2 measurements (resolution=0.313mmx0.446mm) were performed in the medial femur, lateral femur, medial tibia, lateral tibia, and patella compartments. In order to create a reference database, a logarithmic transformation was applied to the data to obtain the 5th-95th percentile values for T2.
Results
Significant differences in mean cartilage T2 values were observed between joint compartments. Although females had slightly higher T2 values than males in a majority of compartments, the differences were only significant in the medial femur (p<0.0001). A weak positive association was seen between age and T2 in all compartments, and was most pronounced in the patella (3.27% increase in median T2/10 years, p=0.009). Significant associations between BMI and T2 were observed, and were most pronounced in the lateral tibia (5.33% increase in median T2/5 kg/m2 increase in BMI, p<0.0001), and medial tibia (4.81% increase in median T2 /5 kg/m2 increase in BMI, p<0.0001).
Conclusions
This study established the first reference database of T2 values in a large sample of morphologically normal cartilage plates in knees without radiographic knee osteoarthritis. While cartilage T2 values were weakly associated with age and gender, they had the highest correlations with BMI.
Keywords: Osteoarthritis, Cartilage, T2 mapping, Magnetic Resonance Imaging, Osteoarthritis Initiative
Introduction
Osteoarthritis (OA) is a degenerative joint disease affecting 37.4% of US adults [1]. Despite the fact that OA is a widespread and debilitating disease, treatment options are currently limited, and established disease-modifying therapies do not exist [2, 3]. Current imaging research focuses on detecting the development of OA, such that preventative measures can be taken at early stages of the disease. Such noninvasive imaging methods are instrumental for the advancement of OA research, as they could provide insight on potential chondroprotective benefits of treatment and prevention.
Magnetic Resonance imaging (MRI) is ideal for non-invasively assessing early signs of osteoarthritic degeneration as MRI depicts morphologic joint structures such as cartilage and menisci, and can also quantify cartilage matrix biochemical changes using techniques such as transverse relaxation time (T2) mapping [4]. Cartilage T2 quantification shows promise in the assessment of early OA, as it is sensitive to changes in collagen fiber orientation[5] and water content[6] which often occur prior to development of macroscopic cartilage defects and thinning, and studies have shown that subjects with OA have elevated cartilage T2 values compared to healthy knees [4]. In addition, cartilage T2 is associated with the progression of cartilage, meniscus and bone marrow lesions in OA [7], as well as with pain[8, 9]. While numerous studies have evaluated cartilage T2 in knees with OA, no studies have examined the natural variations of cartilage T2 in morphologically normal cartilage plates of knees, as seen on MRI. Investigating the relationships between cartilage T2 and demographic features of a sample of knees free from morphological signs of OA would provide a reference database of T2 values for future studies using similar acquisition and scanning methodologies.
This study utilizes data from the Osteoarthritis Initiative (OAI; http://www.oai.ucsf.edu/), a multi-center, longitudinal study aimed at assessing biomarkers in OA including those derived from MR imaging, to establish reference values of cartilage T2 in knees without radiographic OA. The purpose of this study was 1) to establish a gender- and BMI-specific reference database of cartilage T2 values, and 2) to assess the associations between cartilage T2 values and gender, age, and BMI in knees without radiographic OA (KL 0/1) and MRI-based (WORMS 0/1) evidence of cartilage degeneration.
Methods
Sample selection
This study analyzed one knee each from subjects between the ages of 45-65 years participating in the Osteoarthritis Initiative (OAI). The OAI excluded individuals with (i) inflammatory arthropathies (including rheumatoid arthritis and seronegative spondylarthropathies), (ii) MRI contraindications, (iii) use of ambulatory aids and co-morbid conditions that may affect the ability to participate in a 4-year study. Knees included in the present analysis had a baseline Kellgren Lawrence Score (KL) of 0 or 1 and at least one joint compartment (out of 5) with a WORMS cartilage score of 0 or 1. The sample of knees was selected from those included in previous analyses of T2 measurements in knees KL grade<=2 from OAI subjects ages 45-70 years [10-12]. For this analysis we further excluded knees with (i) knee injury with deformity of the knee joint, (ii) total joint replacements at the lower extremities, (iii) MRI evidence of fractures or abnormalities, that do not fit into the spectrum of OA and may indicate other severe disease, such as tumor or inflammation. There were 481 knees meeting all criteria available for analysis.
MR Imaging
MR images were obtained using four identical 3.0 Tesla (Siemens Magnetom Trio, Erlangen, Germany) scanner and quadrature transmit-receive coils (USA Instruments, Aurora, OH, USA) in Columbus, Ohio; Baltimore, Maryland; Pittsburgh, Pennsylvania; Pawtucket, Rhode Island. The following sequences were acquired and used for WORMS scoring: sagittal 2D intermediate-weighted fast spin-echo sequence (TR/TE=3200/30ms, spatial resolution=0.357mmx0.511mm, slice thickness=3.0mm), coronal 2D proton density fast spin-echo sequence (TR/TE=3700/29ms, spatial resolution=0.365mmx0.456mm, slice thickness=3.0mm), and sagittal 3D dual-echo in steady state sequence (TR/TE=16.3/4.7ms, spatial resolution=0.365mmx0.456mm, slice thickness=0.7mm). A sagittal 2D multi-slice multi-echo sequence (MSME, TR=2700ms, TE1-TE7=10-70ms, spatial resolution=0.313mmx0.446mm, slice thickness=3.0mm, and 0.5mm gap) was used for cartilage T2 measurements[13].
Image Analysis
WORMS Scoring
MR images of the right knee obtained at baseline were reviewed on picture archiving communication system (PACS) workstations (Agfa, Ridgefield Park, NJ, USA). Two board certified radiologists with 7- and 6-years of experience read the images independently and graded cartilage and meniscus lesions as well as bone marrow edema pattern (BMEP). Cartilage lesions and BMEP were assessed in five compartments (patella, medial femur, medial tibia, lateral femur and lateral tibia) using a modified semi-quantitative whole-organ magnetic resonance imaging scores (WORMS) [14], with the highest grade of lesion recorded for each region. In case of disagreement between the two readers, a consensus reading was performed with a musculoskeletal radiologist with 23-years of experience (T.M.L.). For calibration purposes, 20 cases were read simultaneously by the three readers in consensus. Compared to the original WORMS grading system, only 5 knee compartments were analyzed as relatively mild lesions were expected. Cartilage signal and morphology were scored using an eight-point scale as described previously [15].
T2 measurements
All baseline images were analyzed using a Sun Workstation (Sun Microsystems, Palo Alto, CA, USA). Knee articular cartilage was segmented manually in five compartments: (patella, medial femur, medial tibia, lateral femur and lateral tibia) as previously reported[16, 17]. We aimed to segment as many slices as possible to cover the entire cartilage but used rigorous criteria to exclude sections with compromised image quality. A slice was only segmented if the cartilage was clearly depicted and the slice did not have evidence of partial volume effects that would have blurred the border of the cartilage. Also sections with artifacts limiting the segmentation of the cartilage were excluded. While the number of slices varied per knee (as this number may depend on knee size), in general, we segmented 3-4 slices for the medial and lateral femur, 5-6 slices for the medial and lateral tibia, and 8-9 slices for the patella. In order to exclude potential chemical shift artifacts or fluid from the region of interest, the user simultaneously examined the T2 map and the first echo of the MSME sequence (in neighboring image panels) with synchronized cursor/slice number/zoom. Areas with fluid or artifacts were not included in the region of interest.
T2 maps were computed from the MSME images on a pixel-by-pixel basis using 6 echoes (TE=20-70ms) and 3 parameter fittings accounting for noise[18, 19], and averaged over all of the slices in each cartilage compartment. The first echo (TE=10ms) was not included in the T2 fitting procedure in order to reduce potential errors resulting from stimulated echoes. A noise-corrected algorithm (which involves fitting the signal and noise to an exponential function) was implemented based on results from a recent study demonstrating increased accuracy and precision of T2 relaxation time when using with a noise correction algorithm as compared to the traditional uncorrected exponential fit[18, 19].
Statistical Analysis
Statistical analysis was performed using STATA version 12 software (StataCorp LP, College Station, TX). Analyses of T2 values in each individual joint compartment were limited to compartments with a WORMS score of 0/1. Quantile-normal plots were utilized to assess the distribution of the cartilage T2 values in each joint compartment. The quantile plots demonstrated that a logarithmic transformation was optimal to obtain a normal distribution of cartilage T2 values; a normally distributed dataset facilitates accurate quantification of percentile values of the data. Thus, a logarithmic transformation was applied to the data to obtain an approximate normal distribution, and percentile values of the log-transformed T2 data were calculated (from means and standard deviations) in each compartment. Finally, the data was reverse-transformed to quantify cartilage T2 values for various percentiles of the study cohort (5%-95%), as presented in Table 2.
The differences in mean cartilage T2 between joint compartments were assessed using mixed random effects models, in order to account for multiple T2 measurements per subject. This analysis was limited to knees that had a cartilage WORMS score of 0 or 1 in all joint compartments (n=273), and was adjusted for age, gender, BMI, WOMAC pain score, and clinical site. Statistical significance was defined as p<0.05.
The associations between mean T2 in each joint compartment (with WORMS 0/1) and 1) age, 2) gender, and 3) BMI were evaluated using linear regression analysis after log transformation. Age was designated as a continuous predictor, while gender was designated as a categorical predictor. Two regression models for BMI were implemented: in the first model, BMI was designated as continuous predictor; in the second model BMI was designated as a categorical predictor in order to investigate clinically relevant cut-points for BMI values. For the categorical analysis, BMI was subdivided into 3 strata (strata 1 - “normal BMI”=18-24.9 kg/m2; strata 2 – “overweight BMI”=25-29.9 kg/m2; strata 3 – “obese BMI” 30-45 kg/m2), in order to assess the effects of obesity on cartilage T2. In a sensitivity analysis, we also evaluated the association of T2 with age, gender and BMI in the subset of knees that had no cartilage lesions (WORMS 0/1) in all joint compartments (n=273).
In addition to descriptive statistics, Chi-squared tests were used to assess whether differences in OA risk factors (history of knee injury, history of knee surgery and family history of knee replacement, race) existed between genders, age groups (5-year increments), and BMI groups, respectively. If significant (p<0.05) differences were found between groups, the risk factor was included as a covariate in the respective regression model. The regression models with age as a predictor were adjusted for gender, BMI, WOMAC pain score, and clinical site. The regression models with BMI as a predictor were adjusted for gender, age, WOMAC pain score, clinical site, and race. The regression models with gender as a predictor were adjusted for BMI, age, WOMAC pain score, clinical site, previous surgery, previous injury, and family history of knee replacement.
The cartilage T2 and WORMS reading reproducibility results have been described previously[20]. The mean T2 values had root mean square (RMS) coefficients of variation (CV) ranging from 0.83% in the medial femur to 3.21% in the patella. For WORMS gradings, the intra-observer reproducibility in all tissues (meniscus, cartilage, bone marrow) was ≥96%, while the inter-observer reproducibility was ≥97%.
Results
Subject Characteristics
Subject characteristics are listed in Table 1. The 481 subjects included in this study have characteristics similar to all subjects in the OAI ages 45-65 with a right knee KL grade of 0-1 (n=1735). The mean (SD) age in this study was 52.2 (4.2) vs. 54.9 (5.5) years in the OAI. The mean BMI in this study was 26.6 (4.5) kg/m2 vs. 27.7 (4.7) kg/m2 in the OAI. The gender distribution in this study was 47.0% males/53.0% females vs. 43.2% males/56.8% females in the OAI. The mean WOMAC pain score in our study was 1.8 (3.4) vs. 1.8 (2.7) in the OAI.
Table 1.
Subject Characteristics
| All Subjects (n = 481) | Males (n = 226) | Females (n = 255) | P value | |
|---|---|---|---|---|
| Age^ | 52.15 ± 4.21 | 51.73 ± 4.04 | 52.52 ± 4.32 | 0.038 |
| BMI * | 26.55 ± 4.52 | 27.02 ± 3.70 | 26.14 ± 5.11 | 0.032 |
| OA Risk Factors # | ||||
| History of knee injury | 188 (39.9%) | 115 (50.88%) | 73 (28.63%) | < 0.0001 |
| Family history of knee replacement surgery | 61 (12.68%) | 25 (11.06%) | 36 (14.12%) | 0.012 |
| History of previous surgery | 76 (15.80%) | 56 (24.78%) | 20 (7.84%) | < 0.0001 |
Values are mean ± SD years
Values are mean ± SD kg/m2
Values are number (%)
Of the 481 knees the following numbers of morphologically normal cartilage compartments were included in this study: lateral femur (LF)=443; lateral tibia (LT)=404; medial femur (MF)=423; medial tibia (MT)=468; patella (PAT)=335.
Reference values for cartilage T2 values
The reference percentile values for mean cartilage T2 in each gender are presented in Table 2A. In each compartment with WORMS scores of 0-1, the cartilage T2 values had a range of ~10ms from the 5th to the 95th percentile. The lowest T2 values were observed in the lateral tibia compartment, ranging from 25.6 ms in the 5th percentile to 36.4 ms in the 95th percentile in females (n=212), and ranging from 25.4 ms in the 5th percentile to 36.9 ms in the 95th percentile in males (n=192). The medial femur had the highest T2 values of all the compartments, ranging from 34.1 ms for the 5th percentile and 42.4 ms for the 95 percentile in females (n=225), and ranging from 33.5 ms for the 5th percentile and 40.8 ms for the 95 percentile in males (n=198). In the 273 knees (137 males and 136 females) with WORMS cartilage score of 0 or 1 in all compartments, we also found significant differences in mean T2 between joint compartments (p<0.0001) in both males and females (data not shown).
Table 2A.
Reference database of percentiles of T2 values (in ms) in subjects with compartment-specific cartilage scores of WORMS 0/1 subdivided by gender*
| N | 5% | 10% | 25% | 50% | 75% | 90% | 95% | |
|---|---|---|---|---|---|---|---|---|
| Females | ||||||||
| Lateral Femur | 236 | 30.9 | 31.7 | 33.1 | 34.6 | 36.9 | 37.8 | 38.8 |
| Lateral Tibia | 212 | 25.6 | 26.7 | 28.4 | 30.6 | 32.9 | 35.1 | 36.4 |
| Medial Femur | 225 | 34.1 | 34.9 | 36.3 | 38.0 | 39.8 | 41.4 | 42.4 |
| Medial Tibia | 250 | 26.8 | 27.8 | 29.4 | 31.4 | 33.5 | 35.5 | 36.7 |
| Patella | 163 | 27.7 | 28.7 | 30.4 | 32.4 | 34.5 | 36.5 | 37.8 |
| Males | ||||||||
| Lateral Femur | 207 | 31.0 | 31.7 | 32.8 | 34.2 | 35.6 | 36.9 | 37.7 |
| Lateral Tibia | 192 | 25.4 | 26.4 | 28.3 | 30.6 | 33.0 | 35.4 | 36.9 |
| Medial Femur | 198 | 33.5 | 34.2 | 35.5 | 37.0 | 38.5 | 40.0 | 40.8 |
| Medial Tibia | 218 | 27.3 | 28.3 | 30.0 | 32.1 | 34.3 | 36.5 | 37.8 |
| Patella | 172 | 27.6 | 28.6 | 30.2 | 32.2 | 34.3 | 36.2 | 37.5 |
A logarithmic transformation was applied to the data to obtain a normal distribution, and percentile values of the log-transformed T2 data were calculated (using means and standard deviations) in each compartment. Finally, the data was reverse-transformed to quantify T2 values for various percentiles of the sample.
The reference values for cartilage T2 in BMI strata (normal, overweight, and obese) are presented in Table 2b. The differences in cartilage T2 values between subjects with normal BMI and obese BMI were greatest in the lateral tibia compartment (~4ms). More specifically, in the 5th percentile, subjects with normal BMI had a T2 of 25.0 ms, while obese subjects had a T2 of 29.3 ms; in the 95th percentile, subjects with normal BMI had a T2 of 34.4 ms and obese subjects had a T2 of 38.2 ms in the lateral tibia. The medial tibia exhibited similar trends: normal and obese subjects had an approximately 3 ms difference in mean T2.
Table 2B.
Reference database of percentiles of T2 values (in ms) in subjects with compartment-specific cartilage scores of WORMS 0/1 subdivided by BMI strata* (strata 1 - “normal BMI” = 18-24.9 kg/m2; strata 2 - “overweight BMI” = 25-29.9 kg/m2; strata 3 - “obese BMI” 30-45 kg/m2).
| N | 5% | 10% | 25% | 50% | 75% | 90% | 95% | |
|---|---|---|---|---|---|---|---|---|
| Normal BMI | ||||||||
| Lateral Femur | 191 | 31.1 | 31.9 | 33.1 | 34.6 | 36.1 | 37.6 | 38.5 |
| Lateral Tibia | 172 | 25.0 | 25.9 | 27.5 | 29.3 | 31.3 | 33.2 | 34.4 |
| Medial Femur | 184 | 33.8 | 34.6 | 35.9 | 37.5 | 39.1 | 40.7 | 41.6 |
| Medial Tibia | 199 | 26.8 | 27.6 | 29.0 | 30.6 | 32.3 | 33.9 | 34.9 |
| Patella | 148 | 27.9 | 28.9 | 30.6 | 32.6 | 34.7 | 36.8 | 38.0 |
| Overweight BMI | ||||||||
| Lateral Femur | 153 | 31.0 | 31.7 | 32.9 | 34.3 | 35.7 | 37.0 | 37.8 |
| Lateral Tibia | 137 | 25.3 | 26.3 | 28.1 | 30.2 | 32.5 | 34.8 | 36.2 |
| Medial Femur | 145 | 33.7 | 34.5 | 35.8 | 37.3 | 38.8 | 40.3 | 41.2 |
| Medial Tibia | 163 | 26.9 | 28.0 | 29.7 | 31.8 | 34.0 | 36.2 | 37.6 |
| Patella | 114 | 27.7 | 28.6 | 30.2 | 32.1 | 34.0 | 35.9 | 37.1 |
| Obese BMI | ||||||||
| Lateral Femur | 99 | 30.5 | 31.3 | 32.7 | 34.4 | 36.1 | 37.7 | 38.8 |
| Lateral Tibia | 95 | 29.3 | 30.2 | 31.7 | 33.4 | 35.3 | 37.1 | 38.2 |
| Medial Femur | 94 | 33.7 | 34.6 | 36.2 | 38.0 | 39.9 | 41.8 | 42.9 |
| Medial Tibia | 106 | 29.2 | 30.2 | 31.9 | 33.8 | 35.9 | 37.9 | 39.1 |
| Patella | 73 | 27.3 | 28.3 | 30.0 | 32.0 | 34.2 | 36.3 | 37.6 |
A logarithmic transformation was applied to the data to obtain a normal distribution, and percentile values of the log-transformed T2 data were calculated (using means and standard deviations) in each compartment. Finally, the data was reverse-transformed to quantify T2 values for various percentiles of the sampl
The relationship between subject demographics and cartilage T2
The association between gender and cartilage T2
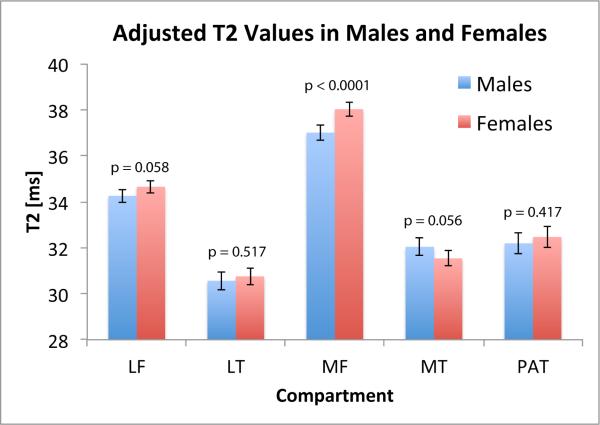
While females had slightly greater cartilage T2 values than males in a majority of compartments (LF, LT, MF, PAT), the differences were only significant in the MF, in which females had 2.74% greater median T2 values compared to males (p<0.0001; CI=1.47%-4.03%) (Figure 1). In the MT, females had slightly lower (−1.56%) median cartilage T2 values than males; however, the difference was not significant (p=0.056; CI=-3.14%-0.04%).
Figure 1.
Adjusted mean cartilage T2 values in males and females in each cartilage compartment (with WORMS scores of 0/1). Error bars represent 95% confidence intervals. (LF = Lateral Femur (Nmales = 207; Nfemales = 236), LT = Lateral Tibia (Nmales = 192; Nfemales = 212), MF = medial femur (Nmales = 198; Nfemales = 225), MT = medial tibia (Nmales = 218; Nfemales = 250), PAT = patella (Nmales = 172; Nfemales = 163)).
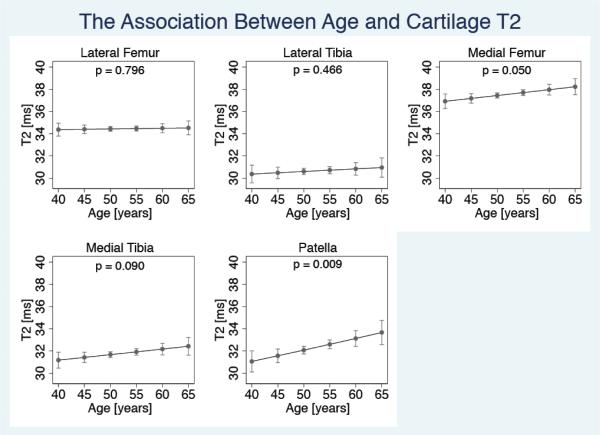
The association between age and cartilage T2
In this cohort with relatively limited age range, a positive yet weak association between age and mean T2 was evident in all compartments, and was most pronounced in the MF (1.40% increase in median T2/10 years, p=0.050, CI=0.00% - 2.82%) and the PAT (3.27% increase in median T2/10 years, p=0.009, CI=0.82% - 5.78%) (Figure 2).
Figure 2.
Association between age and cartilage T2 in each joint compartment (with WORMS scores of 0/1). Figure shows adjusted means with 95% confidence intervals. (NLateral Femur = 443; NLateral Tibia =404; NMedial Femur = 423; NMedial Tibia = 486; NPatella = 335)
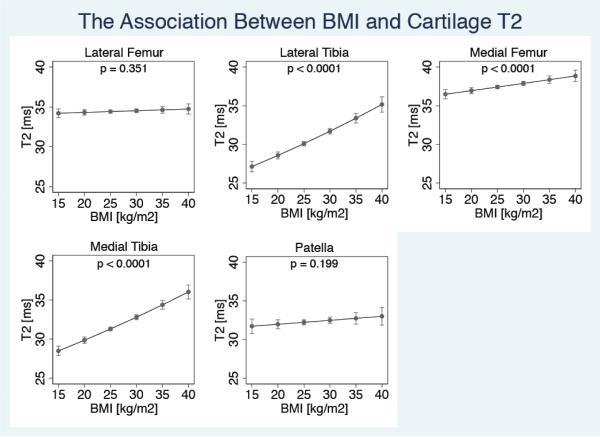
The association between BMI and cartilage T2
A positive association between BMI and mean cartilage T2 was evident in all cartilage compartments – subjects with higher BMI had higher cartilage T2 values. This relationship was significant in the LT (5.33% increase in median T2 /5 kg/m2 increase in BMI, p<0.0001, CI=4.27%-6.39%), the MF (1.26% increase in median T2 /5 kg/m2 increase in BMI, p<0.0001, CI=0.58%-1.95%), and the MT (4.81% increase in median T2 /5 kg/m2 increase in BMI, p<0.0001, CI=3.89% - 5.73%) (Figure 3A).
Figure 3A.
Association between BMI and cartilage T2 in each joint compartment (with WORMS scores of 0/1). Figure shows adjusted means with 95% confidence intervals. (NLateral Femur = 443; NLateral Tibia =404; NMedial Femur = 423; NMedial Tibia = 486; NPatella = 335)
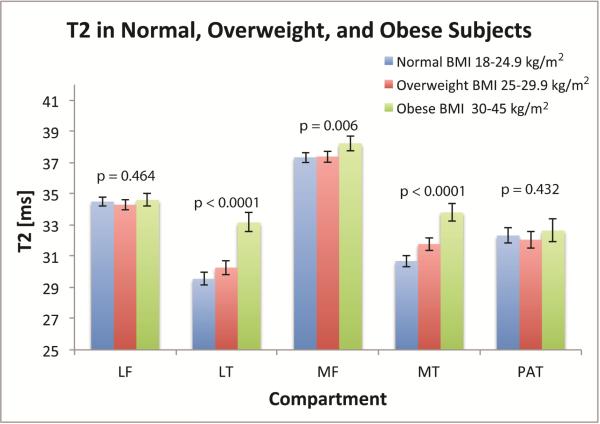
We were particularly interested in the effects of obesity on cartilage integrity. Obese subjects (BMI=30-45 kg/m2) had significantly higher (p<0.02) mean T2 values than subjects with a “normal” BMI” (BMI=18-24.9 kg/m2) and subjects that were “overweight” (BMI=25-29.9 kg/m2) in the LT, MF, and MT compartments. The differences in T2 values between obese and normal subjects were on the order of ~ 3 ms, and were most pronounced in the MT (obese subjects had 10.15% greater median T2 values than healthy subjects; p<0.0001; CI=7.90%-12.44%; obese subjects had 3.55% greater T2 values than overweight subjects; p<0.0001; CI=1.74% - 5.39%) and LT (obese subjects had 12.24% greater median T2 values than healthy subjects; p<0.0001; CI=9.64% - 14.89%; obese subjects had 2.45% greater median T2 values than overweight subjects; p=0.020; CI=0.38% - 4.56%) (Figure 3B).
Figure 3B.
Differences in mean T2 between normal, overweight, and obese subjects. Figure shows adjusted means with 95% confidence intervals. P values represent differences among the BMI strata in each compartment. LF = Lateral Femur (Nnormal = 191; Noverweight = 153; Nobese = 99), LT = Lateral Tibia (Nnormal = 172; Noverweight = 137; Nobese = 95), MF = medial femur (Nnormal = 184; Noverweight = 145; Nobese = 94), MT = medial tibia (Nnormal = 199; Noverweight = 163; Nobese = 106), PAT = patella (Nnormal = 148; Noverweight = 114; Nobese = 73).
After assessing the association between BMI and T2 using linear regression, we included an interaction effect between BMI and gender, which was not significant (p>0.05) in a majority of joint compartments (MF (p=0.22), LF (p=0.068), LT (p=0.75), PAT (p=0.75)). Thus, we did not create a joint reference table subdivided by both gender and BMI due to the fact that interaction effects between BMI and gender were mostly not significant and that sample sizes would be substantially decreased with reduced power for calculation of T2 reference values.
Cartilage T2 and Demographics in subjects with WORMS 0/1 in all joint compartments
In addition to assessing each knee compartment with WORMS 0/1 individually, we evaluated the relationship between T2 and demographic characteristics in a subset of subjects that had no cartilage lesions (WORMS 0/1) in all joint compartments (n total=273) as a sensitivity analysis. The results demonstrate similar demographic relationships to those described above if the compartment of interest had no cartilage lesions or if all joint compartments had no lesions.
Discussion
This study evaluated 481 KL grade 0/1 knees without morphological evidence of cartilage loss on MRI (WORMS 0/1) to establish reference values for cartilage T2 as well as associations with demographics including age, gender, and BMI. Significant differences in the mean cartilage T2 values were observed between joint compartments, and substantial variation was observed among subjects (~10ms range between the 5th and 95th percentiles in each compartment). While an association between cartilage T2 and both age and gender was established, the association with BMI was the most pronounced. These results suggest that the range in mean cartilage T2 values may be influenced by subject demographics and BMI. Thus, normal variation of T2 values according to demographic features should be recognized when studying T2 values in the context of OA. Overall, this database can serve as a reference for future studies performed using the same techniques, and will enable comparisons to studies that focus on cohorts with expected abnormal T2 values.
Our reference values for cartilage T2 in males and females (Table 2A) had two marked features: 1) the large differences in mean cartilage T2 values between joint compartments (as large as 7.4 ms between the medial femur and lateral tibia in females) and 2) the large range of T2 values among subjects within each compartment (as large as 11.5 ms between the 5th and 95th percentiles in the lateral tibia in males). Of all joint compartments, the medial femur had the highest T2 values, which were significantly elevated in females compared to males. This result is not unexpected as the medial compartment is prone to greater loading than the lateral compartment during gait[21], and is 10 times more-often affected by OA than the lateral compartment[22]. It is interesting that the medial femur compartment also had the most pronounced gender differences: the elevations in T2 values in females may represent early signs of OA, which corroborate epidemiologic studies reporting that females have higher prevalence of OA than males [23, 24]. The compartment with the second highest T2 values was the lateral femur demonstrating that the femoral compartment exhibited naturally higher T2 values than the tibia or the patella compartments. The ~10 ms range between the highest and lowest cartilage T2 values suggests that “normal” cartilage may a have large variation in T2 values; thus, quantifying compartment-specific cartilage T2 values and their longitudinal changes on an individual basis may be valuable for assessing disease status and progression.
While many studies have shown that the prevalence of OA increases with age [24-28], the current study is unique as it assesses age-related changes in cartilage T2 in a middle age sample without morphologic evidence of OA. A previous study by Mosher et al.[29] reported that subjects 45 years and younger had no age dependency with bulk T2, but had an age dependent elevation in subjects >45 years in the patella. While our study corroborates the results by Mosher et al., there are large differences in the subject ages between the two studies: Mosher et al. recruited subjects ages 22-86 years old, while we studied subjects 45-65 years old. We purposefully selected the younger subjects in the OAI in order to study large numbers of knees without evidence of radiographic OA or cartilage lesions. Despite the narrow age range in our study, the positive association between cartilage T2 and age was significant in the medial femur and patella compartments; the results from Mosher et al. suggest that the relationship may be more pronounced in a cohort with a larger age range. Overall, our results demonstrate that age-related changes in the collagen structure that result in an increased mobility of water can be detected using cartilage T2, and may occur prior to morphologic cartilage degeneration.
While many previous studies have established that obesity is a risk factor for development of knee OA, our study targets the association between obesity and biochemical cartilage changes that occur in early stages of OA, prior to morphologic cartilage degeneration. Numerous studies have shown that the risk of incident knee OA increases with increasing BMI [30, 31], and being overweight may contribute to an increased the risk of disease progression[32]. In addition, Koff et al. have shown a positive association between BMI and cartilage T2 in subjects with pain and radiographic arthritis in any knee compartment [33]. Several studies have evaluated the association of obesity and MRI abnormalities in knees without radiographic OA. Laberge et al. demonstrated that obesity was associated with an increased prevalence and severity of knee lesions and meniscal tears in subjects with KL<=1 [34], and, Baum et al. reported a positive association between BMI and both cartilage T2 and morphologic cartilage degeneration in subjects with KL<=1 [32]. The current study further expands upon previous ones by targeting a population free from radiographic disease and cartilage lesions. Our results corroborate previous research, demonstrating a positive relationship between BMI and T2 [33] changes representative of cartilage biochemical abnormalities. Collectively, the underlying message of these studies is that increased BMI is associated with both morphologic and biochemical changes in cartilage. Since our study focuses on biochemical properties of cartilage degeneration, the results suggest that changes in joint biomechanics [35, 36] and increases in compression stresses to the cartilage associated with obesity may predispose subjects to early signs of cartilage degeneration in the ECM, and may lead to the development of radiographic OA.
Several limitations are pertinent to this study including 1) the generalizability of our results (i.e. the inability to directly compare our results with those obtained with different techniques or different MRI scanners) and 2) our subject selection process and 3) no sub-compartmental analysis. While our study reports reference values for cartilage T2, it is important to note that cartilage T2 quantification is dependent on the type of MRI scanner[37, 38], MRI field strength[38], radiofrequency coil [39], MRI pulse sequence[40, 41], and T2 fitting method[19] used. In addition, chemical shift misregistration errors may affect the quantified T2 values, especially toward the cartilage surface [42]. Our study aimed to minimize any errors due to scanning and T2 fitting as the OAI has a rigid quality control protocol[43] and by using identical T2 fitting models for all subjects. Also, while we attempted to reduce potential errors resulting from stimulated errors by excluding the first echo in the T2 fitting procedure, recent studies have proposed novel techniques for T2 quantification [44]. Nevertheless, it is important to recognize that the results from our study are specific to the imaging and post-processing methods used. Since the cartilage T2 values are not standardized, directly comparing our results to those from other scanners and MRI pulse sequences may not be accurate. In addition, given the natural variation of T2 values, this reference database may not be able to precisely define which cartilage composition, as quantified by T2, would be considered “normal” and which would be considered early degeneration. However, developing a demographic-specific database is the first step toward a better interpretation of cartilage T2 values. Moreover, the differences in cartilage T2 between compartments and knees may represent a natural variation or differences in integrity; thus, compartment-specific longitudinal monitoring may be required to better understand the evolution of degenerative disease. Another potential limitation of this study is that the subjects were not pain-free. While it would be ideal for the subjects to be asymptomatic, our subject cohort had very low levels of pain (1.80±3.41 WOMAC pain out of a range 0-20, with a median score of 0), which were similar to the mean pain scores in the OAI database overall. In order to address this limitation, we adjusted for pain in the statistical analysis. Also, the subjects in selected in this study had KL 0/1 and WORMS 0/1. It would be ideal to limit the subject groups to only KL 0 and only WORMS 0; however, we opted to include KL 1 and WORMS 1 in order to maximize the number of subjects in the analysis. Another limitation of this study was that sub-compartmental analysis was not included. While sub-compartmental analysis would help quantify the topographical variation of T2 due to both compositional differences and the magic angle effect, we believe that this analysis is beyond the scope of this manuscript and would be better-suited for a future project. Overall, despite these limitations, we believe that our study provides an important contribution to the field, as it establishes the first large dataset of “reference” cartilage T2 values in subjects without morphologic OA characteristics; these results may be helpful for understanding the natural variations in cartilage T2 that are associated with demographic patterns, which occur prior to development of radiographic disease.
We envision this reference database as a first pass for understanding the natural variation in T2 values in subjects free from degenerative disease. Similar to bone mineral density (BMD) measurements, a reference database for cartilage T2 is essential for a definition and classification of “normal” T2 values according to subject demographics. Individuals with elevated T2 (high standard deviations above the median T2 values), as defined by the demographic-specific reference database, would more likely be at risk for OA or have biochemical alterations suggestive of early degeneration. Despite the limitations of T2 quantification across MRI scanners as well as interobserver-related variations, this is the first study attempting to provide normative MRI T2 reference values, and we believe that this is a pre-requisite for the use of cartilage T2 values more widely as an imaging biomarker. A possible next step for future optimization of the cartilage T2 measurements would be to utilize this reference database for standardized comparisons with cohorts at risk or with existing degenerative disease. Overall, we believe that this reference database is a first step for developing cut-off values for a definition of normal and degenerative cartilage composition.
In conclusion, this study established the first set of gender- and age-specific reference values for cartilage T2 in a relatively large cohort without morphologic evidence of OA (KL 0/1 and cartilage WORMS 0/1). Such reference values may be useful for researchers to aid with the interpretation of cartilage T2 values in subjects with early cartilage degeneration without focal abnormalities. While cartilage T2 values were weakly associated with age and gender, they had the highest correlations with BMI. Overall, this study demonstrates that natural variations in cartilage T2 are prevalent in knees without OA, and may be influenced by demographic factors. These variations must be taken into consideration when evaluating cartilage T2 in the context of OA development and progression.
Acknowledgments
Role of Funding Source:
This study was funded by NIH P50-AR060752.
The OAI is a public-private partnership comprised of five contracts (N01-AR-2-2258; N01-AR-2-2259; N01-AR-2-2260; N01-AR-2-2261; N01-AR-2-2262) funded by the National Institutes of Health, a branch of the Department of Health and Human Services, and conducted by the OAI Study Investigators. Private funding partners include Pfizer, Inc.; Novartis Pharmaceuticals Corporation; Merck Research Laboratories; and GlaxoSmithKline. Private sector funding for the OAI is managed by the Foundation for the National Institutes of Health. This manuscript has received the approval of the OAI Publications Committee based on a review of its scientific content and data interpretation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributions:
Study design: GBJ, CEM, MCN, UH, LN, JAL, FL, TB, TML
Subject Selection: GBJ, JAL, FL, MCN, CEM, TML
Image Analysis: GBJ, UH, LN, TB
Statistical analysis: GBJ, CEM
Interpretation of data: GBJ, CEM, MCN, UH, LN, JAL, FL, TB, TML
Drafting of Article: GBJ, TML
Review/revision: GBJ, CEM, MCN, UH, LN, JAL, FL, TB, TML
Final Approval: GBJ, CEM, MCN, UH, LN, JAL, FL, TB, TML
Competing Interests:
The authors declare that they have no conflict of interest.
References
- 1.Dillon CF, Rasch EK, Gu Q, Hirsch R. Prevalence of knee osteoarthritis in the United States: arthritis data from the Third National Health and Nutrition Examination Survey 1991-94. J Rheumatol. 2006;33:2271–2279. [PubMed] [Google Scholar]
- 2.Felson DT, Lawrence RC, Hochberg MC, McAlindon T, Dieppe PA, Minor MA, et al. Osteoarthritis: new insights. Part 2: treatment approaches. Ann Intern Med. 2000;133:726–737. doi: 10.7326/0003-4819-133-9-200011070-00015. [DOI] [PubMed] [Google Scholar]
- 3.Eckstein F, Kwoh CK, Link TM. Imaging research results from the Osteoarthritis Initiative (OAI): a review and lessons learned 10 years after start of enrolment. Ann Rheum Dis. 2014 doi: 10.1136/annrheumdis-2014-205310. [DOI] [PubMed] [Google Scholar]
- 4.Dunn TC, Lu Y, Jin H, Ries MD, Majumdar S. T2 Relaxation Time of Cartilage at MR Imaging: Comparison with Severity of Knee Osteoarthritis. Radiology. 2004;232:592–598. doi: 10.1148/radiol.2322030976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xia Y. Magic-angle effect in magnetic resonance imaging of articular cartilage: a review. Invest Radiol. 2000;35:602–621. doi: 10.1097/00004424-200010000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Liess C, Lusse S, Karger N, Heller M, Gluer CC. Detection of changes in cartilage water content using MRI T2-mapping in vivo. Osteoarthritis Cartilage. 2002;10:907–913. doi: 10.1053/joca.2002.0847. [DOI] [PubMed] [Google Scholar]
- 7.Joseph GB, Baum T, Alizai H, Carballido-Gamio J, Nardo L, Virayavanich W, et al. Baseline mean and heterogeneity of MR cartilage T2 are associated with morphologic degeneration of cartilage, meniscus, and bone marrow over 3 years-- data from the Osteoarthritis Initiative. Osteoarthritis and cartilage. 2012;20:727–735. doi: 10.1016/j.joca.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dautry R, Bousson V, Manelfe J, Perozziello A, Boyer P, Loriaut P, et al. Correlation of MRI T2 mapping sequence with knee pain location in young patients with normal standard MRI. JBR-BTR. 2014;97:11–16. doi: 10.5334/jbr-btr.364. [DOI] [PubMed] [Google Scholar]
- 9.Baum T, Joseph GB, Karampinos DC, Jungmann PM, Link TM, Bauer JS. Cartilage and meniscal T2 relaxation time as non-invasive biomarker for knee osteoarthritis and cartilage repair procedures. Osteoarthritis Cartilage. 2013;21:1474–1484. doi: 10.1016/j.joca.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baum T, Stehling C, Joseph GB, Carballido-Gamio J, Schwaiger BJ, Muller-Hocker C, et al. Changes in knee cartilage T2 values over 24 months in subjects with and without risk factors for knee osteoarthritis and their association with focal knee lesions at baseline: data from the osteoarthritis initiative. Journal of magnetic resonance imaging : JMRI. 2012;35:370–378. doi: 10.1002/jmri.22834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Joseph GB, Baum T, Carballido-Gamio J, Nardo L, Virayavanich W, Alizai H, et al. Texture analysis of cartilage T2 maps: individuals with risk factors for OA have higher and more heterogeneous knee cartilage MR T2 compared to normal controls- -data from the osteoarthritis initiative. Arthritis research & therapy. 2011;13:R153. doi: 10.1186/ar3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stehling C, Lane NE, Nevitt MC, Lynch J, McCulloch CE, Link TM. Subjects with higher physical activity levels have more severe focal knee lesions diagnosed with 3T MRI: analysis of a non-symptomatic cohort of the osteoarthritis initiative. Osteoarthritis Cartilage. 2010;18:776–786. doi: 10.1016/j.joca.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peterfy C, Schneider E, Nevitt M. The osteoarthritis initiative: report on the design rationale for the magnetic resonance imaging protocol for the knee. Osteoarthritis and cartilage/OARS, Osteoarthritis Research Society. 2008;16:1433. doi: 10.1016/j.joca.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peterfy CG, Guermazi A, Zaim S, Tirman PF, Miaux Y, White D, et al. Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage. 2004;12:177–190. doi: 10.1016/j.joca.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 15.Alizai H, Virayavanich W, Joseph GB, Nardo L, Liu F, Liebl H, et al. Cartilage Lesion Score: Comparison of a Quantitative Assessment Score with Established Semiquantitative MR Scoring Systems. Radiology. 2014:122056. doi: 10.1148/radiol.13122056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stehling C, Liebl H, Krug R, Lane NE, Nevitt MC, Lynch J, et al. Patellar cartilage: T2 values and morphologic abnormalities at 3.0-T MR imaging in relation to physical activity in asymptomatic subjects from the osteoarthritis initiative. Radiology. 2010;254:509–520. doi: 10.1148/radiol.09090596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stehling C, Baum T, Mueller-Hoecker C, Liebl H, Carballido-Gamio J, Joseph G, et al. A novel fast knee cartilage segmentation technique for T2 measurements at MR imaging-data from the osteoarthritis initiative. Osteoarthritis and Cartilage. 2011;19:984–989. doi: 10.1016/j.joca.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller AJ, Joseph PM. The use of power images to perform quantitative analysis on low SNR MR images. Magn Reson Imaging. 1993;11:1051–1056. doi: 10.1016/0730-725x(93)90225-3. [DOI] [PubMed] [Google Scholar]
- 19.Raya J, Dietrich O, Horng A, Weber J, Reiser M, Glaser C. T2 measurement in articular cartilage: Impact of the fitting method on accuracy and precision at low SNR. Magnetic Resonance in Medicine. 2010;63:181–193. doi: 10.1002/mrm.22178. [DOI] [PubMed] [Google Scholar]
- 20.Messier SP, Loeser RF, Miller GD, Morgan TM, Rejeski WJ, Sevick MA, et al. Exercise and dietary weight loss in overweight and obese older adults with knee osteoarthritis: the Arthritis, Diet, and Activity Promotion Trial. Arthritis and rheumatism. 2004;50:1501–1510. doi: 10.1002/art.20256. [DOI] [PubMed] [Google Scholar]
- 21.Schipplein OD, Andriacchi TP. Interaction between active and passive knee stabilizers during level walking. J Orthop Res. 1991;9:113–119. doi: 10.1002/jor.1100090114. [DOI] [PubMed] [Google Scholar]
- 22.Ahlback S. Osteoarthrosis of the knee. A radiographic investigation. Acta Radiol Diagn (Stockh) Suppl. 1968;277:277–272. [PubMed] [Google Scholar]
- 23.Felson DT, Naimark A, Anderson J, Kazis L, Castelli W, Meenan RF. The prevalence of knee osteoarthritis in the elderly. The Framingham Osteoarthritis Study. Arthritis Rheum. 1987;30:914–918. doi: 10.1002/art.1780300811. [DOI] [PubMed] [Google Scholar]
- 24.Oliveria SA, Felson DT, Reed JI, Cirillo PA, Walker AM. Incidence of symptomatic hand, hip, and knee osteoarthritis among patients in a health maintenance organization. Arthritis Rheum. 1995;38:1134–1141. doi: 10.1002/art.1780380817. [DOI] [PubMed] [Google Scholar]
- 25.Cicuttini FS. TD. Evidence for the increasing prevalence of osteoarthritis with aging. In: Osteoarthritis: public health implications for an aging population. Baltimore: The Johns Hopkins University Press. 1994:49–62. [Google Scholar]
- 26.Loeser RF. Age-related changes in the musculoskeletal system and the development of osteoarthritis. Clin Geriatr Med. 2010;26:371–386. doi: 10.1016/j.cger.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jarvholm B, Lewold S, Malchau H, Vingard E. Age, bodyweight, smoking habits and the risk of severe osteoarthritis in the hip and knee in men. Eur J Epidemiol. 2005;20:537–542. doi: 10.1007/s10654-005-4263-x. [DOI] [PubMed] [Google Scholar]
- 28.Felson DT, Zhang Y, Hannan MT, Naimark A, Weissman BN, Aliabadi P, et al. The incidence and natural history of knee osteoarthritis in the elderly. The Framingham Osteoarthritis Study. Arthritis Rheum. 1995;38:1500–1505. doi: 10.1002/art.1780381017. [DOI] [PubMed] [Google Scholar]
- 29.Mosher TJ, Liu Y, Yang QX, Yao J, Smith R, Dardzinski BJ, et al. Age dependency of cartilage magnetic resonance imaging T2 relaxation times in asymptomatic women. Arthritis Rheum. 2004;50:2820–2828. doi: 10.1002/art.20473. [DOI] [PubMed] [Google Scholar]
- 30.Felson DT. Epidemiology of hip and knee osteoarthritis. Epidemiol Rev. 1988;10:1–28. doi: 10.1093/oxfordjournals.epirev.a036019. [DOI] [PubMed] [Google Scholar]
- 31.Grotle M, Hagen KB, Natvig B, Dahl FA, Kvien TK. Obesity and osteoarthritis in knee, hip and/or hand: an epidemiological study in the general population with 10 years follow-up. BMC Musculoskelet Disord. 2008;9:132. doi: 10.1186/1471-2474-9-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baum T, Joseph GB, Nardo L, Virayavanich W, Arulanandan A, Alizai H, et al. MRI- based knee cartilage T2 measurements and focal knee lesions correlate with BMI - 36 month follow-up data from the Osteoarthritis initiative. Arthritis care & research. 2012;65:23–33. [Google Scholar]
- 33.Koff MF, Amrami KK, Kaufman KR. Clinical evaluation of T2 values of patellar cartilage in patients with osteoarthritis. Osteoarthritis Cartilage. 2007;15:198–204. doi: 10.1016/j.joca.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 34.Laberge MA, Baum T, Virayavanich W, Nardo L, Nevitt M, Lynch J, et al. Obesity increases the prevalence and severity of focal knee abnormalities diagnosed using 3T MRI in middle-aged subjectsódata from the Osteoarthritis Initiative. Skeletal Radiology. 2011 doi: 10.1007/s00256-011-1259-3. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Messier SP. Osteoarthritis of the knee and associated factors of age and obesity: effects on gait. Med Sci Sports Exerc. 1994;26:1446–1452. [PubMed] [Google Scholar]
- 36.Powell A, Teichtahl AJ, Wluka AE, Cicuttini FM. Obesity: a preventable risk factor for large joint osteoarthritis which may act through biomechanical factors. Br J Sports Med. 2005;39:4–5. doi: 10.1136/bjsm.2004.011841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lammentausta E MJ, Nieminen MT. Differences in T2 values of knee cartilage measured with different scanners.. ISMRM, 17th International Scientific meeting and Exhibition; Honolulu, Hawaii. 2009. [Google Scholar]
- 38.Balamoody S, Williams TG, Wolstenholme C, Waterton JC, Bowes M, Hodgson R, et al. Magnetic resonance transverse relaxation time T2 of knee cartilage in osteoarthritis at 3-T: a cross-sectional multicentre, multivendor reproducibility study. Skeletal Radiol. 2013;42:511–520. doi: 10.1007/s00256-012-1511-5. [DOI] [PubMed] [Google Scholar]
- 39.McVeigh ER, Bronskill MJ, Henkelman RM. Phase and sensitivity of receiver coils in magnetic resonance imaging. Med Phys. 1986;13:806–814. doi: 10.1118/1.595967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pai A, Li X, Majumdar S. A comparative study at 3 T of sequence dependence of T2 quantitation in the knee. Magn Reson Imaging. 2008;26:1215–1220. doi: 10.1016/j.mri.2008.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matzat SJ, McWalter EJ, Kogan F, Chen W, Gold GE. T Relaxation time quantitation differs between pulse sequences in articular cartilage. J Magn Reson Imaging. 2014 doi: 10.1002/jmri.24757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith HE, Mosher TJ, Dardzinski BJ, Collins BG, Collins CM, Yang QX, et al. Spatial variation in cartilage T2 of the knee. J Magn Reson Imaging. 2001;14:50–55. doi: 10.1002/jmri.1150. [DOI] [PubMed] [Google Scholar]
- 43.Schneider E, Nessaiver M. The Osteoarthritis Initiative (OAI) magnetic resonance imaging quality assurance update. Osteoarthritis Cartilage. 2013;21:110–116. doi: 10.1016/j.joca.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ben-Eliezer N, Sodickson DK, Block KT. Rapid and accurate T2 mapping from multi- spin-echo data using Bloch-simulation-based reconstruction. Magn Reson Med. 2014 doi: 10.1002/mrm.25156. [DOI] [PMC free article] [PubMed] [Google Scholar]