Abstract
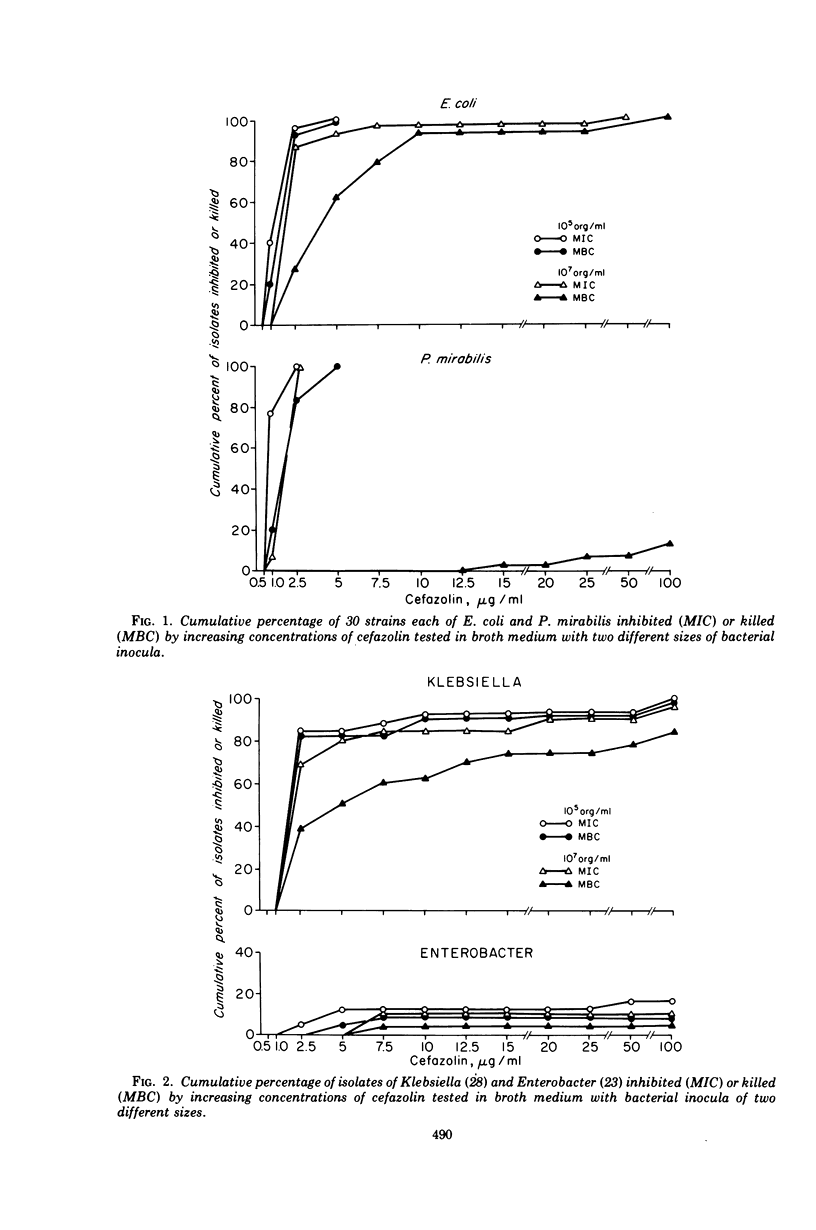
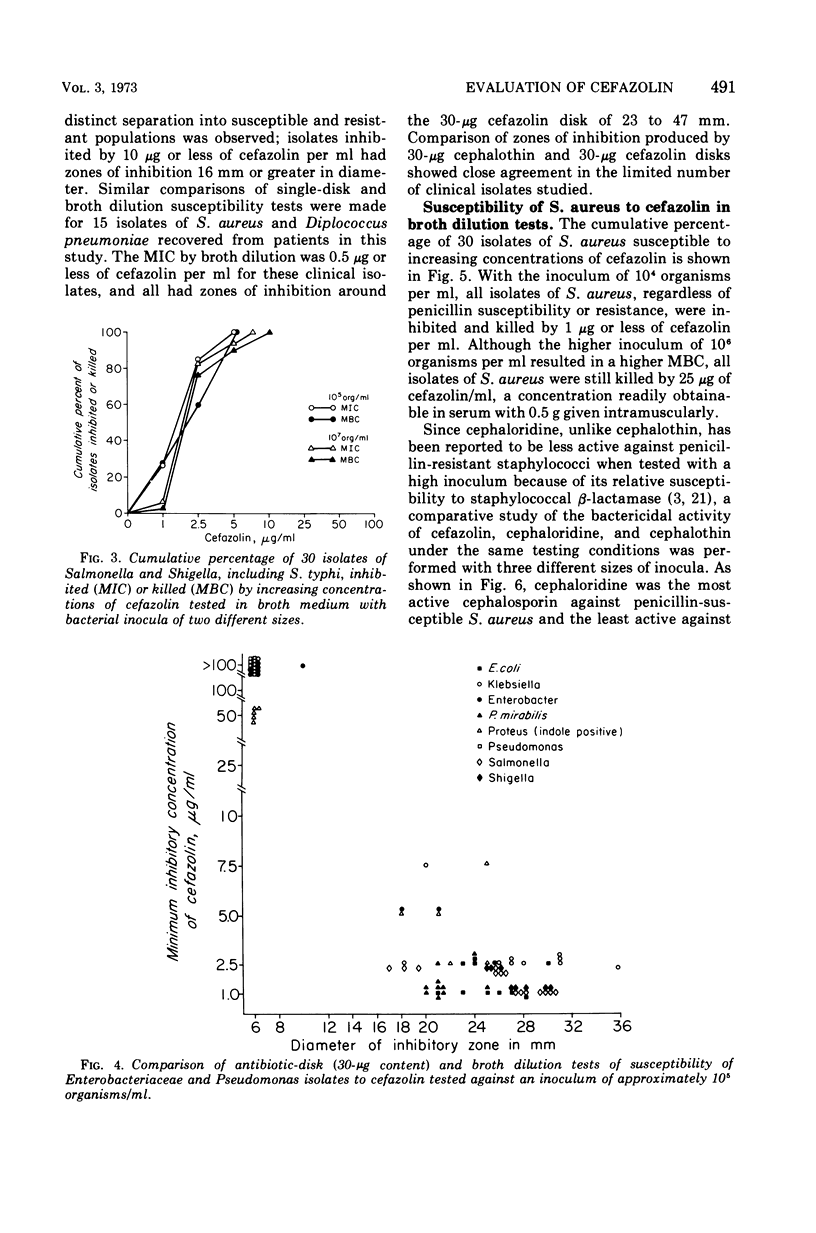
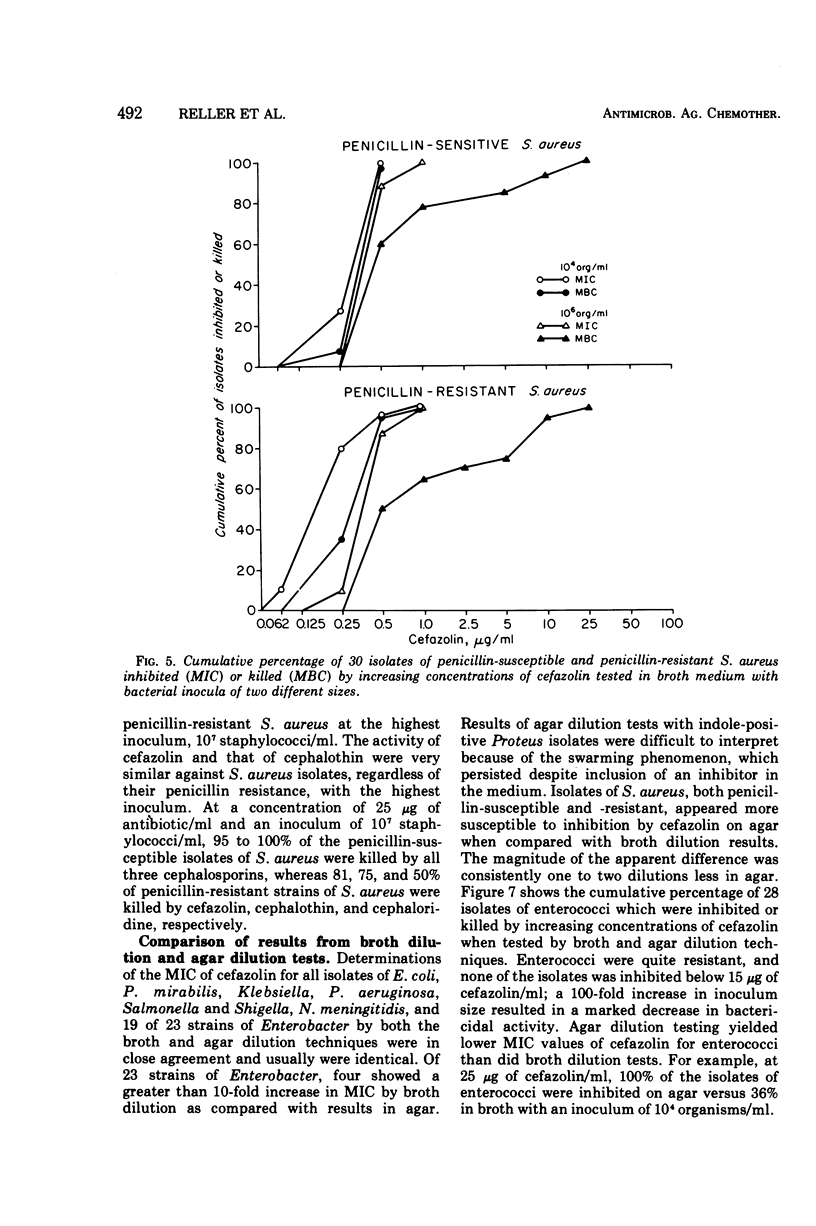
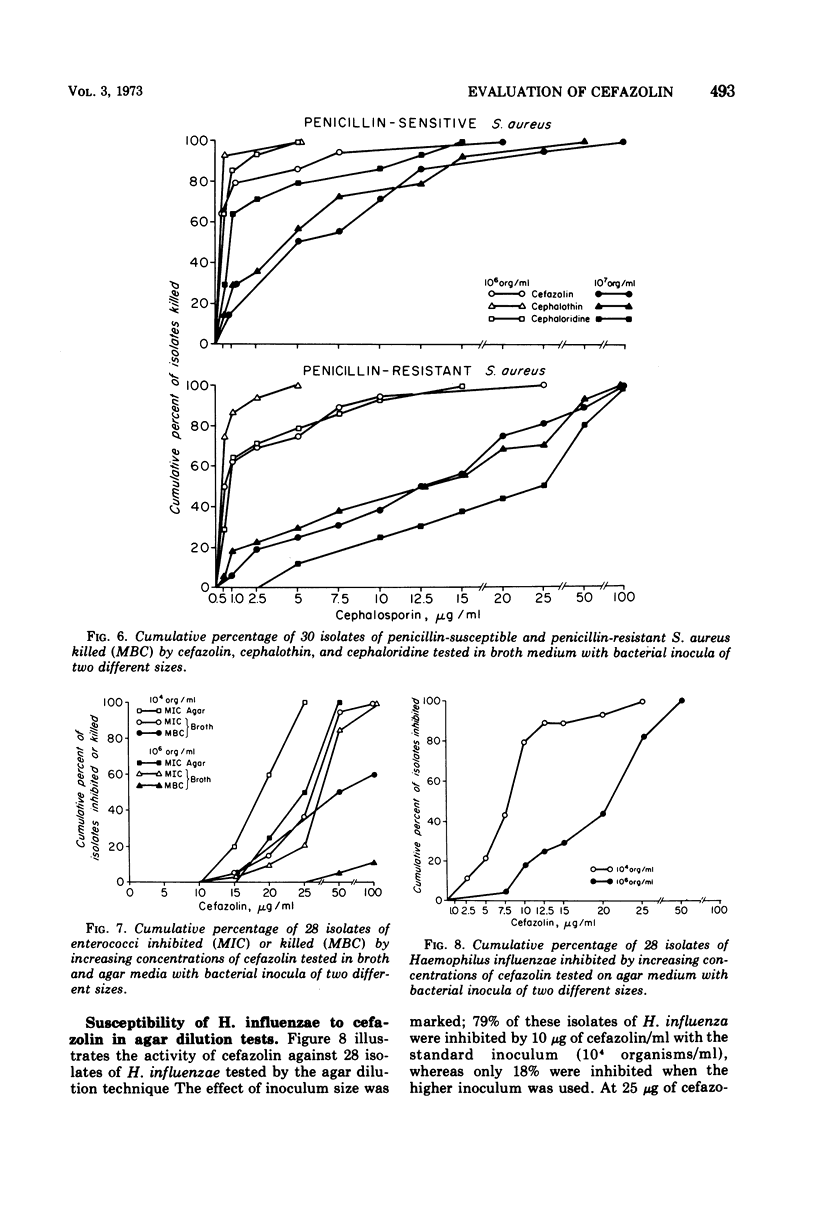
Cefazolin sodium was tested in vitro against 308 isolates of Enterobacteriaceae, Pseudomonas aeruginosa, Neisseria meningitidis, Haemophilus influenzae, Staphylococcus aureus, and enterococcus. Broth and agar dilution and disk diffusion techniques were used with at least two sizes of inocula of organisms. Cefazolin was also studied in the treatment of 85 hospitalized patients with a variety of serious infections. In concentations of 5 μg or less/ml, cefazolin inhibited and killed more than 90% of isolates of Enterobacteriaceae with the exception of indole-positive Proteus and Enterobacter species. No isolate of P. aeruginosa and only a few of Enterobacter and enterococci were killed by 25 μg of cefazolin/ml, a concentration readily attainable in serum with a 500-mg dose given intramuscularly. Penicillin-susceptible as well as penicillin-resistant isolates of S. aureus were killed by 1 μg or less of cefazolin per ml; however, 25 μg/ml was required to kill 100% of the strains when the inoculum size was increased 100-fold. Cefazolin treatment appeared effective in 82 of 85 patients, including four with endocarditis. Pain was minimal after intramuscular injection, and thrombophlebitis was not observed in those treated intravenously. No patient developed a positive Coombs test, and no evidence of renal toxicity was apparent in clinical studies.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acocella G., Mattiussi R., Nicolis F. B., Pallanza R., Tenconi L. T. Biliary excretion of antibiotics in man. Gut. 1968 Oct;9(5):536–545. doi: 10.1136/gut.9.5.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLOUNT J. G. BACTERIAL ENDOCARDITIS. Am J Med. 1965 Jun;38:909–922. doi: 10.1016/0002-9343(65)90010-0. [DOI] [PubMed] [Google Scholar]
- Bauer A. W., Kirby W. M., Sherris J. C., Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966 Apr;45(4):493–496. [PubMed] [Google Scholar]
- Benner E. J., Bennett J. V., Brodie J. L., Kirby W. M. Inactivation of cephalothin and cephaloridine by Staphylococcus aureus. J Bacteriol. 1965 Dec;90(6):1599–1604. doi: 10.1128/jb.90.6.1599-1604.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett J. V., Brodie J. L., Benner E. J., Kirby W. M. Simplified, accurate method for antibiotic assay of clinical specimens. Appl Microbiol. 1966 Mar;14(2):170–177. doi: 10.1128/am.14.2.170-177.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drutz D. J., Schaffner W., Hillman J. W., Koenig M. G. The penetration of penicillin and other antimicrobials into joint fluid. Three case reports with a reappraisal of the literature. J Bone Joint Surg Am. 1967 Oct;49(7):1415–1421. [PubMed] [Google Scholar]
- Fass R. J., Perkins R. L., Saslow S. Positive direct Coombs' tests associated with cephaloridine therapy. JAMA. 1970 Jul 6;213(1):121–123. [PubMed] [Google Scholar]
- Gralnick H. R., McGinniss M., Elton W., McCurdy P. Hemolytic anemia associated with cephalothin. JAMA. 1971 Aug 30;217(9):1193–1197. [PubMed] [Google Scholar]
- Ishiyama S., Nakayama I., Iwamoto H., Iwai S., Okui M. Absorption, tissue concentration, and organ distribution of cefazolin. Antimicrob Agents Chemother (Bethesda) 1970;10:476–480. [PubMed] [Google Scholar]
- Kuwahara S., Mine Y., Nishida M. Immunogenicity of cefazolin. Antimicrob Agents Chemother (Bethesda) 1970;10:374–379. [PubMed] [Google Scholar]
- Nishida M., Matsubara T., Murakawa T., Mine Y., Yokota Y. Cefazolin, a new semisynthetic cephalosporin antibiotic. II. In vitro and in vivo antimicrobial activity. J Antibiot (Tokyo) 1970 Mar;23(3):137–148. doi: 10.7164/antibiotics.23.137. [DOI] [PubMed] [Google Scholar]
- Nishida M., Matsubara T., Murakawa T., Mine Y., Yokota Y., Goto S., Kuwahara S. Cefazolin, a new semisynthetic cephalosporin antibiotic. 3. Absorption, excretion and tissue distribution in parenteral administration. J Antibiot (Tokyo) 1970 Apr;23(4):184–194. [PubMed] [Google Scholar]
- Nishida M., Matsubara T., Murakawa T., Mine Y., Yokota Y., Kuwahara S., Goto S. In vitro and in vivo evaluation of cefazolin, a new cephalosporin C derivative. Antimicrob Agents Chemother (Bethesda) 1969;9:236–243. [PubMed] [Google Scholar]
- Oppenheimer S., Beaty H. N., Petersdorf R. G. Pathogenesis of meningitis. VIII. Cerebrospinal fluid and blood concentrations of methicillin, cephalothin, and cephaloridine in experimental pneumococcal meningitis. J Lab Clin Med. 1969 Apr;73(4):535–543. [PubMed] [Google Scholar]
- Sherris J. C., Rashad A. L., Lighthart G. A. Laboratory determination of antibiotic susceptibility to ampicillin and cephalothin. Ann N Y Acad Sci. 1967 Sep 27;145(2):248–267. doi: 10.1111/j.1749-6632.1967.tb50223.x. [DOI] [PubMed] [Google Scholar]
- Shibata K., Fujii M. Clinical studies of cefazolin in the surgical field. Antimicrob Agents Chemother (Bethesda) 1970;10:467–472. [PubMed] [Google Scholar]
- Silverblatt F., Turck M., Bulger R. Nephrotoxicity due to cephaloridine: a light- and electron-microscopic study in rabbits. J Infect Dis. 1970 Jul-Aug;122(1):33–44. doi: 10.1093/infdis/122.1-2.33. [DOI] [PubMed] [Google Scholar]
- Simpson I. J. Nephrotoxicity and acute renal failure associated with cephalothin and cephaloridine. N Z Med J. 1971 Nov;74(474):312–315. [PubMed] [Google Scholar]
- TURCK M., ANDERSON K. N., SMITH R. H., WALLACE J. F., PETERSDORF R. G. LABORATORY AND CLINICAL EVALUATION OF A NEW ANTIBIOTIC--CEPHALOTHIN. Ann Intern Med. 1965 Aug;63:199–211. doi: 10.7326/0003-4819-63-2-199. [DOI] [PubMed] [Google Scholar]
- Turck M., Belcher D. W., Ronald A., Smith R. H., Wallace J. F. New cephalosporin antibiotic--cephaloridine. Clinical and laboratory evaluation. Arch Intern Med. 1967 Jan;119(1):50–59. doi: 10.1001/archinte.119.1.50. [DOI] [PubMed] [Google Scholar]
- Wick W. E., Preston D. A. Biological properties of three 3-heterocyclic-thiomethyl cephalosporin antibiotics. Antimicrob Agents Chemother. 1972 Mar;1(3):221–234. doi: 10.1128/aac.1.3.221. [DOI] [PMC free article] [PubMed] [Google Scholar]



