Supplemental Digital Content is available in the text.
Keywords: adipokines, atherosclerosis, fibroblast growth factor 21, hormones
Abstract
Background—
Fibroblast growth factor 21 (FGF21) is a metabolic hormone with pleiotropic effects on glucose and lipid metabolism and insulin sensitivity. It acts as a key downstream target of both peroxisome proliferator-activated receptor α and γ, the agonists of which have been used for lipid lowering and insulin sensitization, respectively. However, the role of FGF21 in the cardiovascular system remains elusive.
Methods and Results—
The roles of FGF21 in atherosclerosis were investigated by evaluating the impact of FGF21 deficiency and replenishment with recombinant FGF21 in apolipoprotein E−/− mice. FGF21 deficiency causes a marked exacerbation of atherosclerotic plaque formation and premature death in apolipoprotein E−/− mice, which is accompanied by hypoadiponectinemia and severe hypercholesterolemia. Replenishment of FGF21 protects against atherosclerosis in apolipoprotein E−/−mice via 2 independent mechanisms, inducing the adipocyte production of adiponectin, which in turn acts on the blood vessels to inhibit neointima formation and macrophage inflammation, and suppressing the hepatic expression of the transcription factor sterol regulatory element-binding protein-2, thereby leading to reduced cholesterol synthesis and attenuation of hypercholesterolemia. Chronic treatment with adiponectin partially reverses atherosclerosis without obvious effects on hypercholesterolemia in FGF21-deficient apolipoprotein E−/− mice. By contrast, the cholesterol-lowering effects of FGF21 are abrogated by hepatic expression of sterol regulatory element-binding protein-2.
Conclusions—
FGF21 protects against atherosclerosis via fine tuning the multiorgan crosstalk among liver, adipose tissue, and blood vessels.
Fibroblast growth factor (FGF) 21 is a member of the endocrine FGF subfamily that is produced predominantly in the liver.1 Physiologically, FGF21 plays a key role in mediating the metabolic responses to fasting/starvation by enhancing fatty acid oxidation and ketogenesis and inducing growth hormone resistance.2,3 Pharmacologically, therapeutic intervention with recombinant FGF21 has been shown to counteract obesity and its related metabolic disorders in both rodents and nonhuman primates, including reduction of adiposity and amelioration of hyperglycemia, hyperinsulinemia, insulin resistance, dyslipidemia, and fatty liver disease.4,5 Furthermore, FGF21 is the downstream target of both peroxisome proliferator-activated receptor (PPAR) α and PPARγ, and a growing body of evidence suggest that the glucose-lowering and insulin-sensitizing effects of the PPARγ agonists (thiazolidinediones)and the therapeutic benefits of the PPARα agonists (fenofibrates) on lipid profiles are mediated in part by induction of FGF21.6
Clinical Perspective on p 1871
FGF21 exerts its metabolic actions by binding to the complex receptor between the FGF receptor (FGFR) and β-klotho, a single transmembrane protein that is highly expressed in adipose tissue, liver, pancreas, and hypothalamus.4,7,8 Adipocytes are the primary target of FGF21, where it increases glucose uptake, modulates lipolysis,9 enhances mitochondrial oxidative capacity, enhances PPARγ activity,10 and promotes browning of white adipose tissue.11 Furthermore, therapeutic administration of FGF21 has been shown to increase the production of adiponectin,12,13 an adipocyte-secreted hormone with insulin-sensitizing, anti-inflammatory, and vascular protective activity. Adiponectin knockout mice are resistant to the effects of FGF21 on alleviation of insulin resistance, hyperglycemia, dyslipidemia, and fatty liver disease associated with dietary or genetic obesity,12 suggesting that adiponectin acts as an obligatory downstream mediator of FGF21 on energy metabolism and insulin sensitivity. In addition, FGF21 has also been shown to exert its direct actions on the pancreas, hypothalamus, heart, and liver,14–18 acting as a mediator to coordinate the multiorganic crosstalk under various pathophysiological conditions.
Although the metabolic functions of FGF21 are well characterized, little is known about its pathophysiological roles in atherosclerosis, a chronic inflammatory disease intimately associated with metabolic syndrome. A number of clinical studies have observed an increased circulating level of FGF21 in patients with atherosclerosis or those individuals who are at high risk of developing this disease.19,20 In both rhesus monkeys and humans with obesity and diabetes mellitus, chronic administration of FGF21 decreases low-density lipoprotein (LDL) cholesterol and increases high-density lipoprotein cholesterol.5,21,22 However, whether such a beneficial effect of FGF21 on lipid profiles is sufficient to render a protection against atherosclerotic diseases has not been explored. To address this issue, we investigated the impact of both FGF21 deficiency and replenishment on the pathogenesis of atherosclerosis in apolipoprotein (apo) E−/− mice. Our results showed a markedly aggravated atherosclerotic phenotype of FGF21 knockout mice, which can be reversed by replenishment of FGF21. Therefore, we further investigated the mechanisms whereby FGF21 protects atherosclerosis via its multiple actions in both adipose tissue and liver.
Methods
Additional details of mice and experimental procedures are included in the online-only Data Supplement. All of the animal studies were approved by the animal research ethics committees of Wenzhou Medical University and the University of Hong Kong.
Statistical analysis was performed using either the Mann-Whitney U test or the Kruskal-Wallis test when more than 2 experimental conditions were compared. When the global Kruskal-Wallis test was significant, pairwise comparisons were performed with the Dunn-Sidak procedure for multiple corrections. Repeated-measure ANOVA was used to compare circulating FGF21 levels between wild-type and apoE−/− mice at different time points, as well as serum levels of FGF21 and adiponectin in FGF21 and apoE−/− double deficiency (DKO) mice at different time points after administration with FGF21 or adiponectin. The survival of mice was compared using Kaplan-Meier survival analysis with a log-rank test. All of the statistical analyses were performed with IBM SPSS version 20.0 (IBM Corporation, Armonk, NY). A value of P<0.05 was considered statistically significant.
Results
FGF21 Deficiency Accelerates Atherosclerotic Plaque Formation in ApoE−/− Mice
Several clinical studies have observed a significantly elevated serum level of FGF21 in patients with atherosclerosis.19,20 Consistently, both circulating levels of FGF21 and its hepatic mRNA expression were progressively elevated in apoE−/− mice with spontaneous development of hypercholesterolemia and atherosclerosis (Figure I in the online-only Data Supplement). To explore the pathophysiological roles of FGF21 in atherosclerosis, we generated DKO mice by backcrossing FGF21 knockout mice into apoE−/− mice in C57BL/J background for more than 10 generations. DKO mice were confirmed by both polymerase chain reaction analysis and Western blot analysis of the liver tissue (Figure II in the online-only Data Supplement). There were no obvious differences in food intake and body weight between apoE−/− mice and DKO mice on standard chow (Figure IIIA in the online-only Data Supplement). However, the atherosclerotic lesion area in DKO, as determined by oil red O staining of the entire aorta, was 1.6-fold and 1.8-fold greater at 24 weeks and 52 weeks than age- and sex-matched apoE−/− mice (P<0.01; Figure 1A). Additional histological evaluation showed that the plaque areas in the aortic sinus and brachiocephalic artery of 24-week-old DKO mice were 2.1-fold and 2.9-fold greater than in apoE−/− mice (Figure 1B and 1C). Likewise, both macrophage infiltration and smooth muscle proliferation in the atherosclerotic lesion area of the aortic sinus in DKO mice were significantly higher than in apoE−/− mice (Figure 1D and 1E). Cholesterol ester contents extracted from the brachiocephalic artery of DKO mice were also much higher than those in apoE−/− mice (Figure 1F), suggesting that FGF21 deficiency renders apoE−/− mice more susceptible to atherosclerosis.
Figure 1.
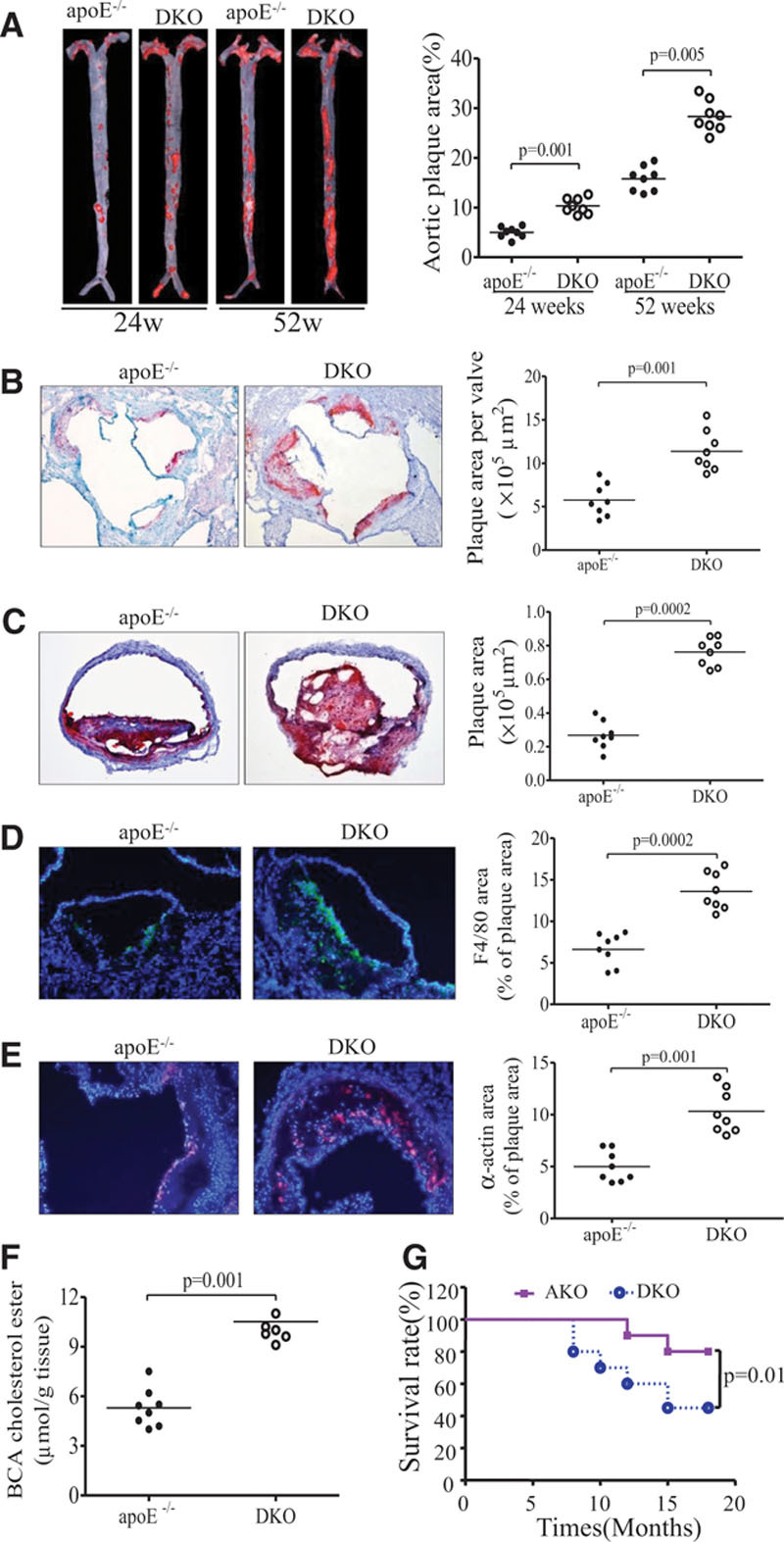
Apolipoprotein (apo) E−/− mice with fibroblast growth factor (FGF) 21 deficiency exhibit exacerbated atherosclerosis and premature death. Aortas were dissected from 24- and 52-week-old apoE−/− mice and apoE−/−FGF21−/− (DKO) mice. n=8 in each group. A, En face staining of entire aortas of 24-week-old mice with oil red O. B and C, Cross-sections of aortic sinuses and brachiocephalic arteries of 24-week-old mice, respectively. D and E, Macrophage infiltration and smooth muscle proliferation in aortic sinus as determined by immunostaining for F4/80 and α-actin, respectively. F, Cholesterol ester levels in brachiocephalic arteries (BCA) of 24-week-old mice. G, The surviving rate of apoE−/− mice (n=20) and DKO mice (n=20) on standard chow was monitored for 18 months. Data are presented as dot plots with the line indicating the median. The Mann-Whitney U test was used for 2-group comparisons (A–F); the survivals of mice were compared using Kaplan-Meier survival analysis with the log-rank test (G).
To investigate whether accelerated atherosclerosis in DKO mice decreases longevity, we monitored DKO (n=20) and apoE−/− mice (n=20) on standard chow for 18 months. The surviving rate of DKO was decreased to ≈45%, which was significantly lower than that in apoE−/− mice (80%; Figure 1G).
DKO Mice Display Exacerbated Hyperlipidemia and Augmented Inflammation
Because FGF21 is an important metabolic regulator, we next investigated whether the atherosclerosis-prone phenotype of DKO mice is attributed to impaired glucose or lipid metabolism. Glucose and insulin levels were comparable between DKO and apoE−/− mice (Figure IIIB in the online-only Data Supplement). A glucose tolerance test showed a similar glucose excursion in response to intraperitoneal glucose challenge (Figure IIIC and IIID in the online-only Data Supplement). On the other hand, DKO mice exhibited a 1.5-fold and 2.1-fold increase in plasma levels of total triglyceride and cholesterol, respectively (Figure 2A and 2B). Additional analysis of lipoprotein compositions demonstrated a significantly increased LDL and very LDL but decreased high-density lipoprotein levels in DKO mice as compared with apoE−/− controls (Figure 2C through 2E).
Figure 2.
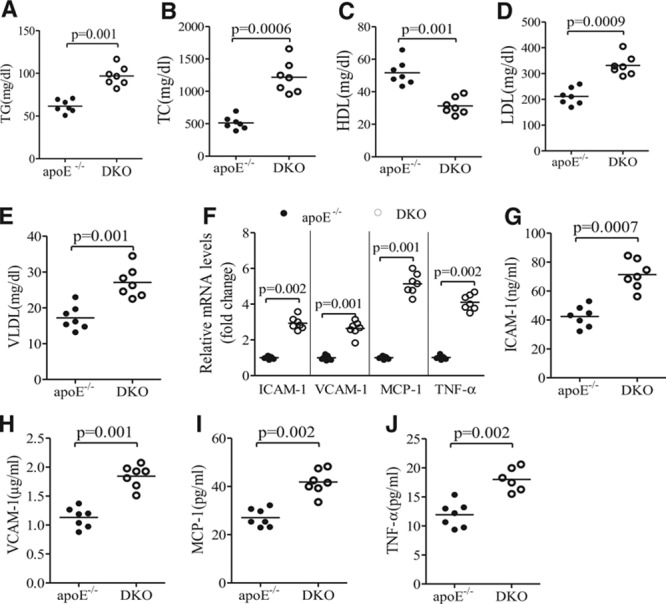
Fibroblast growth factor (FGF) 21 deficiency worsens lipid profiles and exacerbates inflammation in apolipoprotein (apo) E−/− mice. ApoE−/−FGF21−/− (DKO) and apoE−/− mice fed with standard chow were euthanized at 24 weeks after birth. Plasma samples were collected for measurement of triglycerides (TG; A), total cholesterol (TC; B), high-density lipoprotein (HDL; C), low-density lipoprotein (LDL; D), and very LDL (VLDL; E). F, The mRNA expression of intercellular adhesion molecule-1 (ICAM-1) vascular cell adhesion protein-1 (VCAM-1), tumor necrosis factor-α (TNFα), and monocyte chemotactic protein-1 (MCP-1) in aortic tissue, as well as (G–J) plasma levels of these proinflammatory chemokines and cytokines, were measured with real-time polymerase chain reaction and ELISA, respectively. n=6 to 7. Data are presented as dot plots with the line indicating the median. The Mann-Whitney U test was used for comparison of 2 groups.
Quantitative real-time polymerase chain reaction analysis demonstrated a significantly increased expression of the adhesion molecules intercellular adhesion molecule-1 and vascular cell adhesion protein-1 and the proinflammatory cytokines monocyte chemotactic protein-1 and tumor necrosis factor-α in aortic tissues of DKO mice as compared with apoE−/− mice (Figure 2F). Likewise, the circulating levels of these proinflammatory chemokines and cytokines in DKO mice were much higher than those in apoE−/− mice (Figure 2G through 2J), suggesting that FGF21 deficiency exacerbates both local inflammation in atherosclerotic lesions and systemic inflammation.
Similar to the above findings in chow-fed mice, high-fat, high-cholesterol–induced atherosclerotic plaque formation, hypertriglyceridemia, hypercholesterolemia, and production of the proinflammatory cytokines were significantly exacerbated in DKO mice as compared with apoE−/− mice (Figure IV in the online-only Data Supplement), suggesting that FGF21 is also an important protector against Western diet–induced dyslipidemia and atherosclerosis in mice.
FGF21 Exerts Its Antiatherosclerotic Effects via Both Adiponectin-Dependent and -Independent Mechanisms
Adipocytes are the primary target of FGF21, where it induces the expression and secretion of adiponectin, an adipokine with insulin-sensitizing, anti-inflammatory, and antiatherosclerotic activities.23–25 Because the insulin-sensitizing actions of FGF21 are mediated by adiponectin,12,13 we next investigated whether FGF21 exerts its antiatherosclerotic activities via induction of adiponectin. As expected, both circulating levels of adiponectin and its mRNA expression in different adipose depots, including epididymal, subcutaneous, perivascular, and perirenal adipose tissues, were significantly reduced in DKO mice as compared with apoE−/− mice (Figure 3A and 3B). Daily administration of recombinant mouse FGF21 (rmFGF21) for a period of 16 weeks led to higher circulating levels of adiponectin in DKO mice (Figure VA and VB in the online-only Data Supplement), which was accompanied by a significant reduction of atherosclerotic lesion area, as determined by both oil red O staining of the entire aorta and histological quantification of plaque areas between the sinus aorta and brachiocephalic arteries (Figure 3C through 3E). Chronic administration of recombinant mouse adiponectin (Figure VC in the online-only Data Supplement) also alleviated atherosclerotic plaque formation in DKO mice, whereas the magnitude of reduction in atherosclerosis by adiponectin was significantly smaller than that by rmFGF21.
Figure 3.
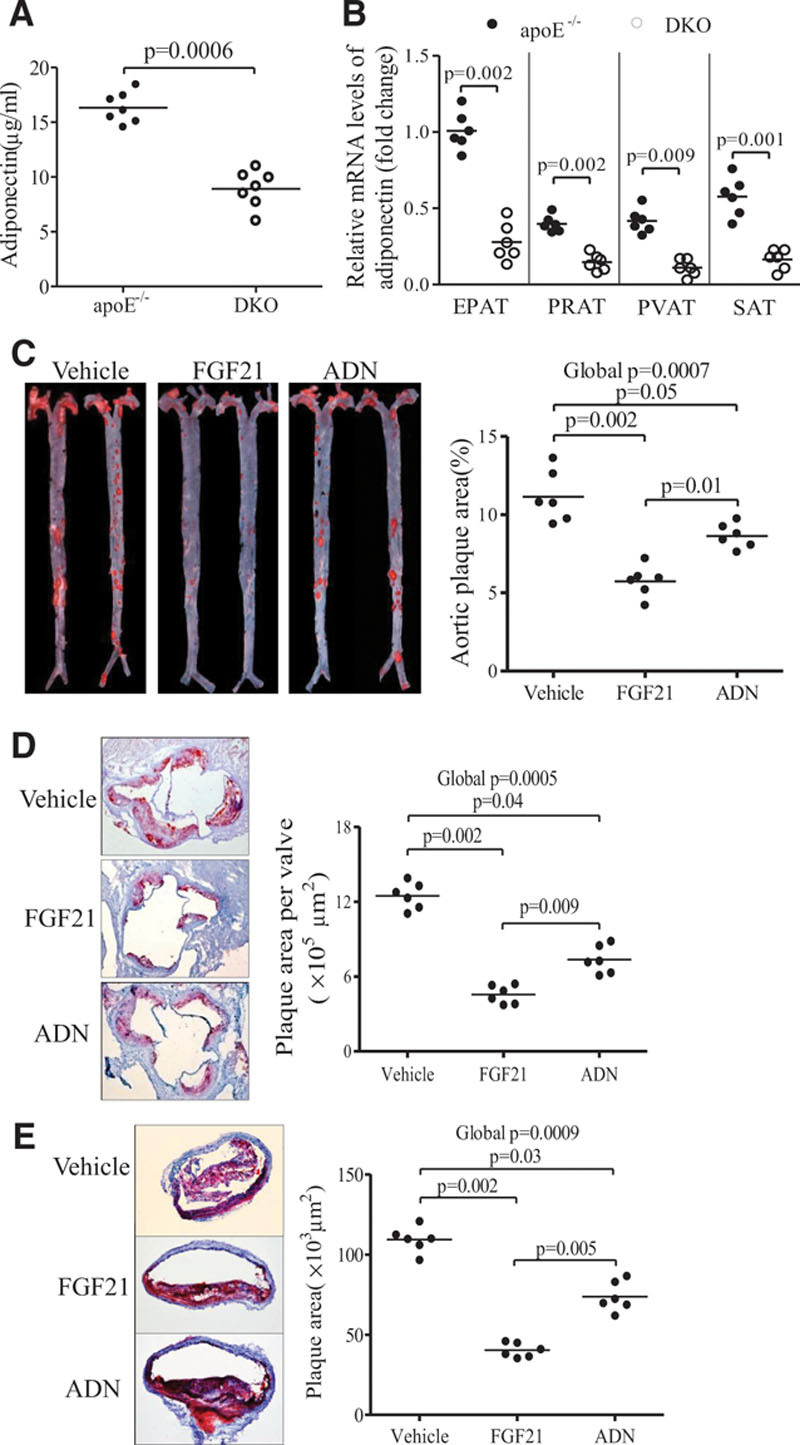
Recombinant mouse fibroblast growth factor (FGF) 21 and adiponectin (ADN) attenuate the atherosclerotic plaque formation in apolipoprotein E−/−FGF21−/− (DKO) mice. Eight-week-old DKO mice were treated with recombinant mouse FGF21 (0.1 mg/kg per day), adiponectin (10 mg/kg per day), or vehicle by daily intraperitoneal injection for a period of 16 weeks. A and B, Plasma levels of adiponectin and its mRNA expression in epididymal adipose tissues (EPAT), subcutaneous (SAT), perivascular (PVAT), and perirenal (PRAT) adipose tissues. C, En face staining of entire aortas with oil red O. D and E, Cross-section analysis of aortic sinuses and brachiocephalic arteries with oil red O, respectively. n=6 to 7. Data are presented as dot plots with the line indicating the median. The Mann-Whitney U test was used to compare 2 groups (A and B). The global significance among 3 groups was determined by Kruskal-Wallis test, followed by pairwise comparisons with the Dunn-Sidak procedure (C–E).
Further histological analysis demonstrated that rmFGF21 and adiponectin caused a similar degree of decrease in collagen composition, smooth muscle proliferation, and macrophage infiltration (Figure 4A). The magnitude of reduction in expression of proinflammatory chemokines intercellular adhesion molecule-1 and vascular cell adhesion protein-1 and cytokines tumor necrosis factor-α and monocyte chemotactic protein-1 was also comparable between rmFGF21- and adiponectin-treated DKO mice (Figure 4B through 4F). However, in adiponectin-treated DKO mice, cholesterol ester contents in brachiocephalic arteries were reduced only by 22%, which was significantly lower than rmFGF21-mediated reduction (56%; Figure 4G). Notably, whereas rmFGF21 decreased total cholesterol in DKO mice to a level comparable with apoE−/− mice, adiponectin had no effect on hypercholesterolemia caused by FGF21 deficiency (Figure 4H), despite that both rmFGF21 and adiponectin had a similar potency in decreasing hypertriglyceridemia in DKO mice (Figure 4I).
Figure 4.
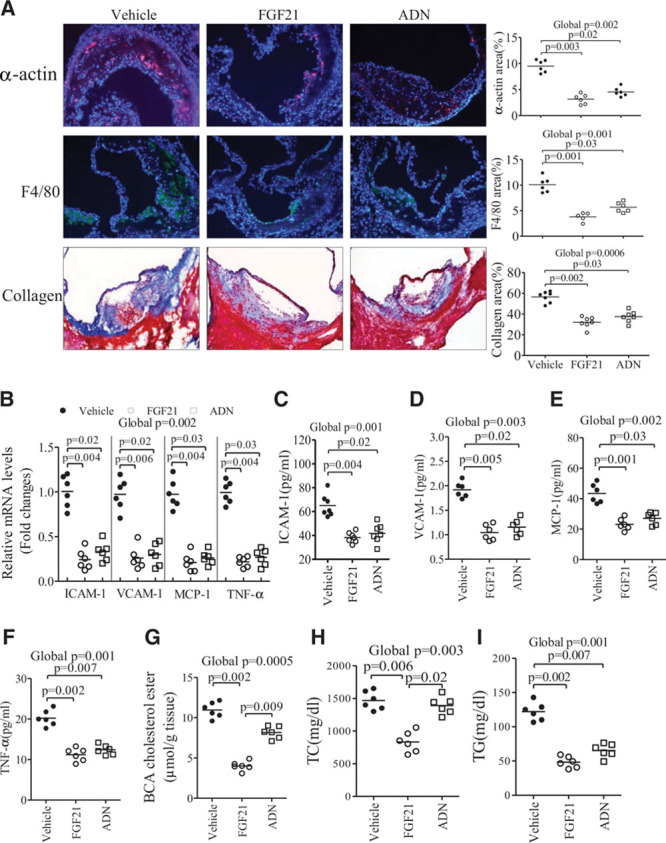
Differential effects of fibroblast growth factor (FGF) 21 and adiponectin on lipid profiles and atherosclerotic plaque composition in apolipoprotein E−/−FGF21−/− (DKO) mice. DKO mice were treated with recombinant mouse FGF21, adiponectin (ADN), or vehicle for 16 weeks as in Figure 3. A, Immunohistological analysis of atherosclerotic lesion areas in aortic sinuses with antibodies against the smooth muscle marker α-actin, the macrophage marker F4/80, or with Masson trichrome staining for the collagen composition as indicated. B–F, The mRNA expression of several proinflammatory chemokines and cytokines in the aortic sinus and their plasma levels as determined by real-time polymerase chain reaction and ELISA, respectively. G, Cholesterol ester content in the brachiocephalic arteries. H and I, Plasma cholesterol and triglyceride levels in DKO mice treated with recombinant mouse (rm) FGF21, ADN, or vehicle, respectively. n=5 to 7. Data are presented as dot plots with the line indicating the median. The global significance among 3 groups was determined by Kruskal-Wallis test, followed by pairwise comparisons with the Dunn-Sidak procedure.
We next compared the direct effects of adiponectin and rmFGF21 in several types of blood vessel cells. Consistent with previous reports,26,27 recombinant adiponectin directly inhibited platelet-derived growth factor–induced proliferation and migration of human smooth muscle cells (Figure VIA and VIB in the online-only Data Supplement) and also reduced the uptake of acetylated LDL in peritoneal macrophages (Figure VIC in the online-only Data Supplement). However, rmFGF21 had no direct effect on these cells.
FGF21 Suppresses Cholesterol Biosynthesis and Enhances Cholesterol Efflux in Mice
Because our data suggest that the cholesterol-lowering effects of rmFGF21 are independent of adiponectin, we further explored the mechanisms by which FGF21 modulates cholesterol metabolism in mice. The intestinal absorption of cholesterol, as measured by the fecal dual isotope ratio of 14C:3H in feces, was comparable between apoE−/− mice and DKO mice and was not affected by treatment with either rmFGF21 or adiponectin (Figure 5A). There was a modest but significant decrease in cholesterol contents in the feces of DKO mice, and this change was reversed by treatment with rmFGF21 but not adiponectin (Figure 5B). On the other hand, the excretion of bile acids into the feces was not altered by either FGF21 deficiency or replenishment with rmFGF21 (Figure 5C). The de novo biosynthesis of cholesterol in the liver, as measured with the amount of [1-14C]-acetate incorporated into sterols in liver, was markedly increased by 1.49-fold in DKO mice as compared with apoE−/− mice, and this augmented cholesterol synthesis was completely rectified by replenishment with rmFGF21 but not adiponectin (Figure 5D). Likewise, hepatic cholesterol accumulation was elevated by FGF21 deficiency but was suppressed by treatment with rmFGF21 (Figure 5E).
Figure 5.
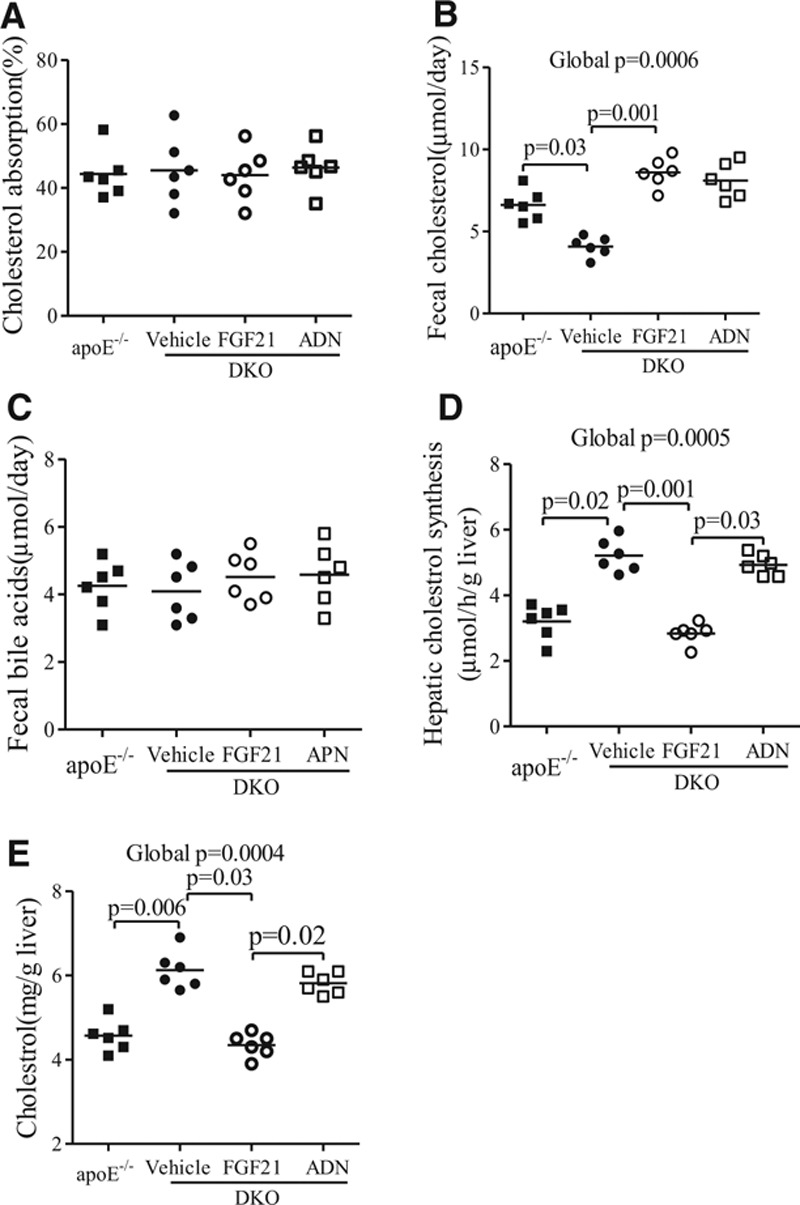
Effects of fibroblast growth factor (FGF) 21 and adiponectin (ADN) on cholesterol metabolism in mice. Apolipoprotein (apo) E−/−FGF21−/− (DKO) mice were treated with recombinant mouse FGF21, ADN, or vehicle for 4 weeks as in Figure 4. ApoE−/− mice were used as a control. A, The absorption rate of dietary cholesterol was determined by oral gavage with [14C] cholesterol and [3H] sitostanol, followed by measurement of the ratio of the 2 isotopes in feces. B, Fecal cholesterol and (C) bile acids were measured with the corresponding commercial kits, respectively. D, The rate of de novo cholesterol synthesis as measured by determining the amount of [1-14C]-acetate incorporated into sterols per minute per gram liver tissue. E, Hepatic cholesterol contents determined by a cholesterol assay kit. n=6 to 7. Data are presented as dot plots with the line indicating the median. The global significance among 4 groups was determined by Kruskal-Wallis test, followed by pairwise comparisons with the Dunn-Sidak procedure.
We next evaluated the impact of FGF21 on the expression of key genes involved in cholesterol metabolism in the liver. In DKO mice, hepatic expression of 3-hydroxy-3-methylglutaryl-CoA reductase (a rate-limiting enzyme involved in cholesterol synthesis) and several other cholesterologenic genes was significantly elevated when compared with apoE−/− mice, whereas this elevation in DKO mice was inhibited by administration of rmFGF21 but not adiponectin (Figure 6A through 6B). On the other hand, the expression levels of key genes involved in bile acid metabolism and secretion, including cholesterol 7-α-monooxygenase, sterol 27-hydroxylase, sterol 12-α-hydroxylase, and small heterodimer partner, were not altered by either FGF21 deficiency or administration (Figure 6C). DKO mice exhibited a modest elevation in the expression of ABC5 and ABCG8 (Figure 6D), the 2 ATP-binding cassette transporters that promote cholesterol secretion.28 The reduced expression of ABCG5 and ABCG8 was reversed by replenishment with rmFGF21 but not adiponectin.
Figure 6.
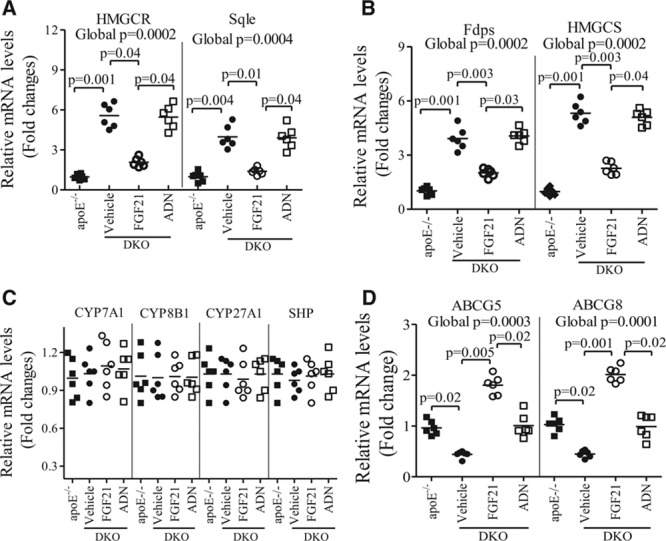
Fibroblast growth factor (FGF) 21, but not adiponectin (ADN), alters hepatic expression of the key genes involved in cholesterol biosynthesis and transport. Total RNA extracted from the liver of apolipoprotein (apo) E−/− mice or apoE−/−FGF21−/− (DKO) mice treated with recombinant mouse FGF21, ADN, or vehicle as in Figure 4 was subjected to real-time polymerase chain reaction analysis. Figure shows the relative mRNA expression levels of genes involved in cholesterol synthesis, including 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR), 3-hydroxy-3-methylglutaryl-CoA synthetase (HMGCS), squalene synthase (Sqle), and farnesyl diphosphate synthetase (Fdps; A and B) genes involved in bile acids metabolism including cholesterol 7-α-monooxygenase (CYP7A1), sterol 12-α-hydroxylase (CYP8B1), sterol 27-hydroxylase (CYP27A1), and small heterodimer partner (SHP; C), as well as genes involved in cholesterol transports including ABCG5 and ABCG8 (D). n=5 to 7. Data are presented as dot plots with the line indicating the median. The global significance among 4 groups was determined by Kruskal-Wallis test, followed by pairwise comparisons with the Dunn-Sidak procedure.
FGF21 Inhibits Cholesterol Biosynthesis via Suppression of Sterol Regulatory Element-Binding Protein-2
Cholesterol homeostasis is orchestrated by a number of transcriptional factors, including sterol regulatory element-binding protein (Srebp)-1a, -1c, and -2; liver X receptors; and farnesoid X receptor.29,30 We next investigated whether FGF21 modulates cholesterol metabolism via these transcription factors. There was no obvious difference in either mRNA or protein expression of liver X receptor α, farnesoid X receptor, and Srebp-1 between DKO mice and apoE−/− mice (Figure 7A and 7B). In contrast, DKO mice exhibited a marked elevation in both mRNA and protein expression of Srebp-2, and this change was reversed by administration of rmFGF21 but not adiponectin (Figure 7C and 7D). Consistently, the transcriptional activity of nuclear Srebp-2 in the liver of DKO mice was ≈2.9-fold higher than in apoE−/− mice, as determined by the binding of Srebp-2 in the nuclear extracts to the specific DNA sequences (Figure 7E). Elevated transcriptional activity of Srebp-2 in DKO mice was largely reversed by treatment with rmFGF21 but not adiponectin. On the other hand, the transcriptional activity of nuclear Srebp-1 in the liver was not altered by either FGF21 deficiency or supplementation (Figure 7F).
Figure 7.
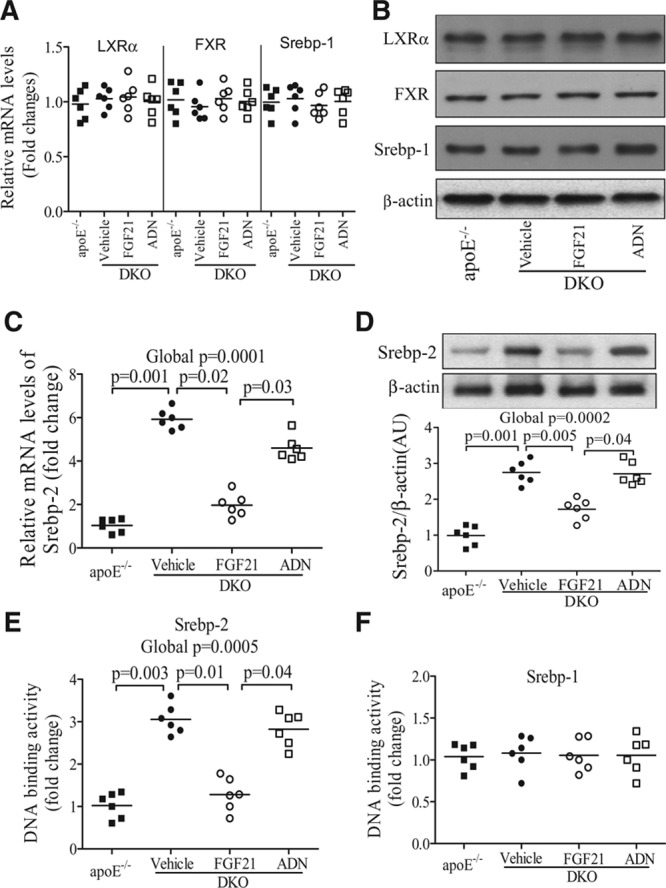
Effects of fibroblast growth factor (FGF) 21 and adiponectin (ADN) on several key transcription factors involved in cholesterol metabolism. The liver samples from apolipoprotein (apo) E−/− mice or apoE−/−FGF21−/− (DKO) mice treated with recombinant mouse FGF21, ADN, or vehicle as in Figure 4 were subjected to real-time polymerase chain reaction or Western blot analysis. A and B, The relative mRNA and protein expression levels of liver X receptor (LXR) α, farnesoid X receptor (FXR), and sterol regulatory element-binding protein (Srebp)-1. C and D, The relative mRNA and protein expression of Srebp-2. E and F, The DNA binding activities of Srebp-1 and Srebp-2 in the nuclear extracts of liver tissues. n=5 to 7. Data are presented as dot plots with the line indicating the median. The global significance among 4 groups was determined by Kruskal-Wallis test, followed by pairwise comparisons with the Dunn-Sidak procedure.
To explore whether FGF21 lowers cholesterol via inhibition of Srebp-2, adenovirus delivery system was used to knock down or overexpress Srebp-2 in the liver. After tail-vein injection of recombinant adenovirus encoding Srebp-2–specific small interfering RNA, an obvious reduction in Srebp-2 expression was observed at 2 days postinfection (data not shown), and its expression continued to decline to a level comparable with apoE−/− mice at days 6 and 12 after adenoviral Srebp-2 small interfering RNA infection (Figure 8A and 8B). Notably, suppression of Srebp-2 expression reversed hypercholesterolemia in DKO mice caused by FGF21 deficiency and concurrently reduced the expression of several cholesterologenic genes, including 3-hydroxy-3-methylglutaryl-CoA reductase, farnesyl diphosphate synthetase, squalene synthase, and 3-hydroxy-3-methylglutaryl-CoA synthetase, which are all well-known downstream targets of Srebp-2 (Figure 8C). Conversely, the effects of rmFGF21 administration on the alleviation of hypercholesterolemia and suppression of cholesterologenic gene expression were abrogated by adenovirus-mediated expression of Srebp-2 (Figure 8D through 8F).
Figure 8.
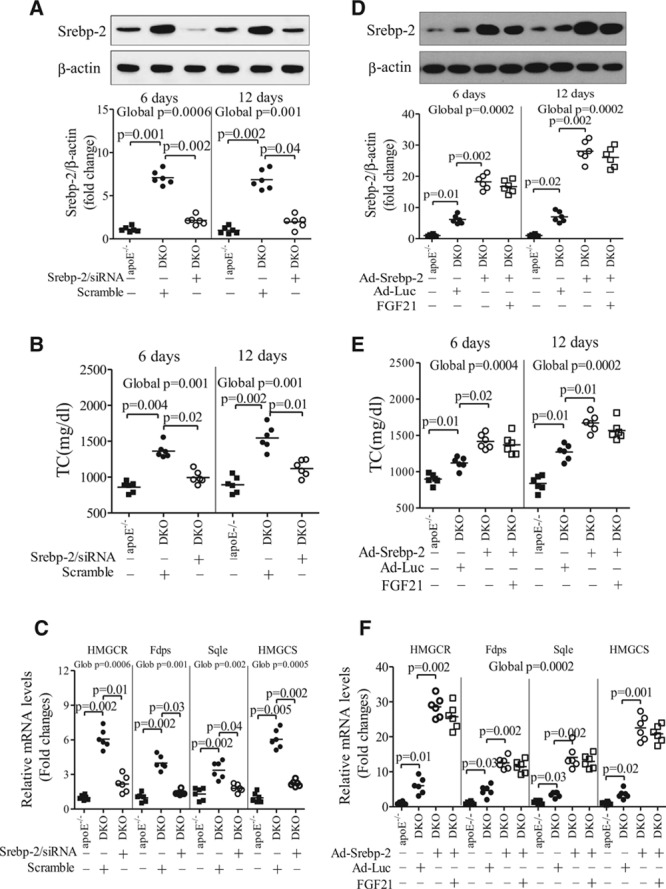
Fibroblast growth factor (FGF) 21 decreases hypercholesterolemia via inhibition of hepatic sterol regulatory element-binding protein (Srebp)-2. A–C, Apolipoprotein (apo) E−/−FGF21−/− (DKO) mice were infected with adenovirus encoding small interfering RNA (siRNA) specific to Srebp-2 or scrambled control (5×108 plaque-forming units per mouse) for various periods. Age-matched apoE−/− mice were used as a control. A, Protein expression levels of hepatic Srebp-2 at day 6 and day 12 after adenoviral infection. B, Circulating levels of total cholesterol (TC) and (C) the expression levels of cholesterologenic genes determined by real-time polymerase chain reaction analysis at day 12 (n=6). D and E, DKO mice were infected with adenovirus encoding Srebp-2 (Ad-Srebp-2) or luciferase (Ad-Luc) for 6 days (as control), followed by treatment with daily intraperitoneal injection of recombinant mouse (rm) FGF21 (0.1 mg/kg per day) for another 6 days. D, The protein expression levels of Srebp-2 in the liver and (E) serum levels of total cholesterol. F, The mRNA expression of cholesterologenic genes at 12 days after adenoviral infection (n=6). Data are presented as dot plots with the line indicating the median. The global significance among 3 groups was determined by Kruskal-Wallis test, followed by pairwise comparisons with the Dunn-Sidak procedure.
Suppressive Effects of FGF21 on Cholesterol Biosynthesis Are Mediated by β-Klotho and FGFR2 in the Liver
FGF21 exerts its actions by binding to FGFR and its coreceptor β-klotho, the latter of which is highly expressed in the liver.31 To determine whether the regulatory effects of FGF21 on cholesterol homeostasis are attributed to its direct hepatic actions, we generated the β-klotho liver-specific knockout (β-klotho-LKO) mice by intravenous injection of adenovirus-associated virus encoding Cre recombinase into β-klotho-floxed mice (Figure VIIA and VIIB in the online-only Data Supplement). Daily administration of FGF21 significantly decreased high-fat, high-cholesterol diet–induced hypercholesterolemia, which was accompanied by decreased expression of Srebp-2 and several cholesterologenic genes in β-klotho-floxed mice injected with AAV encoding green fluorescent protein as wild-type control, whereas these effects of FGF21 were largely abrogated in β-klotho-LKO mice. By contrast, the stimulatory effects of FGF21 on adiponectin production were comparable between β-klotho-LKO mice and β-klotho-floxed mice, suggesting that hepatic β-klotho mediates the effects of FGF21 on lowering cholesterol but not on elevating adiponectin levels (Figure VIIC through VIIH in the online-only Data Supplement).
Among 4 major subtypes of FGFRs, FGFR1 plays a key role in mediating the FGF21 actions in adipose tissues.31 However, hepatic expression levels of FGFR1 were hardly detectable (Figure VIIIA in the online-only Data Supplement). Instead, FGFR4 and FGFR2 were abundantly present in the liver, followed by FGFR3.31 We next explored the role of these FGFRs in mediating the hepatic actions of FGF21 on cholesterol metabolism using adenovirus-mediated knockdown of their expression. Notably, the inhibitory effects of FGF21 on the expression of Srebp-2 and cholesterologenic genes and hypercholesterolemia were significantly compromised in mice with reduced hepatic expression of FGFR2 (Figure VIIIB through VIIIG in the online-only Data Supplement). By contrast, these FGF21 actions on cholesterol metabolism were little affected by knocking down the expression of the other 3 FGFRs despite >70% knocking down efficiency (data not shown). Taken together, these findings suggest that the regulatory effects of FGF21 on cholesterol homeostasis are mediated at least in part by the FGFR2–β-klotho complex.
Discussion
Despite intensive research on metabolic functions of FGF21, its role in the cardiovascular system has scarcely been explored. This study provides novel evidence that FGF21 deficiency causes a marked exacerbation of atherosclerosis and increased mortality of apoE−/− mice, suggesting that FGF21 is a physiological protector against vascular diseases. In this connection, elevated circulating FGF21 levels in patients and rodents with atherosclerosis may represent the defense mechanism of the body to prevent vascular damage. In support of this notion, upregulated FGF21 has been shown to act as a compensatory mechanism to protect against cerulein-induced pancreatitis,15 endotoxin-induced sepsis,32 and acetaminophen-induced acute liver injury.33
Atherosclerosis is a chronic inflammatory disease involving multiple cell types at various stages of plaque formation, including endothelial cells, lymphocytes, monocytes/macrophages, and smooth muscle cells.34 Our histological and immunologic analysis demonstrated that depletion of FGF21 in apoE−/− mice causes a markedly increased endothelial activation (as determined by expression of endothelial adhesion molecules), augmented macrophage infiltration and foam cell formation, exacerbated smooth muscle cell proliferation, and collagen deposition, all of which can be reversed by the replenishment of exogenous rmFGF21, suggesting that FGF21 is able to inhibit almost every key pathogenic event of atherosclerosis. However, these antiatherosclerotic effects of FGF21 are not attributed to its direct actions on the vascular walls but attributed to the ability of FGF21 in the induction of adiponectin in adipocytes and reduction of cholesterol biosynthesis in the liver. In support of this notion, the expression of β-klotho, an obligatory coreceptor of FGF21, is hardly detectable in any type of blood vessel cells (Z.L. and A.X., unpublished data, 2014), despite its high abundance in adipose tissue and liver.7,8
Recent studies have demonstrated the effects of FGF21 on the elevation of circulating adiponectin in both rodents and humans.12,13 In adipocytes, FGF21 can stimulate the gene expression, as well as the protein secretion, of adiponectin in a PPARγ-dependent manner.12 Adiponectin possesses potent anti-inflammatory and antiatherosclerotic activities via its multiple actions on blood vessels.35 In humans, hypoadiponectinemia is an independent risk factor for vascular inflammation and atherosclerosis.36 In contrast, elevation of circulating adiponectin by either pharmacological or genetic intervention can decrease neointima formation and atherosclerosis in both rodents and rabbits.24,37 Adiponectin accumulates in the atherosclerotic lesion area, where it protects the vascular endothelium by promoting nitric oxide and alleviating oxidative stress, suppresses smooth muscle cell proliferation and migration, inhibits macrophage infiltration and foam cell formation, and ameliorates the collagen deposition.35 In line with these reports, our results demonstrated that adiponectin, but not FGF21, suppresses platelet-derived growth factor–induced proliferation and migration of smooth muscle cells and blocks LDL uptake and cholesterol accumulation in macrophages. On the other hand, the exacerbated smooth muscle proliferation and macrophage infiltration in the atherosclerotic plaques of DKO mice can largely be reversed by replenishment with adiponectin. Taken together, these findings suggest that the effects of FGF21 on smooth muscle cells and macrophages in the vessel walls are indirect, mediated in part by the induction of adiponectin.
Dyslipidemia, especially elevated LDL cholesterol, is a major contributor to atherosclerotic plaque formation. The cholesterol-lowering drugs, such as statins, have been used clinically to reduce the risk of coronary heart disease. Therapeutic administration of FGF21 has been shown to alleviate dyslipidemia in rodents,4 obese monkeys,22 and patients with type 2 diabetes mellitus,38 including reductions in total and LDL cholesterol and triglycerides, elevations in high-density lipoprotein cholesterol, and a shift to a less atherogenic apolipoprotein profile. Consistent with these pharmacological studies, our present study showed that FGF21 deficiency in apoE−/− mice causes a further aggravation of hypercholesterolemia and a shift of apolipoprotein profiles from high-density lipoprotein to LDL. Notably, the severe hypercholesterolemia in DKO mice is accompanied by augmented de novo cholesterol biosynthesis and increased expression of several cholesterologenic genes in the liver, suggesting that endogenous FGF21 is a physiological suppressor of hepatic cholesterol production. However, whereas adiponectin replenishment reverses hypertriglyceridemia, it has little effect on hypercholesterolemia and augmented hepatic cholesterogenesis in DKO mice, suggesting that the cholesterol-lowering activity of FGF21 is independent of adiponectin. Given that hepatic FGF21 expression is progressively elevated with the development of hypercholesterolemia in apoE−/− mice, it is possible that FGF21 acts as a sensor of cholesterol overload, which in turn prevents further worsening of hypercholesterolemia via its autocrine inhibition of hepatic cholesterogenesis.
Srebps, which structurally belong to the basic helix-loop-helix-leucine zipper transcription factor family, are the principal regulator of lipid synthesis.29 Unlike other members of this class of transcription factor, Srebps are synthesized as membrane-bound precursors that require cleavage by a 2-step proteolytic process to release their amino-terminal transactivation domain into the nucleus to bind to a specific DNA sequence (sterol regulatory element) and activate their target genes.29 Hepatic expression and activity of Srebps are tightly regulated at both transcriptional and posttranslational levels by metabolic hormones and nutritional factors.29 Srebp-1a and 1c preferably activate transcription of genes involved in fatty acid synthesis, whereas Srebp-2 displays strong specificity for genes involved in cholesterol biosynthesis.39,40 Our present study demonstrated that the expression and transcriptional activity of Srebp-2, but not Srebp-1, is significantly enhanced by FGF21 deficiency but is markedly suppressed by FGF21 treatment. Furthermore, adenovirus-mediated silencing of hepatic Srebp-2 expression is sufficient to counteract exacerbation of hypercholesterolemia and augmentation of hepatic cholesterol biosynthesis caused by FGF21 deficiency, whereas the therapeutic benefits of systemic FGF21 administration on the inhibition of hepatic cholesterogenesis and reduction of hypercholesterolemia are abrogated by overexpression of Srebp-2. Thus, our study identifies hepatic Srebp-2 as a key intracellular mediator conferring the regulatory effects of FGF21 on cholesterol homeostasis.
Although the precise signaling pathways whereby FGF21 selectively suppresses hepatic Srebp-2 remain unclear, differential regulation of Srebp-1 and Srebp-2 has been reported in several previous studies.41,42 A high-carbohydrate diet induces the mRNA and protein expression of Srebp-1 but not Srebp-2,41 whereas dietary cholesterol enhances the expression of Srebp-2 and Srebp-1c but not Srebp-1a.42 The NAD+-dependent deacetylase sirtuin (Sirt) 6 and FOXO3 suppress the transcriptional activation of the Srebp-2 gene without any obvious effect on Srebp-1.43 Notably, FGF21 has been shown to form a regulatory loop with Sirt1 to reduce diet-induced fatty liver disease.44 Additional investigation is warranted to interrogate the role of the sirtuin family members in mediating FGF21-induced suppression of hepatic Srebp-2.
There are several limitations in our study. First, our observations are solely based on rodent models. In light of the fact that there is a difference in lipid metabolism and cardiovascular structure between rodents and humans, the pathophysiological relevance of our findings remains to be confirmed in humanoid large animals (eg, pigs) and in clinical studies. Second, although our data demonstrated the obligatory role of β-klotho and FGFR2 in mediating the cholesterol-lowering effects of FGF21 via suppression of Srebp-2 in the liver, the signaling pathways that link the FGF21 receptor with its regulation of cholesterol metabolism need further investigation.
In summary, our present study uncovers the protective effects of FGF21 against atherosclerosis via the induction of adiponectin in adipose tissue, reduction of hypercholesterolemia by suppression of hepatic Srebp-2, and augmentation of cholesterol efflux possibly by increasing ABCG5/8 expression (Figure IX in the online-only Data Supplement). Consistent with our animal data, a recent clinical trial in obese patients with type 2 diabetes mellitus showed that chronic administration of a long-acting form of FGF21 causes a marked elevation of adiponectin and an obvious reduction in total and LDL cholesterol but has little effect on hyperglycemia.38 Therefore, our present study, together with these clinical data, raises the possibility that FGF21 or its agonists might be more effective for the treatment of atherosclerosis, instead of diabetes mellitus.
Acknowledgments
We thank Dr Ruby C. L. Hoo from the University of Hong Kong Faculty of Medicine for technical assistance.
Sources of Funding
This work was supported by the National Natural Science Foundation of China (Major Program 91439123; General Program 81471075); General Research Fund (784111), Collaborative Research Fund (CUHK2/CRF/12G and C7055-14G), and Theme-Based Research Scheme Grant T12-705/11 from the Research Grant Council of Hong Kong; and Qatar National Research Fund (NPRP 6-428-3-113).
Disclosures
None.
Supplementary Material
Footnotes
The online-only Data Supplement is available with this article at http://circ.ahajournals.org/lookup/suppl/doi:10.1161/CIRCULATIONAHA.115.015308/-/DC1.
CLINICAL PERSPECTIVE
Atherosclerosis is a chronic inflammatory disease with many risk factors, including obesity, insulin resistance, diabetes mellitus, and dyslipidemia. Therefore, the therapeutic interventions targeting a single risk factor (eg, the use of statins to decrease hypercholesterolemia) are often insufficient to block the progression of atherosclerotic disease. In this study, we demonstrated that the liver-secreted hormone fibroblast growth factor (FGF) 21 potently alleviates atherosclerotic plaque formation and decreases premature death in apolipoprotein E−/− mice via several mechanisms. First, FGF21 induces the production of adiponectin, which in turn acts on the blood vessels directly to inhibit neointima formation and macrophage inflammation. Second, FGF21 exerts its autocrine actions in the liver to suppress lipid biosynthesis, thereby reducing hypertriglyceridemia and hypercholesterolemia. Consistent with our animal findings, chronic administration of an FGF21 analog in type 2 diabetic patients with obesity has been shown to reduce several cardiovascular risk factors, including insulin resistance, dyslipidemia, and hypoadiponectinemia. Notably, FGF21 is a downstream effector for both the nuclear receptors peroxisome proliferator-activated receptor (PPAR) α and γ, the agonists of which are effective for treatment of both metabolic and vascular diseases. Therefore, our results raise the possibility that FGF21 or its analogs may represent a promising cure for atherosclerotic diseases via its multiple actions in adipose tissue, blood vessels, and liver. Furthermore, our findings suggest that elevated circulating FGF21 levels in patients with atherosclerosis and cardiovascular disorders, which has been observed in both cross-sectional and prospective studies on several ethnic groups, may represent the body’s compensatory responses to defend these diseases.
References
- 1.Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, Galbreath EJ, Sandusky GE, Hammond LJ, Moyers JS, Owens RA, Gromada J, Brozinick JT, Hawkins ED, Wroblewski VJ, Li DS, Mehrbod F, Jaskunas SR, Shanafelt AB. FGF-21 as a novel metabolic regulator. J Clin Invest. 2005;115:1627–1635. doi: 10.1172/JCI23606. doi: 10.1172/JCI23606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Badman MK, Pissios P, Kennedy AR, Koukos G, Flier JS, Maratos-Flier E. Hepatic fibroblast growth factor 21 is regulated by PPARα and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab. 2007;5:426–437. doi: 10.1016/j.cmet.2007.05.002. doi: 10.1016/j.cmet.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 3.Potthoff MJ, Inagaki T, Satapati S, Ding X, He T, Goetz R, Mohammadi M, Finck BN, Mangelsdorf DJ, Kliewer SA, Burgess SC. FGF21 induces PGC-1α and regulates carbohydrate and fatty acid metabolism during the adaptive starvation response. Proc Natl Acad Sci U S A. 2009;106:10853–10858. doi: 10.1073/pnas.0904187106. doi: 10.1073/pnas.0904187106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coskun T, Bina HA, Schneider MA, Dunbar JD, Hu CC, Chen Y, Moller DE, Kharitonenkov A. Fibroblast growth factor 21 corrects obesity in mice. Endocrinology. 2008;149:6018–6027. doi: 10.1210/en.2008-0816. doi: 10.1210/en.2008-0816. [DOI] [PubMed] [Google Scholar]
- 5.Kharitonenkov A, Wroblewski VJ, Koester A, Chen YF, Clutinger CK, Tigno XT, Hansen BC, Shanafelt AB, Etgen GJ. The metabolic state of diabetic monkeys is regulated by fibroblast growth factor-21. Endocrinology. 2007;148:774–781. doi: 10.1210/en.2006-1168. doi: 10.1210/en.2006-1168. [DOI] [PubMed] [Google Scholar]
- 6.Li H, Gao Z, Zhang J, Ye X, Xu A, Ye J, Jia W. Sodium butyrate stimulates expression of fibroblast growth factor 21 in liver by inhibition of histone deacetylase 3. Diabetes. 2012;61:797–806. doi: 10.2337/db11-0846. doi: 10.2337/db11-0846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ito S, Kinoshita S, Shiraishi N, Nakagawa S, Sekine S, Fujimori T, Nabeshima YI. Molecular cloning and expression analyses of mouse βklotho, which encodes a novel Klotho family protein. Mech Dev. 2000;98:115–119. doi: 10.1016/s0925-4773(00)00439-1. [DOI] [PubMed] [Google Scholar]
- 8.Adams AC, Cheng CC, Coskun T, Kharitonenkov A. Fgf21 requires βklotho to act in vivo. PLoS One. 2012;7:e49977. doi: 10.1371/journal.pone.0049977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen W, Hoo RL, Konishi M, Itoh N, Lee PC, Ye HY, Lam KS, Xu A. Growth hormone induces hepatic production of fibroblast growth factor 21 through a mechanism dependent on lipolysis in adipocytes. J Biol Chem. 2011;286:34559–34566. doi: 10.1074/jbc.M111.285965. doi: 10.1074/jbc.M111.285965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murata Y, Konishi M, Itoh N. FGF21 as an endocrine regulator in lipid metabolism: from molecular evolution to physiology and pathophysiology. J Nutr Metab. 2011;2011:981315. doi: 10.1155/2011/981315. doi: 10.1155/2011/981315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fisher FM, Kleiner S, Douris N, Fox EC, Mepani RJ, Verdeguer F, Wu J, Kharitonenkov A, Flier JS, Maratos-Flier E, Spiegelman BM. Fgf21 regulates pgc-1α and browning of white adipose tissues in adaptive thermogenesis. Genes Dev. 2012;26:271–281. doi: 10.1101/gad.177857.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin Z, Tian H, Lam KS, Lin S, Hoo RC, Konishi M, Itoh N, Wang Y, Bornstein SR, Xu A, Li X. Adiponectin mediates the metabolic effects of FGF21 on glucose homeostasis and insulin sensitivity in mice. Cell Metab. 2013;17:779–789. doi: 10.1016/j.cmet.2013.04.005. doi: 10.1016/j.cmet.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 13.Holland WL, Adams AC, Brozinick JT, Bui HH, Miyauchi Y, Kusminski CM, Bauer SM, Wade M, Singhal E, Cheng CC, Volk K, Kuo MS, Gordillo R, Kharitonenkov A, Scherer PE. An FGF21-adiponectin-ceramide axis controls energy expenditure and insulin action in mice. Cell Metab. 2013;17:790–797. doi: 10.1016/j.cmet.2013.03.019. doi: 10.1016/j.cmet.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu J, Lloyd DJ, Hale C, Stanislaus S, Chen M, Sivits G, Vonderfecht S, Hecht R, Li YS, Lindberg RA, Chen JL, Jung DY, Zhang Z, Ko HJ, Kim JK, Véniant MM. Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice. Diabetes. 2009;58:250–259. doi: 10.2337/db08-0392. doi: 10.2337/db08-0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson CL, Weston JY, Chadi SA, Fazio EN, Huff MW, Kharitonenkov A, Köester A, Pin CL. Fibroblast growth factor 21 reduces the severity of cerulein-induced pancreatitis in mice. Gastroenterology. 2009;137:1795–1804. doi: 10.1053/j.gastro.2009.07.064. doi: 10.1053/j.gastro.2009.07.064. [DOI] [PubMed] [Google Scholar]
- 16.Planavila A, Redondo I, Hondares E, Vinciguerra M, Munts C, Iglesias R, Gabrielli LA, Sitges M, Giralt M, van Bilsen M, Villarroya F. Fibroblast growth factor 21 protects against cardiac hypertrophy in mice. Nat Commun. 2013;4:2019. doi: 10.1038/ncomms3019. doi: 10.1038/ncomms3019. [DOI] [PubMed] [Google Scholar]
- 17.Berglund ED, Li CY, Bina HA, Lynes SE, Michael MD, Shanafelt AB, Kharitonenkov A, Wasserman DH. Fibroblast growth factor 21 controls glycemia via regulation of hepatic glucose flux and insulin sensitivity. Endocrinology. 2009;150:4084–4093. doi: 10.1210/en.2009-0221. doi: 10.1210/en.2009-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wente W, Efanov AM, Brenner M, Kharitonenkov A, Köster A, Sandusky GE, Sewing S, Treinies I, Zitzer H, Gromada J. Fibroblast growth factor-21 improves pancreatic β-cell function and survival by activation of extracellular signal-regulated kinase ½ and Akt signaling pathways. Diabetes. 2006;55:2470–2478. doi: 10.2337/db05-1435. doi: 10.2337/db05-1435. [DOI] [PubMed] [Google Scholar]
- 19.Lin Z, Wu Z, Yin X, Liu Y, Yan X, Lin S, Xiao J, Wang X, Feng W, Li X. Serum levels of FGF-21 are increased in coronary heart disease patients and are independently associated with adverse lipid profile. PLoS One. 2010;5:e15534. doi: 10.1371/journal.pone.0015534. doi: 10.1371/journal.pone.0015534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chow WS, Xu A, Woo YC, Tso AW, Cheung SC, Fong CH, Tse HF, Chau MT, Cheung BM, Lam KS. Serum fibroblast growth factor-21 levels are associated with carotid atherosclerosis independent of established cardiovascular risk factors. Arterioscler Thromb Vasc Biol. 2013;33:2454–2459. doi: 10.1161/ATVBAHA.113.301599. doi: 10.1161/ATVBAHA.113.301599. [DOI] [PubMed] [Google Scholar]
- 21.Adams AC, Halstead CA, Hansen BC, Irizarry AR, Martin JA, Myers SR, Reynolds VL, Smith HW, Wroblewski VJ, Kharitonenkov A. LY2405319, an engineered FGF21 variant, improves the metabolic status of diabetic monkeys. PLoS One. 2013;8:e65763. doi: 10.1371/journal.pone.0065763. doi: 10.1371/journal.pone.0065763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Véniant MM, Komorowski R, Chen P, Stanislaus S, Winters K, Hager T, Zhou L, Wada R, Hecht R, Xu J. Long-acting FGF21 has enhanced efficacy in diet-induced obese mice and in obese rhesus monkeys. Endocrinology. 2012;153:4192–4203. doi: 10.1210/en.2012-1211. doi: 10.1210/en.2012-1211. [DOI] [PubMed] [Google Scholar]
- 23.Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N, Ezaki O, Akanuma Y, Gavrilova O, Vinson C, Reitman ML, Kagechika H, Shudo K, Yoda M, Nakano Y, Tobe K, Nagai R, Kimura S, Tomita M, Froguel P, Kadowaki T. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7:941–946. doi: 10.1038/90984. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- 24.Okamoto Y, Kihara S, Ouchi N, Nishida M, Arita Y, Kumada M, Ohashi K, Sakai N, Shimomura I, Kobayashi H, Terasaka N, Inaba T, Funahashi T, Matsuzawa Y. Adiponectin reduces atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2002;106:2767–2770. doi: 10.1161/01.cir.0000042707.50032.19. [DOI] [PubMed] [Google Scholar]
- 25.Xu A, Wang Y, Keshaw H, Xu LY, Lam KS, Cooper GJ. The fat-derived hormone adiponectin alleviates alcoholic and nonalcoholic fatty liver diseases in mice. J Clin Invest. 2003;112:91–100. doi: 10.1172/JCI17797. doi: 10.1172/JCI17797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Y, Lam KS, Xu JY, Lu G, Xu LY, Cooper GJ, Xu A. Adiponectin inhibits cell proliferation by interacting with several growth factors in an oligomerization-dependent manner. J Biol Chem. 2005;280:18341–18347. doi: 10.1074/jbc.M501149200. doi: 10.1074/jbc.M501149200. [DOI] [PubMed] [Google Scholar]
- 27.Ouchi N, Kihara S, Arita Y, Nishida M, Matsuyama A, Okamoto Y, Ishigami M, Kuriyama H, Kishida K, Nishizawa H, Hotta K, Muraguchi M, Ohmoto Y, Yamashita S, Funahashi T, Matsuzawa Y. Adipocyte-derived plasma protein, adiponectin, suppresses lipid accumulation and class A scavenger receptor expression in human monocyte-derived macrophages. Circulation. 2001;103:1057–1063. doi: 10.1161/01.cir.103.8.1057. [DOI] [PubMed] [Google Scholar]
- 28.Yu L, Li-Hawkins J, Hammer RE, Berge KE, Horton JD, Cohen JC, Hobbs HH. Overexpression of ABCG5 and ABCG8 promotes biliary cholesterol secretion and reduces fractional absorption of dietary cholesterol. J Clin Invest. 2002;110:671–680. doi: 10.1172/JCI16001. doi: 10.1172/JCI16001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown MS, Goldstein JL. The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell. 1997;89:331–340. doi: 10.1016/s0092-8674(00)80213-5. [DOI] [PubMed] [Google Scholar]
- 30.Calkin AC, Tontonoz P. Transcriptional integration of metabolism by the nuclear sterol-activated receptors LXR and FXR. Nat Rev Mol Cell Biol. 2012;13:213–224. doi: 10.1038/nrm3312. doi: 10.1038/nrm3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fisher FM, Estall JL, Adams AC, Antonellis PJ, Bina HA, Flier JS, Kharitonenkov A, Spiegelman BM, Maratos-Flier E. Integrated regulation of hepatic metabolism by fibroblast growth factor 21 (FGF21) in vivo. Endocrinology. 2011;152:2996–3004. doi: 10.1210/en.2011-0281. doi: 10.1210/en.2011-0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feingold KR, Grunfeld C, Heuer JG, Gupta A, Cramer M, Zhang T, Shigenaga JK, Patzek SM, Chan ZW, Moser A, Bina H, Kharitonenkov A. FGF21 is increased by inflammatory stimuli and protects leptin-deficient ob/ob mice from the toxicity of sepsis. Endocrinology. 2012;153:2689–2700. doi: 10.1210/en.2011-1496. doi: 10.1210/en.2011-1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ye D, Wang Y, Li H, Jia W, Man K, Lo CM, Wang Y, Lam KS, Xu A. Fibroblast growth factor 21 protects against acetaminophen-induced hepatotoxicity by potentiating peroxisome proliferator-activated receptor coactivator protein-1α-mediated antioxidant capacity in mice. Hepatology. 2014;60:977–989. doi: 10.1002/hep.27060. [DOI] [PubMed] [Google Scholar]
- 34.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 35.Hui X, Lam KS, Vanhoutte PM, Xu A. Adiponectin and cardiovascular health: an update. Br J Pharmacol. 2012;165:574–590. doi: 10.1111/j.1476-5381.2011.01395.x. doi: 10.1111/j.1476-5381.2011.01395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shioji K, Moriguchi A, Moriwaki S, Manabe K, Takeuchi Y, Uegaito T, Mutsuo S, Matsuda M. Hypoadiponectinemia implies the development of atherosclerosis in carotid and coronary arteries [in Japanese]. J Cardiol. 2005;46:105–112. [PubMed] [Google Scholar]
- 37.Li CJ, Sun HW, Zhu FL, Chen L, Rong YY, Zhang Y, Zhang M. Local adiponectin treatment reduces atherosclerotic plaque size in rabbits. J Endocrinol. 2007;193:137–145. doi: 10.1677/JOE-06-0173. doi: 10.1677/JOE-06-0173. [DOI] [PubMed] [Google Scholar]
- 38.Gaich G, Chien JY, Fu H, Glass LC, Deeg MA, Holland WL, Kharitonenkov A, Bumol T, Schilske HK, Moller DE. The effects of LY2405319, an FGF21 analog, in obese human subjects with type 2 diabetes. Cell Metab. 2013;18:333–340. doi: 10.1016/j.cmet.2013.08.005. doi: 10.1016/j.cmet.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 39.Shimano H, Horton JD, Shimomura I, Hammer RE, Brown MS, Goldstein JL. Isoform 1c of sterol regulatory element binding protein is less active than isoform 1a in livers of transgenic mice and in cultured cells. J Clin Invest. 1997;99:846–854. doi: 10.1172/JCI119248. doi: 10.1172/JCI119248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Horton JD, Shimomura I, Brown MS, Hammer RE, Goldstein JL, Shimano H. Activation of cholesterol synthesis in preference to fatty acid synthesis in liver and adipose tissue of transgenic mice overproducing sterol regulatory element-binding protein-2. J Clin Invest. 1998;101:2331–2339. doi: 10.1172/JCI2961. doi: 10.1172/JCI2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Horton JD, Bashmakov Y, Shimomura I, Shimano H. Regulation of sterol regulatory element binding proteins in livers of fasted and refed mice. Proc Natl Acad Sci U S A. 1998;95:5987–5992. doi: 10.1073/pnas.95.11.5987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Field FJ, Born E, Murthy S, Mathur SN. Regulation of sterol regulatory element-binding proteins in hamster intestine by changes in cholesterol flux. J Biol Chem. 2001;276:17576–17583. doi: 10.1074/jbc.M010917200. doi: 10.1074/jbc.M010917200. [DOI] [PubMed] [Google Scholar]
- 43.Tao R, Xiong X, DePinho RA, Deng CX, Dong XC. Hepatic SREBP-2 and cholesterol biosynthesis are regulated by FoxO3 and Sirt6. J Lipid Res. 2013;54:2745–2753. doi: 10.1194/jlr.M039339. doi: 10.1194/jlr.M039339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee J, Hong SW, Park SE, Rhee EJ, Park CY, Oh KW, Park SW, Lee WY. Exendin-4 regulates lipid metabolism and fibroblast growth factor 21 in hepatic steatosis. Metabolism. 2014;63:1041–1048. doi: 10.1016/j.metabol.2014.04.011. doi: 10.1016/j.metabol.2014.04.011. [DOI] [PubMed] [Google Scholar]


