Summary
Constitutive or aberrant signalling of the B cell receptor signalling cascade has been implicated in the propagation and maintenance of a variety of B cell malignancies. Small molecule inhibitors of Bruton tyrosine kinase (BTK), a protein early in this cascade and specifically expressed in B cells, have emerged as a new class of targeted agents. There are several BTK inhibitors, including ONO-WG-307, LFM-A13, dasatinib, CC-292, and PCI-32765 (ibrutinib), in preclinical and/or clinical development of which ibrutinib is currently in phase III trials. Recent clinical data suggest significant activity of ibrutinib as a first in class oral inhibitor of BTK. This review provides an overview of ongoing clinical studies of BTK inhibitors.
Keywords: Bruton tyrosine kinase, B cell receptor signalling, refractory non-Hodgkin lymphoma, ibrutinib, CC-292
B cell receptor signalling pathway
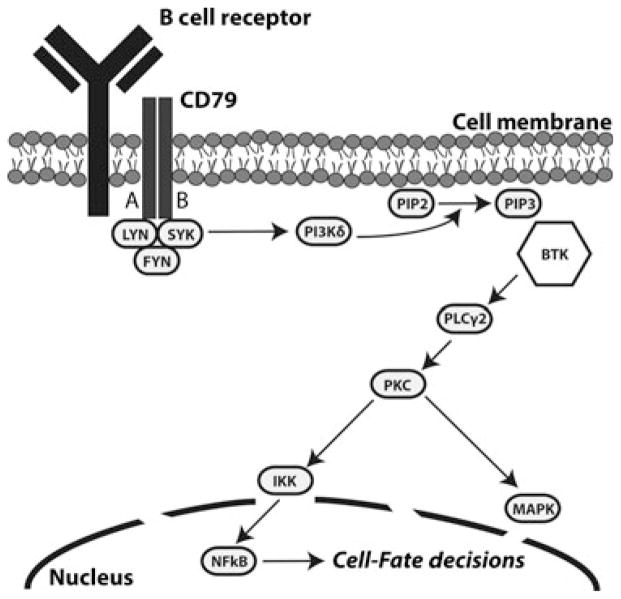
The B cell receptor (BCR) signalling pathway plays a fundamental role in determining B cell fate and function by regulating cellular selection, maturation, proliferation, and antibody production (Fig 1) (Dal Porto et al, 2004). The receptor consists of a surface transmembrane immunoglobulin (Ig) associated with the Igα (CD79A) and Igβ (CD79B) chains (Wiestner, 2012). In normal B cells, antigenic binding to the BCR results in receptor aggregation and subsequent phosphorylation of the receptor’s cytoplasmic tyrosine-based activation motifs (ITAMs) by the recruited SRC-family kinases LYN and SYK (Wiestner, 2013). SYK propagates the signal through activation of phosphoinositide 3-kinase (PI3Kδ), which in turn mediates the conversion of phosphatidylinositol 4,5 bisphosphate (PIP2) to phosphatidylinositol 3,4,5 triphosphate (PIP3) (Wiestner, 2013). The ensuing recruitment of Bruton tyrosine kinase (BTK) further transmits and amplifies the signal via phosphorylation of phospholipase C gamma 2 (PLCγ2), mobilization of calcium secondary messengers, and eventual activation of MAP kinase pathways, nuclear factor of activated T cells (NFAT), and nuclear factor κB (NFκB). These signals regulate patterns of gene expression necessary for B cell survival and proliferation (Brown, 2012; Wiestner, 2013).
Fig 1.
The BCR signalling cascade. Antigen binding to the B cell receptor initiates kinase-mediated signal transduction resulting in activation of secondary messengers and, ultimately, transcription factors that regulate cell fate.
While the BCR signalling cascade is generally antigen-dependent in normal B cells, antigen-independent signalling, also known as ‘tonic’ signalling, has been shown to exist (de Rooij et al, 2012). An overactive antigen-independent pathway is thought to be a contributing factor to B cell malignancies characterized by constitutively or aberrantly active BCR signalling (Monroe, 2006; Rinaldi et al, 2006; Chen et al, 2008; Davids & Brown, 2012; Dühren-von Minden et al, 2012). Specifically, overactive signalling promotes the development of a supportive tumour microenvironment by modulating chemokine-controlled migration and integrin-mediated adhesion, while lack of such support leads to rapid apoptosis in B cells (de Rooij et al, 2012; Wiestner, 2012). Consistent with this, several inhibitors of protein kinases involved in BCR signalling have achieved notable clinical results: a LYN inhibitor (Bafetinib), a SYK inhibitor (Fostamatinib), and a PI3Kδ inhibitor (Idelalisib) (Robak & Robak, 2013).
Bruton tyrosine kinase as a unique target of inhibition
Among the many kinases involved in BCR signalling, BTK, a tyrosine kinase member of the Tec kinase family, is a unique therapeutic target. Loss of gene function mutations of BTK in humans results in X-linked agammaglobulinaemia (XLA), characterized by a complete lack of B cells, low levels of serum Ig, and recurring infections. This suggests that BTK is required for B cell development and immunoglobulin production (Maas & Hendriks, 2001). As with other kinases in the BCR pathway, inhibition of BTK has been shown to inhibit NFκB DNA binding, reduce integrin-mediated cell adhesion and migration, limit cell production of chemokines, diminish cellular response to chemotactic factors, and ultimately induce apoptosis (Herman et al, 2011; Ponader et al, 2012; de Rooij et al, 2012).
The druggability of BTK is supported by the clinical phenotype of patients with XLA, where a lack of functioning BTK is both nonlethal and selective to only B cells as opposed to T cells (Herman et al, 2011). This selectivity for B lymphocytes is promising as inhibitors of BTK will hypothetically take less of a toll on a patient’s naturally occurring immune cells. These properties have made inhibition of BTK an attractive target in pharmacology with several drugs in the development pipeline. Published data are available for at least five BTK inhibitors that vary in specificity and mechanism of binding: ONO-WG-307, LFM-A13, dasatinib, CC-292, and ibrutinib (PCI-32765) (Table I).
Table I.
Preclinical and phase I study data.
| Agent | ONO-WG-307 | LFM-A13 | Dasatinib | CC-292 | Ibrutinib |
|---|---|---|---|---|---|
| Developer | ONO Pharmaceutical | University of Southern California | Bristol-Myers Squibb | Celgene Corporation | Pharmacyclics and Janssen Pharmaceuticals |
| Mechanism, Target | Reversible, Tyr223 | Reversible, Unknown | Reversible, Unknown | Irreversible, Cys481 | Irreversible, Cys481 |
| Potency | IC50 = 2 nmol/l | IC50 = 7·5 μmol/l | IC50 = 5 nmol/l | IC50 <0·5 nmol/l | IC50 = 0·5 nmol/l |
| Clinical Data From Phase I Trials in Relapsed/Refractory B Cell Malignancies | N/A | N/A | Multiple Histologies n = 19 ORR 32%, CR 11% |
Multiple Histologies n = 17 SD: 94%, PR: 6% CLL n = 50 ORR 34%, CR 0% |
Multiple Histologies n = 56 (% ORR/CR) MCL: 78/33 CLL/SLL: 69/13 FL: 55/27 DLBCL: 29/0 WM: 75/0 MZL: 25/0 |
B-NHL, B cell non-Hodgkin lymphoma; CLL, chronic lymphocytic leukaemia; SLL, small lymphocytic lymphoma; DLBCL, diffuse large B cell lymphoma; FL, follicular lymphoma; MCL, mantle cell lymphoma; MZL, marginal zone lymphoma; WM, Waldenström macroglobulinaemia; ORR, objective response rate; CR, complete response; PR, partial response; PFS, progression-free survival; R/R, relapsed/refractory; N/A: not available.
ONO-WG-307 (ONO Pharmaceutical Co., Osaka, Japan)
ONO-WG-307 is an oral, potent (50% inhibitory concentration [IC50] = 2 nmol/l), reversible inhibitor of BTK that acts by blocking auto-phosphorylation at the Tyr223 position (Yasuhiro et al, 2012). ONO-WG-307 displays high specificity for BTK given that its IC50 values for related tyrosine kinases (LCK, LYN, FYN) are above 1 μmol/l. The drug exhibits significant activity against the activated B cell-like (ABC) subtype of diffuse large B-cell lymphoma (DLBCL) in TMD-8 mouse xenograft models, with dose-dependent tumour volume growth inhibition of up to 84% over 24 d (Kozaki et al, 2011). ONO-WG-307 demonstrates in vitro anti-proliferative effects in follicular lymphoma (FL), mantle cell lymphoma (MCL), and chronic lymphocytic leukaemia (CLL) cell lines and its combination with the anti-CD20 antibody rituximab shows promise as an effective combination therapy (Kozaki et al, 2011, 2012). To date, no clinical data are available; however, ONO-WG-307’s reversible mechanism may result in fewer off-target effects in comparison to irreversible inhibitors.
LFM-A13 (University of Southern California)
LFM-A13 is an intraperitoneally injectable reversible inhibitor of BTK (IC50 = 7·5 μmol/l) that binds to the rectangular catalytic pocket defined by Leu460, Tyr476, Arg525, and Asp539 (Mahajan et al, 1999; Uckun et al, 2002). LFM-A13 does not affect the enzymatic activity of tyrosine kinases JAK1, JAK3, HCK, epidermal growth factor receptor kinase, and insulin receptor kinase and was shown to increase sensitivity of B-lineage leukaemic cells to ceramide- and vincristine-induced apoptosis in vitro (Mahajan et al, 1999). In leukaemic mouse models, LFM-A13 was non-toxic when administered at dose levels ranging from 10 to 80 mg/kg and significantly prolonged survival when delivered in combination with vincristine, methylprednisolone, and L-asparaginase (VPL) compared to only VPL therapy (Uckun et al, 2002). Preliminary data suggest that chemoresistance in relapsed B cell precursor acute lymphoblastic leukaemia can be overcome with LFM-A13 in combination with chemotherapy (Uckun et al, 2011). No clinical data are available to date.
Dasatinib (Bristol-Myers Squibb, New York, NY, USA)
Dasatinib is an orally available, reversible tyrosine kinase inhibitor currently approved for treatment of chronic myeloid leukaemia (CML) and Philadelphia chromosome-positive acute lymphoblastic leukaemia (ALL) (Steinberg, 2007). At the time of approval, only BCR-ABL and SRC kinases had been considered as relevant targets, but dasatinib was later discovered to also be a potent inhibitor of BTK (IC50 = 5 nmol/l) in vitro with selective activity over ITK (Hantschel et al, 2007). Mutation of the gatekeeper residue Thr474 to Ile in BTK confers complete resistance to dasatinib and is suggestive of its mode of interaction with BTK (Hantschel et al, 2007).
Preliminary phase I/II data has been reported on 27 patients with relapsed/refractory (R/R) non-Hodgkin lymphoma (NHL) (William et al, 2010). Patients received dasatinib at doses of 100, 150, or 200 mg once daily for 28-day cycles. The median age was 58 years (range: 34–87), and patients had received a median of 4 (range: 1–20) prior therapies. The maximum tolerable dose (MTD) was determined to be 150 mg. At a median follow up of 24 months, of the 19 evaluable patients the objective response rate (ORR) was 32%: 2 complete responses (CRs), 4 partial responses (PRs), and 8 with stable disease (SD) (Table I). Progression-free survival (PFS) was 17% at 1 year and 13% at 2 years. Grade 3 and 4 adverse events (AEs) included pleural effusion (22%), thrombocytopenia (19%), neutropenia (11%), anaemia (7%), diarrhoea (7%), leucopenia (4%), rash (4%), weakness/orthostasis (4%), prolonged QTc interval (4%), flash pulmonary oedema (4%), and skin graft failure (4%).
Dasatinib has also been evaluated in a phase II study of R/R CLL (Amrein et al, 2011). 15 patients, median age 59 years (range: 40–78) and median 3 prior therapies, were enrolled with a median time on study of 14 weeks. Seventy three percent had either del(11q) or del(17p). 3 patients achieved a PR for an ORR of 20% (Table II). Of the remaining 12 patients, 6 had nodal responses. Grade 3 and 4 haematological toxicities were frequent including neutropenia (67%) and thrombocytopenia (40%). Two patients developed grade 3 pneumonia and four developed pleural effusions. 33% of patients were removed from the study within 4 months due to severe myelosuppression. 73% patients had treatment interrupted at some point in the study due to toxicity.
Table II.
Clinical data from phase II studies with BTK inhibitors.
| Regimen | Disease | n | %ORR (CR) | Outcome |
|---|---|---|---|---|
| Ibrutinib | CLL, TN | 31 | 67 (2) | 26-month PFS: 96% |
| CLL, R/R | 85 | 71 (3) | 26-month PFS: 75% | |
| MCL, R/R | 111 | 68 (21) | 13·9 months | |
| GCB DLBCL, R/R | 20 | 5·3 (0) | Median OS: 3·35 months | |
| ABC DLBCL, R/R | 29 | 40 (8) | Median OS: 9·76 months | |
| Ibrutinib + Ofatumumab | CLL, R/R | 27 | 100 (4) | 89% remain on study |
| Ibrutinib + Bendamustine-Rituximab | CLL, R/R | 30 | 93 (13) | 8·1-month PFS: 90% |
| Dasatinib | CLL, R/R | 15 | 20 (0) | N/A |
TN, treatment naïve; R/R, relapsed/refractory; CLL, chronic lymphocytic leukaemia; MCL, mantle cell lymphoma; DLBCL, Diffuse large B cell lymphoma; GCB, Germinal centre B cell, ABC, activated B cell; ORR, objective response rate; CR, complete response; PFS, progression-free survival; OS, overall survival; N/A, not available.
CC-292 (Celgene Corporation, Summit, NJ, USA)
CC-292, formerly known as AVL-292, is an orally available, potent (IC50 apparent = 0·5 nmol/l, half maximal effective concentration [EC50] = 8 nmol/l), irreversible small molecule inhibitor of BTK that forms a covalent bond with Cys481 (Evans et al, 2013). CC-292 displays high levels of specificity for BTK in comparison to other SRC family kinases. The inhibitory activity of CC-292 has been demonstrated in both immunoblot analysis, which revealed that CC-292 strongly inhibits auto-phosphorylation in human naïve primary B cells, and flow cytometry tracking of CD69 upregulation (a surrogate for B cell activation) which exhibited a dose-dependent reduction in CD69 expression with increasing concentrations of CC-292 in vitro. Covalent probe analysis of human B cells coupled with enzyme linked immunosorbent assay (ELISA) quantification methods demonstrated 42% BTK occupancy in a 1-h incubation with 10 nmol/l CC-292. In mice models, % BTK occupancy was positively correlated with reduced signs of arthritic disease.
CC-292 was evaluated in healthy adult subjects and was found to be safe and well tolerated following oral administration at dose levels ranging from 0·5 mg/kg to 7·0 mg/kg. (Evans et al, 2011). PK data was reported for the 2·0 mg/kg single oral dose (Evans et al, 2013). CC-292 was rapidly absorbed (Tmax c. 30–120 min), achieving >84% BTK occupancy and mean peak plasma levels (Cmax = 542 ng/ml) at this dose. The T1/2 = 1·9 h, and BTK occupancy was sustained over a 24-h period.
Preliminary phase Ib data with CC-292 was reported on 12 patients with R/R B cell NHL (B-NHL) (8 CLL, 1 DLBCL, 1 FL, 1 marginal zone lymphoma (MZL)) (Brown et al, 2012a). Escalating cohorts of 125, 250, and 400 mg/d for 28-day cycles were evaluated. Full BTK occupancy was reached at 250 mg/d and the MTD was >400 mg/d. The median age of patients was 68 years (range: 45–79), median number of prior therapies 2·5 (range: 1–10), and reported median time on treatment was 65 d. While the duration of response (DOR) was not available at the time of the report, 10 of the 12 patients were continuing on treatment. All 8 CLL patients had SD with a median 28% decrease from baseline lymph node measurement. The MZL patient also had SD. The DLBCL patient had progressive disease (PD) and the FL patient was not evaluable due to dose limiting toxicity (DLT). The drug was generally well tolerated with no grade 4 AEs reported and only a single grade 3 AE of a low absolute neutrophil count, probably treatment-related.
Updated results were recently presented on 86 patients (23 B-NHL, 6 Waldenström macroglobulinaemia (WM), 57 CLL/small lymphocytic lymphoma (SLL)) treated with CC-292 at doses of 125, 250, 400, 625, 750, and 1000 mg QD or 375 and 500 mg BID (Table I) (Brown et al, 2013). An expansion cohort of CLL patients at 750 mg QD was also tested. All patients received continuous dosing in 28-day cycles until PD or intolerable toxicity. The median age was 65 years (range: 29–89) and median number of prior therapies 3 (range: 1–12). Of the 57 CLL patients, 39 (68·4%) had at least 1 high risk factor, including 30 (52·6%) with unmutated IGHV, and 26 (45·6%) with del11q22 (n = 12), del17p (n = 14), or both (n = 2). The median time on therapy was 144 d (range: 13–515). Three DLTs were reported, including thrombocytopenia (400 mg), pneumonitis (1000 mg), and altered mental status (500 mg BID). The MTD has not been reached. The most frequent treatment emergent AEs (≥10% of patients regardless of causality) were grade 1–2.
All 17 efficacy-evaluable B-NHL patients had SD except for a single PR in a patient with MZL who started at 250 mg and escalated sequentially up to 750 mg QD, achieving a PR at cycle 16. Of 50 efficacy-evaluable CLL patients, 17 (34%) achieved a PR and 24 (45%) showed lymph node reduction. Lymphocytosis, a class effect of these agents (discussed under the ibrutinib section) was noted in 10 patients and resolved in 5 patients. At the time of reporting, the median duration of treatment was 176 d (range: 16–473), and 2 CLL patients had been on treatment for over 15 cycles, both initiating treatment at 400 mg QD and experiencing nodal reductions of 32% and 27%.
The ORR was 31% at 750 mg QD, 50% at 1000 mg QD and 66·7% at 375 mg BID, suggesting that BID dosing maybe more efficacious. Most patients at 500 mg BID were not eligible for response evaluation at the time of publication. Among the responding patients, poor risk factors included unmutated IGHV (n = 8), del11q (n = 3), and/or del17p (n = 2) suggesting efficacy in these subgroups.
Currently there is an ongoing phase Ib study of CC-292 in combination with lenalidomide in patients with R/R B cell lymphoma, but no data has been reported to date.
Ibrutinib (PCI-32765) (Pharmacyclics, Inc., Sunnyvale, CA, USA and Janssen Pharmaceuticals, Inc., Titusville, NJ, USA)
Ibrutinib is the most advanced of the BTK inhibitors in clinical development (Burger & Buggy, 2013). It is an orally available, potent (IC50 = 0·5 nmol/l), irreversible inhibitor of BTK that forms a covalent bond with Cys481 (Honigberg et al, 2010). In vitro, treatment of DOHH2 cells with ibrutinib prevents phosphorylation of BTK’s immediate substrate PLCγ2 and the further downstream kinase ERK, while not affecting upstream kinases like SYK. Specificity for BTK was confirmed by showing a lack of significant activity against a sample of 19 other related kinases, including seven kinases that share the cysteine residue targeted by ibrutinib. BTK occupancy can be measured with a fluorescently tagged affinity probe, PCI-33380, which consists of the core inhibitor molecule linked with a Bodipy FL fluorophore via a piperazine linker. Levels of irreversible probe binding to BTK can be detected by denaturing gel electrophoresis and fluorescent gel staining of BTK-transfected cell lysates treated with PCI-33380 such that increased target occupancy by drug results in lower probe signalling (Honigberg et al, 2010). Continuous exposure to ibrutinib inhibits upregulation of CD69 while maintaining over 1000-fold selectivity for B cells over T cells (Honigberg et al, 2010).
In vivo data confirmed the therapeutic potential of the drug (Honigberg et al, 2010). In mice models, reduction in signs of arthritic disease correlated with levels of inhibition of BTK by ibrutinib and was marked by reductions in production of anticollagen autoantibodies and total IgG levels. Similarly, in canine models with both naïve and previously treated naturally occurring B-NHL, objective clinical responses were achieved with full BTK occupancy at dosages of 2·5–20 mg/kg/d.
In a phase Ia dose escalation trial, 56 patients with R/R B-NHL of variable histologies (16 FL, 16 CLL/SLL, 9 MCL, 7 DLBCL, 4 MZL, and 4 WM) were enrolled to determine MTD, safety, PK/pharmacodynamics and tumour response (Advani et al, 2013). MTD was defined as a dose three levels above the dose level with full BTK occupancy, as measured by a fluorescent affinity probe. Two dosing schedules were evaluated: 35-day cycles with 28 d on and 7 d off and a once daily continuous dosing. The median age of patients was 65 years (range: 41–82), median number of prior therapies was 3 (range: 1–10), and median treatment time was 5 cycles. Ibrutinib was tolerable at doses of 1·0–12·5 mg/kg/d with no DLTs. Pharmacodynamic studies using the competitive binding assay with a fluorescent probe showed that 1·25 mg/kg/d of ibrutinib achieved sustained BTK engagement over 24 h and 2·5 mg/kg/d achieved near complete BTK engagement at 4 and 24 h post-dosing. PK data suggested that at dose levels of 1·25 mg/kg/d, the Cmax = 82 ng/ml, Tmax median = 1·14 h, and T1/2 = 4·92 h (Pollyea et al, 2009). Ibrutinib was well tolerated with the majority of AEs being mainly grade 1 or 2 with limited occurrences of grade 3 and 4 toxicities including neutropenia (12·5%), thrombocytopenia (7·2%), and anaemia (7·1%). Only two DLTs were reported, a grade 3 allergic reaction in a patient with a history of similar reactions and a dose interruption for more than 7 d due to transient grade 2 neutropenia. Continuous dosing was just as effective as the interrupted dosing schedule in terms of PK/pharmacodynamics and a fixed dose of 560 mg/d was identified as the dose to move forward with in phase II trials.
Efficacy data was reported on 50 evaluable patients with an ORR of 60% (CR 16%) (Advani et al, 2013). Responses were noted across histologies: MCL (7 of 9 patients, 3 CRs), CLL/SLL (11 of 16 patients, 2 CRs), FL (6 of 16 patients, 3 CRs), DLBCL (2 of 7 patients), WM (3 of 4 patients), and MZL (1 of 4 patients) (Table I). The median PFS was 13·6 months and 20 patients remained on treatment at the time of the report.
Consistent with observations of other agents that affect BCR signalling inhibition in patients with CLL and MCL, along with nodal shrinkage there was a simultaneous dramatic spike in plasma lymphocyte levels occurring in cycles 1 and 2 of treatment with ibrutinib which slowly declined and stabilized over time (Herman et al, 2011; Brown, 2012). The apparent increase in lymphocyte counts was a result of the egress of CLL cells from the lymph nodes and recirculation in the blood (Brown, 2012). BCR signalling is implicated in integrin-mediated adhesion of cells to fibronectin and, therefore, inhibition reduces cellular retention within the tumour microenvironment (Herman et al, 2011).
An update of the 16 patients with FL treated in the phase I study has also been reported (Fowler et al, 2012). The median age was 60 years (range: 41–71) with a median of 3 prior therapies (range: 1–5). The Follicular Lymphoma International Prognostic Index (FLIPI) scores were high in 44% of patients and intermediate in 38%. Of the 11 patients that received treatment with a dose of 2·5 mg/kg or higher (the minimum dose necessary for full BTK occupancy), 3 patients achieved a CR and 3 a PR for an ORR of 54·5%. The DOR was 12·3 months with a median PFS of 13·4 months. The 9 patients treated at doses of at least 5 mg/kg had a median PFS of 19·6 months with 2 patients remaining on the study at 25 and 29 months.
Collectively, these phase I results suggest that ibrutinib is well tolerated and has significant activity in a variety of histologies (Brown, 2013). These data have led to several phase II studies evaluating its efficacy in specific B cell histologies (Table II).
Phase II studies with ibrutinib
Relapsed/refractory chronic lymphocytic leukaemia and small lymphocytic lymphoma
In a phase Ib/II study, 85 patients (median age 64 years) with R/R disease were enrolled in three cohorts, with 51 patients receiving 420 mg/d and 34 patients receiving 840 mg/d in 28-day cycles (Byrd et al, 2013). Median number of prior treatments was 4 (range: 1–12). Of note, 52% of patients had bulky adenopathy (>5 cm), 33% had 17p deletion, and 81% unmutated IGHV. The ORR was 71% (4% CR) in the 420 mg cohorts and 71% (0% CR) in the 820 mg cohort. A nodal PR (defined as a >50% reduction in total lymph node size) with lymphocytosis was seen in 20% and 15% of patients in the 420 mg and 840 mg cohorts respectively. The lymphocytosis resolved over time. 13% of patients had PD while taking ibrutinib. The estimated 26 months PFS was 75%. The best response was very similar across the two doses and therefore 420 mg was selected as the dose for future evaluation. Ibrutinib was well tolerated and the majority of reported AEs were grade 2. The most common grade ≥3 AEs were pneumonia (12%) and dehydration (6%). Grade ≥3 haematological toxicities were limited: anaemia (6%), neutropenia (15%), and thrombocytopenia (6%).
Therapy naïve chronic lymphocytic leukaemia
A phase Ib/II study evaluated treatment of naïve elderly CLL patients (≥65 years) (Byrd et al, 2012a). 31 patients were enrolled in two cohorts with 26 patients receiving 420 mg/d and 5 receiving 840 mg/d in 28 d cycles. The 840 mg cohort was eventually discontinued due to improved safety of the 420 mg dose and comparable efficacy (Byrd et al, 2012b). The median age was 71 years (range: 65–84) and 55% of patients had unmutated IGHV. In the 420 mg cohort at a median 16·6 months follow-up, the ORR was 71% (10% CR), with an additional 10% of patients achieving PR with lymphocytosis. The 22-month estimated PFS was 96%. The majority of AEs were grade 2, easily managed, and included diarrhoea, fatigue, upper respiratory tract infection, rash, nausea and arthralgias. Grade ≥3 haematological AEs were infrequent.
The very promising single agent activity and tolerability of ibrutinib in CLL have paved the way for trials in combination with chemoimmunotherapy or obviating the need for chemoimmunotherapy, especially in elderly patients. Early results of ibrutinib and ofatumumab on 27 patients reported an ORR of 100% (4% CR) (Jaglowski et al, 2012). Ibrutinib in combination with bendamustine-rituximab (n = 30) had an ORR of 93% (13% CR) at a median follow up of 8·1 months with an estimated PFS at 8·1 months of 90% (Brown et al, 2012b). Several phase III studies are ongoing including the RESONATE trial that evaluates ibrutinib versus ofatumumab in R/R patients ineligible for purine analogue-based therapy (Table III).
Table III.
Ongoing phase III trials with ibrutinib.
| Disease | Trial | Eligibility | Regimen |
|---|---|---|---|
| MCL | SHINE NCT01776840 | Untreated elderly | BR + Ibrutinib vs. BR + Placebo |
| RAY NCT01646021 | R/R, ≥1 prior therapy | Ibrutinib vs. Temsirolimus | |
| CLL | RESONATE NCT01578707 | R/R not eligible for purine analogues | Ibrutinib vs. Ofatumumab |
| RESONATE 2 NCT01722487 | Untreated elderly | Ibrutinib vs. Chlorambucil | |
| HELIOS NCT01611090 | R/R, ≥1 prior therapy | BR + Ibrutinib vs. BR + placebo | |
| US Intergroup Alliance A041202 NCT01886872 | Untreated elderly | BR vs. Ibrutinib vs. Ibrutinib + Rituximab | |
| US Intergroup ECOG E1912 (Protocol in development) | Untreated fit patients | Ibrutinib–based Therapy vs. Fludarabine + Cyclophosphamide + Rituximab | |
| UK NCRI CLL10 (Protocol in development) | Untreated fit patients | Ibrutinib + Rituximab vs. Fludarabine + Cyclophosphamide + Rituximab |
BR, bendamustine-rituximab; CLL, chronic lymphocytic leukaemia; MCL, mantle cell lymphoma; R/R, relapsed/refractory.
Relapsed/refractory mantle cell lymphoma
A phase II trial evaluated 111 patients with median age of 68 years (range: 40–84) and a median of 3 prior therapies (range: 1–5) (Wang et al, 2013). 45% of patients had refractory disease. 63 patients were bortezomib-naïve. Patients received 560 mg/d as a continuous dose in 28-day cycles. Similar to the experience with other trials of ibrutinib, most AEs were grade 1 or 2. Grade ≥3 AEs included neutropenia (16%), thrombocytopenia (11%), anaemia (10%) and pneumonia (6%). At a median treatment time of 15·3 months, 63 evaluable bortezomib-naïve patients achieved an ORR of 68% (19% CR) and 48 evaluable bortezomib-exposed patients achieved a comparable ORR of 67% (23% CR). The median PFS across all patients was 13·9 months.
Relapsed/refractory diffuse large B cell lymphoma
Interim results of a phase II trial reported responses in germinal centre B cell-like (GCB) and ABC subtypes of DLBCL (Wilson et al, 2012). Unlike GCB-DLBCL, the ABC subtype is sustained by chronically active BCR signalling (Davis et al, 2010). Gain-of-function mutations in the CD79B BCR subunit, as well as a constitutively active MYD88 (an adapter for Toll-like receptors) mutation are common in the ABC but not the GCB subtype, leading to the hypothesis that ibrutinib might be more active in ABC-subtype patients (Ngo et al, 2011; Wilson et al, 2012). A total of 70 patients were enrolled with a median age of 63 years (range: 28–92) and a median of 3 prior therapies (range: 1–7). 54% had refractory disease and 23% a prior stem cell transplant. All patients received a fixed 560 mg/d dose of ibrutinib. The evaluable patients with the GCB subtype did not respond well to treatment with an ORR of only 5·3% and a median overall survival (OS) of 3·35 months. In contrast, the ORR in the ABC subtype patients was 40% (8% CR) with a median OS of 9·76 months. Responses were also correlated with specific molecular subtypes. Five of 7 patients with mutated CD79B responded as well as 10 of 29 patients without the mutation, suggesting alternative mechanisms of BCR signalling. Patients with CARD11 and MYD88 mutations without CD79B mutations did not respond to treatment suggesting that perhaps CD79B-driven activation of the BCR pathway is more dominant compared to the Toll-like receptor pathway. Future studies targeting the ABC subtype are planned including a frontline trial in combination with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) (Trial Number: NCT01855750).
Conclusion
The BCR signalling pathway plays a major role in the development of B cell malignancies. Several active agents that target this pathway are being evaluated. The impressive clinical efficacy and tolerability of ibrutinib to date across multiple B cell lymphomas has paved the path for small molecule BTK inhibitors as novel agents in the management of B cell lymphomas and CLL. Several other BTK inhibitors have variable activity, however, they are relatively early in development. Earlier this year, the US Food and Drug Administration granted ibrutinib a “breakthrough therapy designation” in MCL, WM, and CLL with 17p deletion. It is likely that this agent has the potential to replace traditional chemoimmunotherapy treatments in elderly patients. Studies are also ongoing to evaluate the combination of ibrutinib with rituximab and/or chemotherapy in multiple lymphoma subtypes. A better understanding of the mechanism of resistance to ibrutinib will allow for the development of rational combinations with other inhibitors of BCR signalling. The considerable progress in understanding the link between BCR signalling and B cell malignancies will probably result in further expansion of the current therapeutic options targeting this pathway. Future challenges include how to best combine or sequence the various inhibitors of the BCR signalling pathway with each other or with chemoimmunotherapy.
Footnotes
Author contributions
Amin Aalipour and Ranjana H. Advani wrote, reviewed, and edited the manuscript.
Disclosures
Amin Aalipour declares no conflict of interests. Ranjana H. Advani has received research funding from Pharmacyclics, Inc. and Janssen Pharmaceuticals, Inc.
References
- Advani RH, Buggy JJ, Sharman JP, Smith SM, Boyd TE, Grant B, Kolibaba KS, Furman RR, Rodriguez S, Chang BY, Sukbuntherng J, Izumi R, Hamdy A, Hedrick E, Fowler NH. Bruton tyrosine kinase inhibitor ibrutinib (PCI-32765) has significant activity in patients with relapsed/refractory B-cell malignancies. Journal of Clinical Oncology. 2013;31:88–94. doi: 10.1200/JCO.2012.42.7906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amrein PC, Attar EC, Takvorian T, Hochberg EP, Ballen KK, Leahy KM, Fisher DC, LaCasce AS, Jacobsen ED, Armand P, Hasserjian RP, Werner L, Neuberg D, Brown JR. Phase II study of dasatinib in relapsed or refractory chronic lymphocytic leukemia. Clinical Cancer Research. 2011;17:2977–2986. doi: 10.1158/1078-0432.CCR-10-2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JR. Ibrutinib (PCI-32765), the first BTK (Bruton’s Tyrosine Kinase) inhibitor in clinical trials. Current Hematologic Malignancy Reports. 2012;8:1–6. doi: 10.1007/s11899-012-0147-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JR. Ibrutinib in chronic lymphocytic leukemia and B cell malignancies. Leukemia and Lymphoma. 2013 doi: 10.3109/10428194.2013.803226. [DOI] [PubMed] [Google Scholar]
- Brown JR, Sharman JP, Harb WA, Kelly KR, Schreeder MT, Sweetenham JW, Barr PM, Foran JM, Gabrilove JL, Kipps TJ, Ma S, O’Brien SM, Evans E, Lounsbury H, Silver BA, Singh J, Stiede K, Westlin W, Witowski S, Mahadevan D. Phase Ib trial of AVL-292, a covalent inhibitor of Bruton’s tyrosine kinase (Btk), in chronic lymphocytic leukemia (CLL) and B-non-Hodgkin lymphoma (B-NHL)[abstract] Journal of Clinical Oncology (ASCO meeting abstracts) 2012a;30:8032. [Google Scholar]
- Brown JR, Barrientos J, Flinn I, Barr P, Burger J, Navarro T, James D, Hedrick E, Friedberg J, Brown JR. The Bruton’s Tyrosine Kinase (BTK) inhibitor ibrutinib combined with bendamustine and rituximab is active and tolerable in patients with relapsed/refractory CLL, interim results of a phase Ib/II study [abstract] Haematologica. 2012b;97:0543. [Google Scholar]
- Brown J, Harb W, Sharman J, Hill B, Ma S, Miller T, Schreeder M, Barr P, Foran J, Gabrilove J, Kelly K, Burger J, Barnett E, Marine J, Nava-Parada P, Azaryan A, Mei J, Kipps T. Phase 1 study of single agent cc-292, a highly selective bruton’s tyrosine kinase (btk) inhibitor, in relapsed/refractory chronic lymphocytic leukemia (CLL) and B-cell non-Hodgkin lymphoma (b-NHL) [abstract] Haematologica. 2013;98:S520. doi: 10.3324/haematol.2015.140806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger JA, Buggy JJ. Emerging Drug Profiles: bruton tyroine kinase (BTK) inhibitor ibrutinib (PCI-32765) Leukemia and Lymphoma. 2013 doi: 10.3109/10428194.2013.777837. [DOI] [PubMed] [Google Scholar]
- Byrd JC, Furman RR, Coutre SE, Flinn IW, Burger JA, Blum KA, Sharman JP, Grant B, Jones JA, Wierda WG, Zhao W, Heerema NA, Johnson AJ, Tran A, Clow F, Kunkel L, James DF, O’Brien S. The Bruton’s tyrosine kinase (BTK) inhibitor ibrutinib (PCI-32765) promotes high response rate, durable remissions, and is tolerable in treatment naïve (TN) and relapsed or refractory (RR) chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL) patients including patients with high-risk (HR) disease: new and updated results of 116 patients in a phase Ib/II study [abstract] Blood (ASH Annual Meeting Abstracts) 2012a;120:189. [Google Scholar]
- Byrd JC, Furman RR, Coutre SE, Burger JA, Blum KA, Sharman JP, Flinn IW, Grant BW, Heerema NA, Johnson AJ, Navarro T, James DF, Hedrick E, O’Brien SM. The Bruton’s tyrosine kinase (BTK) inhibitor PCI-32765 (P) in treatment-naive (TN) chronic lymphocytic leukemia (CLL) patients (pts): interim results of a phase Ib/II study [abstract] Journal of Clinical Oncology (ASCO meeting abstracts) 2012b;30:6507. [Google Scholar]
- Byrd JC, Furman RR, Coutre SE, Flinn IW, Burger JA, Blum KA, Grant B, Sharman JP, Coleman M, Wierda WG, Jones JA, Zhao W, Heerema NA, Johnson AJ, Sukbuntherng J, Chang BY, Clow F, Hedrick E, Buggy JJ, James DF, O’Brien S. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. The New England Journal of Medicine. 2013;369:32–42. doi: 10.1056/NEJMoa1215637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Monti S, Juszczynski P, Daley J, Chen W, Witzig TE, Habermann TM, Kutok JL, Shipp MA. SYK-dependent tonic B-cell receptor signaling is a rational treatment target in diffuse large B-cell lymphoma. Blood. 2008;111:2230–2237. doi: 10.1182/blood-2007-07-100115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Porto JM, Gauld SB, Merrell KT, Mills D, Pugh-Bernard AE, Cambier J. B cell antigen receptor signaling 101. Molecular Immunology. 2004;41:599–613. doi: 10.1016/j.molimm.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Davids MS, Brown JR. Targeting the B cell receptor pathway in chronic lymphocytic leukemia. Leukemia and Lymphoma. 2012;53:2362–2370. doi: 10.3109/10428194.2012.695781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RE, Ngo VN, Lenz G, Tolar P, Young RM, Romesser PB, Kohlhammer H, Lamy L, Zhao H, Yang Y, Xu W, Shaffer AL, Wright G, Xiao W, Powell J, Jiang JK, Thomas CJ, Rosenwald A, Ott G, Muller-Hermelink HK, Gascoyne RD, Connors JM, Johnson NA, Rimsza LM, Campo E, Jaffe ES, Wilson WH, Delabie J, Smeland EB, Fisher RI, Braziel RM, Tubbs RR, Cook JR, Weisenburger DD, Chan WC, Pierce SK, Staudt LM. Chronic active B-cell-receptor signalling in diffuse large B-cell lymphoma. Nature. 2010;463:88–92. doi: 10.1038/nature08638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dühren-von Minden M, Übelhart R, Schneider D, Wossning T, Bach MP, Buchner M, Hofmann D, Surova E, Follo M, Köhler F, Wardemann H, Zirlik K, Veelken H, Jumaa H. Chronic lymphocytic leukaemia is driven by antigen-independent cell-autonomous signalling. Nature. 2012;489:309–312. doi: 10.1038/nature11309. [DOI] [PubMed] [Google Scholar]
- Evans E, Tester R, Aslanian S, Chaturvedi P, Mazdiyasni H, Ponader S, Tesar B, Sheets M, Nacht M, Stiede K, Witowski S, Lounsbury H, Petter R, Brown JR, Burger JA, Singh J, Westlin WF. Clinical Development of AVL-292; A Potent, Selective Covalent Btk Inhibitor for the Treatment of B Cell Malignancies [abstract] Blood (ASH Annual Meeting Abstracts) 2011;118:3485. [Google Scholar]
- Evans EK, Tester R, Aslanian S, Karp R, Sheets M, Labenski MT, Witowski SR, Lounsbury H, Chaturvedi P, Mazdiyasni H, Zhu Z, Nacht M, Freed MI, Petter RC, Dubrvskiy A, Singh J, Westlin WF. Inhibition of Btk with CC-292 Provides early pharmacodynamic assessment of activity in mice and humans. Journal of Pharmacology and Experimental Therapeutics. 2013;346:219–228. doi: 10.1124/jpet.113.203489. [DOI] [PubMed] [Google Scholar]
- Fowler NH, Advani RH, Sharman JP, Smith SM, McGreivy J, Kunkel L, Truong V, Zhou C, Boyd TE. The Bruton’s tyrosine kinase inhibitor ibrutinib (PCI-32765) is active and tolerated in relapsed follicular lymphoma [abstract] Blood (ASH Annual Meeting Abstracts) 2012;120:156. [Google Scholar]
- Hantschel O, Rix U, Schmidt U, Bürckstümmer T, Kneidinger M, Schütze G, Colinge J, Bennett KL, Ellmeier W, Valent P, Superti-Furga G. The Btk tyrosine kinase is a major target of the Bcr-Abl inhibitor dasatinib. Proceedings of the National Academy of Sciences. 2007;104:13283–13288. doi: 10.1073/pnas.0702654104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman SE, Gordon AL, Hertlein E, Ramanunni A, Zhang X, Jaglowski S, Flynn J, Jones J, Blum KA, Buggy JJ, Hamdy A, Johnson AJ, Byrd JC. Bruton tyrosine kinase represents a promising therapeutic target for treatment of chronic lymphocytic leukemia and is effectively targeted by PCI-32765. Blood. 2011;117:6287–6296. doi: 10.1182/blood-2011-01-328484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honigberg LA, Smith AM, Sirisawad M, Verner E, Loury D, Chang B, Li S, Pan Z, Thamm DH, Miller RA, Buggy JJ. The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy. Proceedings of the National Academy of Sciences. 2010;107:13075–13080. doi: 10.1073/pnas.1004594107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaglowski SM, Jones JA, Flynn JM, Andritsos LA, Maddocks KJ, Blum KA, Grever MR, Geyer SM, Woyach JA, Johnson AJ, Heerema NA, Molnar E, Stefanos M, Devlin S, Navarro T, James DF, Lowe AM, Hedrick E, Byrd JC. A phase Ib/II study evaluating activity and tolerability of BTK inhibitor PCI-32765 and ofatumumab in patients with chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) and related diseases [abstract] Journal of Clinical Oncology (ASCO Annual Meeting Abstracts) 2012;30:6508. [Google Scholar]
- Kozaki R, Yoshizawa T, Tohda S, Yasuhiro T, Hotta S, Ariza Y, Ueda Y, Narita M, Kawabata K. Development of a Bruton’s tyrosine kinase (Btk) Inhibitor, ONO-WG-307: efficacy in ABC-DLBCL xenograft model – potential treatment for B-cell malignancies [abstract] Blood (ASH Annual Meeting Abstracts) 2011;118:3731. [Google Scholar]
- Kozaki R, Yoshizawa T, Yashuhiro T, Mirjolet J, Birkett J, Narita M, Kawabata K. Development of a Bruton’s tyrosine kinase (Btk) inhibitor - ONO-WG-307, a potential treatment for B-cell malignancies [abstract] Cancer Research. 2012;72:857. [Google Scholar]
- Maas A, Hendriks RW. Role of Bruton’s tyrosine kinase in B cell development. Developmental Immunology. 2001;8:171–181. doi: 10.1155/2001/28962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahajan S, Ghosh S, Sudbeck EA, Zheng Y, Downs S, Hupke M, Uckun FM. Rational design and synthesis of a novel antileukemic agent targeting Bruton’s tyrosine kinase (BTK), LFM-A13 [alpha-cyano-betahydroxy-beta-methyl-N-(2, 5-dibromophenyl) propenamide] The Journal of Biological Chemistry. 1999;274:9587–9599. doi: 10.1074/jbc.274.14.9587. [DOI] [PubMed] [Google Scholar]
- Monroe JG. ITAM-mediated tonic signaling through pre-BCR and BCR complexes. Nature Reviews Immunology. 2006;6:283–294. doi: 10.1038/nri1808. [DOI] [PubMed] [Google Scholar]
- Ngo VN, Young RM, Schmitz R, Jhavar S, Xiao W, Lim KH, Kohlhammer H, Xu W, Yang Y, Zhao H, Shaffer AL, Romesser P, Wright G, Powell J, Rosenwald A, Muller-Hermelink HK, Ott G, Gascoyne RD, Connors JM, Rimsza LM, Campo E, Jaffe ES, Delabie J, Smeland EB, Fisher RI, Braziel RM, Tubbs RR, Cook JR, Weisenburger DD, Chan WC, Staudt LM. Oncogenically active MYD88 mutations in human lymphoma. Nature. 2011;470:115–119. doi: 10.1038/nature09671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollyea DA, Smith S, Fowler N, Boyd TE, Smith AM, Sirisawad M, Honigberg LA, Hamdy A, Advani RH. A phase I dose escalation study of the BtkInhibitor PCI-32765 in relapsed and refractory B cell non-Hodgkin Lymphoma and use of a novel fluorescent probe pharmacodynamic assay [abstract] Blood. 2009;114:3713. [Google Scholar]
- Ponader S, Chen SS, Buggy JJ, Balakrishnan K, Gandhi V, Wierda WG, Keating MJ, O’Brien S, Chiorazzi N, Burger JA. Bruton’s tyrosine kinase inhibitor PCI-32765 thwarts chronic lymphocytic leukemia cell survival and tissue homing in vitro and in vivo. Blood. 2012;119:1182–1189. doi: 10.1182/blood-2011-10-386417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaldi A, Kwee I, Taborelli M, Largo C, Uccella S, Martin V, Poretti G, Gaidano G, Calabrese G, Martinelli G, Baldini L, Pruneri G, Capella C, Zucca E, Cotter FE, Cigudosa JC, Catapano CV, Tibiletti MG, Bertoni F. Genomic and expression profiling identifies the B-cell associated tyrosine kinase Syk as a possible therapeutic target in mantle cell lymphoma. British Journal of Haematology. 2006;132:303–316. doi: 10.1111/j.1365-2141.2005.05883.x. [DOI] [PubMed] [Google Scholar]
- Robak T, Robak P. BCR signaling in chronic lymphocytic leukemia and related inhibitors currently in clinical studies. International Reviews of Immunology. 2013;32:358–376. doi: 10.3109/08830185.2013.786711. [DOI] [PubMed] [Google Scholar]
- de Rooij MF, Kuil A, Geest CR, Eldering E, Chang BY, Buggy JJ, Pals ST, Spaargaren M. The clinical active BTK inhibitor PCI-32765 targets B-cell receptor- and chemokine-controlled adhesion and migration in chronic lymphocytic leukemia. Blood. 2012;119:2590–2594. doi: 10.1182/blood-2011-11-390989. [DOI] [PubMed] [Google Scholar]
- Steinberg M. Dasatinib: a tyrosine kinase inhibitor for the treatment of chronic myelogenous leukemia and philadelphia chromosome-positive acute lymphoblastic leukemia. Clinical Therapeutics. 2007;29:2289–2308. doi: 10.1016/j.clinthera.2007.11.005. [DOI] [PubMed] [Google Scholar]
- Uckun FM, Zheng Y, Cetkovic-Cvrlje M, Vassilev A, Lisowski E, Waurzyniak B, Chen H, Carpenter R, Chen CL. In vivo pharmacokinetic features, toxicity profile, and chemosensitizing activity of alpha-cyano-beta-hydroxy-beta- methyl-N-(2,5-dibromophenyl) propenamide (LFM-A13), a novel antileukemic agent targeting Bruton’s tyrosine kinase. Clinical Cancer Research. 2002;8:1224–1233. [PubMed] [Google Scholar]
- Uckun F, Dibirdik I, Sarkissian A, Qazi S. In vitro and in vivo chemosensitizing activity of LFM-A13, a dual-function inhibitor of Bruton’s tyrosine kinase and polo-like kinases, against human leukemic B-cell precursors. Arzneimittel-Forschung. 2011;61:252–259. doi: 10.1055/s-0031-1296196. [DOI] [PubMed] [Google Scholar]
- Wang ML, Rule S, Martin P, Goy A, Auer R, Kahl BS, Jurczak W, Advani RH, Williams ME, Barrientos JC, Chmielowska E, Radford J, Stilgenbauer S, Dreyling M, Jedrzejczak WW, Johnson P, Spurgeon SE, Li L, Zhang L, Newberry K, Ou Z, Cheng N, Fang B, McGreivy J, Clow F, Buggy JJ, Chang BY, Beaupre DM, Kunkel LA, Blum KA. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. The New England Journal of Medicine. 2013;369:507–516. doi: 10.1056/NEJMoa1306220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiestner A. Emerging role of kinase-targeted strategies in chronic lymphocytic leukemia. Blood. 2012;120:4684–4691. doi: 10.1182/blood-2012-05-423194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiestner A. Targeting B-cell receptor signaling for anticancer therapy: the Bruton’s tyrosine kinase inhibitor ibrutinib induces impressive responses in B-cell malignancies. Journal of Clinical Oncology. 2013;31:128–130. doi: 10.1200/JCO.2012.44.4281. [DOI] [PubMed] [Google Scholar]
- William BM, Hohenstein M, Loberiza FR, Jr, Caponetti GC, Bociek RG, Bierman P, Armitage JO, Chan W, Vose JM. Phase I/II study of dasatinib in relapsed or refractory non-Hodgkin’s lymphoma (NHL) [abstract] Blood (ASH Annual Meeting Abstracts) 2010;116:288. [Google Scholar]
- Wilson WH, Gerecitano JF, Goy A, de Vos S, Kenkre VP, Barr PM, Blum KA, Shustov AR, Advani RH, Lih J, Williams M, Schmitz R, Yang Y, Pittaluga S, Wright G, Kunkel LA, McGrievy J, Balasubramanian S, Cheng M, Moussa D, Buggy JJ, Staudt LM. The Bruton’s tyrosine kinase (BTK) inhibitor, ibrutinib (PCI-32765), has preferential activity in the ABC subtype of relapsed/refractory de novo diffuse large B-cell lymphoma (DLBCL): interim results of a multicenter, open-label, phase 2 study [abstract] Blood (ASH Annual Meeting Abstracts) 2012;120:686. [Google Scholar]
- Yasuhiro T, Yoshizawa T, Daub H, Weber C, Narita M, Kawabata K. ONO-WG-307, a novel, potent and selective inhibitor of Bruton’s tyrosine kinase (Btk), results in sustained inhibition of the ERK, AKT and PKD signaling pathways [abstract] Cancer Research. 2012;72:2021. [Google Scholar]