Abstract
Purpose
This study assessed the trial of boost intracavitary brachytherapy (ICBT) for the patients of uterine cervical cancer with residual malignant cells detected at the final stage of ICBT.
Material and methods
This is a retrospective analysis of 75 patients with cervical cancer treated radically with external beam radiotherapy and high-dose-rate intracavitary brachytherapy between August 2004 and December 2008. Eighteen patients (24%) out of 75 received one additional ICBT and five patients (7%) had two additional ICBT. All 75 patients were retrospectively analyzed. The median follow-up time was 30 months. The median age was 59 years (range 28-85 years). There were 12 patients (16%) in stage IB, 27 (36%) stage II, 22 (29%) stage III, and 14 (19%) stage IV.
Results
The 5-year overall survival rate was 65%. Non-hematological toxicities greater than grade 2 occurred in 12 patients (16%). Of these, only two patients received on additional ICBT. No significant difference was found in grade 3 toxicity between patients who did and did not receive additional ICBT (p = 0.8).
Conclusions
The method to perform dose escalation should be examined depending on the treatment response.
Keywords: cervical cancer, high-dose-rate brachytherapy, radiation therapy, toxicity
Purpose
Cervical cancer is one of the malignancies showing an increasing incidence over the past 20 years in Japan, with an estimated 15 new cases per 100,000 females every year. Though radical surgery was the usually adopted treatment in Japan, radiotherapy with or without chemotherapy began to be selected for many patients with uterine cervical cancer. Notable differences have been observed between American and Japanese radiotherapeutic techniques. At most institutions in Japan, a midline block was inserted at the latter half of the pelvic external radiation treatment. The total biologically effective dose (BED) delivered to the primary lesion in Japan was approximately 80% of that in the United States (85.5 Gy in Japan vs. 103 Gy in the United States) [1]. Meanwhile, some randomized clinical trials reported a significant improvement in pelvic disease control and survival when concurrent chemotherapy consisting of cisplatin (CDDP)-containing regimens was added to radiotherapy (RT) in treating patients with locally advanced cervical cancer [2–4].
In our institution, the patients with residual malignant cells detected at the final stage of intracavitary brachytherapy (ICBT) were treated with boost ICBT. The results of this selective boost ICBT for cervical cancer in our department were assessed in this retrospective study.
Material and methods
Patients
Patients with cervical cancer treated with primary radical radiotherapy with or without chemotherapy at the University of Tokyo Hospital between August 2004 and December 2008 were evaluated. A total of 75 patients in the International Federation of Gynaecology and Obsterics (FIGO) stages IB1 (n = 10), IB2 (n = 2), IIA (n = 8), IIB (n = 19), IIIA (n = 3), IIIB (n = 19), IVA (n = 2), IVB (n = 12) were treated. Patients with para-aortic lymph node swelling (n = 12) were eligible. Patients treated palliatively and patients treated post-operatively were excluded. Patients who underwent external beam radiation therapy (EBRT) at other institutions were not excluded. Median age was 59 years (range 28-85 years). Seventeen patients (23%) were treated with radiation therapy (RT) alone and 58 patients (77%) were treated with concurrent chemoradiation therapy (cCRT). Table 1 shows the patients’ characteristics.
Table 1.
Patient characteristics
| Characteristics | n (%) |
|---|---|
| Age (y) | |
| Median | 59 |
| Range | 28-85 |
| FIGO Stage | |
| IB1 | 10 (13) |
| IB2 | 2 (3) |
| IIA | 8 (11) |
| IIB | 19 (25) |
| IIIA | 3 (4) |
| IIIB | 19 (25) |
| IVA | 2 (3) |
| IVB | 12 (16) |
| N stage | |
| N0 | 51 (68) |
| N1 | 24 (32) |
| M stage | |
| M0 | 63 (84) |
| M1 (paraaortic lymph node) | 12 (16) |
| Pathological type | |
| Squamous cell carcinoma | 65 (87) |
| Adenocarcinoma | 4 (5) |
| Adenosquamous cell carcinoma | 6 (8) |
| Treatment | |
| Radiation therapy – alone | 17 (23) |
| Chemoradiotherapy | 58 (77) |
Patients were evaluated with a physical and pelvic examination without anesthesia, routine blood counts, blood chemistry profile, chest radiograph, intravenous urogram, proctoscopy, and cystoscopy. Computed tomography (CT) scan, magnetic resonance imaging (MRI), and positron emission tomography (PET) were used for detecting lymphadenopathy and distant metastases. Neither lymphangiography nor surgical evaluations of lymph nodes were performed. All patients were previously untreated and had a histological diagnosis.
External-beam radiation therapy
All patients underwent radical radiotherapy as primary treatment for cervical cancer with intent to cure. External-beam radiation therapy (EBRT) of whole pelvic irradiation ranged from 30.6 Gy for non-bulky stages I or II to 41.4 Gy for bulky stages I or II, and over stage II. These regimens were applied in 17 to 22 fractions and whole pelvic irradiation with a midline block from 9.0 Gy to 19.8 Gy in 6 to 11 fractions (1.8 Gy fractions from Monday to Friday). Whole pelvic irradiation was administered using a linear accelerator with four fields of a 10 MV photon beam (two anterior-posterior opposed fields, and two lateral opposed fields). Whole pelvic irradiation with a 4 cm wide midline block was administered using two anterior-posterior opposed fields to decrease the dose to the rectum and the bladder. The midline block did not extend to the top of the pelvic field.
All patients undergoing three-dimensional (3D) radiation treatment planning were positioned on the couch of the CT simulator in a supine position. The gross tumor volume (GTV) consisted of the primary cervical mass of the uterus and metastatic lymph nodes if present. The clinical target volume (CTV) included all areas of gross and potentially microscopic disease, and included the upper half of the vagina, parametria, uterus, presacral region, and regional lymph node regions (common, internal and external iliacs, para-aortic, obturator regions). The CTV in the para-aortic region was contiguous with the pelvic lymph node stations and generously encompassed the aorta and the inferior vena cava with at least a 1.5-cm margin. The upper border was individualized depending on the patients’ known extent of the disease. The superior field border was usually a bifurcation of the common iliac artery and the inferior border was usually a tuber of the ischium. The lateral border was about 1.5 cm lateral to the bony pelvis. For the lateral fields, the anterior margin was the anterior edge of the symphysis or 3 cm in front of the sacral promontory. The posterior margin was the S2–S3 interspace, or the posterior border of the uterine cervix assessed by CT for treatment planning plus a 2 cm margin.
Intracavitary brachytherapy
Three to four weeks after starting EBRT, Iridium-192 insertions were performed 4 times weekly or twice-weekly. External-beam radiation therapy was given on the same day as ICBT. High-dose-rate intracavitary therapy was delivered by placing after-loading applicators (tandem & two ovoids) in the uterine cavity and vagina. A Manchester system applicator (Nucletron, an Elekta company, Elekta AB, Stockholm, Sweden) was used. The dose distribution was calculated with the CT planning system (Plato planning system®, Nucletron, an Elekta company, Elekta AB, Stockholm, Sweden) for each individual patient and placement. Applications were usually done under intravenous anesthesia with midazoram under O2 saturation monitoring. Anterior and posterior vaginal packing was used in an attempt to displace the bladder and rectum from the applicators. An external fixation device was used to prevent applicator movement. High-dose-rate ICBT was performed with a dose of 6 Gy at point A. Doses in ICRU points in rectum and bladder were within 70% and 80% of the prescription dose at point A.
For each case of ICBT, pelvic examination was performed with biopsy of the external os of uterus at the third and fourth applications of ICBT. Biopsies were obtained at a depth of within 5 mm at the external os because of the shape of the device for biopsy. If residual malignant cells were detected, these patients were treated with additional ICBT dose of 4.5 Gy at the uterine cervix, which was detected by the CT. Although additional ICBT and biopsy were performed with tandem and ovoids, a cylindrical type applicator was used for additional ICBT in case there was a sufficient distance between rectum, bladder, and the uterine cervix. If the judgment on the presence of residual malignant cells was not clear, biopsy at the cervical os was repeated. The treatment was concluded after confirming the absence of residual malignant cells in two consecutive biopsies.
Chemotherapy
All of the 64 patients treated with chemotherapy and concurrent platinum-based chemotherapies were combined with RT. In the cases of early stage (FIGO stages IA & IB1), advanced age, poor renal or heart function, or in patients declining chemotherapy, the treatment was with RT – alone. Thirty six patients (56%) received tri-weekly CDDP or nedaplatin (75-80 mg/m2 in a bolus infusion on days 1, 22 and 43). This was performed mainly between August 2004 and March 2006. Sixteen patients (25%) received weekly CDDP or nedaplatin (30-40 mg/m2 in a bolus infusion) after April 2006. Other patients who were treated only with ICBT at our institution received 5FU and CDDP (n = 1), paclitaxel and carboplatin (n = 1), and CDDP by arterial infusion and weekly 5FU/CDDP (n = 1). About these patients, the types of chemotherapy were decided by the gynecologist at their mainly treatment institutions. Chemotherapy regimens were not specified for two patients (3%) who were treated only with ICBT at our institution.
Follow-up
Both radiation and gynecological oncologists followed up the treated patients. The patients were seen every 1-3 months for the first year, every 3-4 months for the next 2 years, and 3-12 months thereafter. Patients without regular visits were followed-up via telephone. No patients were lost to follow-up.
Follow-up procedures, included pelvic examination, palpation of supraclavicular nodes, cervical Papanicolaou smear, and review of serum tumor marker values such as squamous cell carcinoma antigen, carcinoembryonic antigen, and p53. When central and/or parametrial recurrence was suspected by pelvic examination and/or Papanicolaou smear, a biopsy was taken for confirmation. Intravenously enhanced chest, abdominal and pelvic CTs were performed at least every 6 months. Other imaging studies, such as MRI, ultrasound bone scintigraphy and PET were not routinely performed.
Late complications were graded in accordance with the National Cancer Institute Common Toxicity Criteria (NCI-CTC) version 3.0, especially for bladder and bowel complications.
Statistical analysis
Statistical analyses were performed using StatView Dataset File version 5.0J for Windows computers. Overall survival (OS) was calculated from the first date of EBRT. Survival time was plotted using the Kaplan-Meier method. Differences in the incidences of toxicities were analyzed by the chi-square test. Differences in survival by treatment were evaluated using the log-rank test. The proportional hazards model was used in univariate and multivariate analyses.
Results
Median follow-up time for all 75 patients was 30 months (range 3.1-72.9 months). No patients were lost to follow-up. All patients had a Karnovsky performance status of at least 80%. Most patients (n = 65, 87%) were diagnosed with squamous cell carcinoma (SqCC). Four patients (5%) had adenocarcinoma (AC) and six patients (8%) had adenosquamous cell carcinoma (ASC). All patients completed RT treatment.
Survival
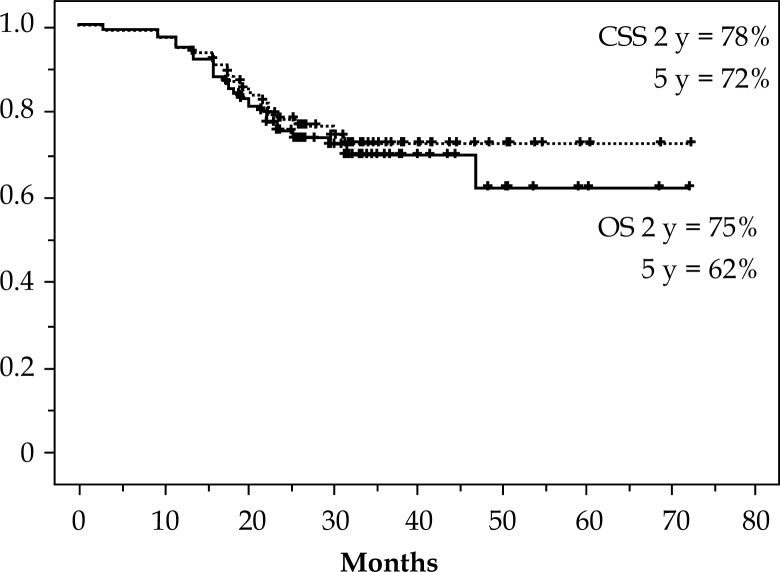
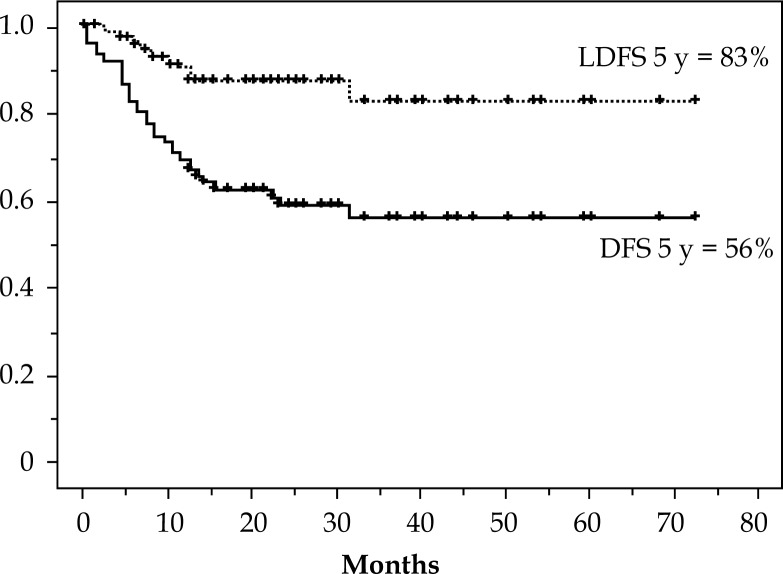
When this analysis was closed, 53 patients out of 75 were alive. Twenty patients died from recurrent disease. Two patients (stage IIB and IIIB) died from coincidental disease. No patient had a treatment-related death. Figure 1 shows outcomes of OS and cause specific survival (CSS). The 2 and 5-year OS rates were 75% and 62%, respectively. For CSS, the 2- and 5-year rates were 78% and 72%, respectively. Figure 2 shows the 5-year outcomes of 56% and 83% for the disease free survival (DFS) and local disease free survival (LDFS), respectively.
Fig. 1.
Overall survival (OS) and cause specific survival (CSS) curves
Fig. 2.
Disease free survival (DFS) and local disease free survival (LDFS) curves
Boost intracavitary brachytherapy
Table 2 shows the relationship between additional ICBT treatments and FIGO stages. Eighteen patients (24%) out of 75 received one additional ICBT and five patients (7%) had two additional ICBT. The differences in the number of additional ICBT performed among FIGO stages were not significant (p = 0.6). Among the patients treated with additional ICBT, the differences in factors such as: (cCRT vs. RT alone, FIGO stage I/II vs. III/IV, hemoglobin < 12 mg/ml vs. > 12 mg/ml, age < 60 years vs. > 60 years, SqCC vs. AC vs. ASC), were also not significant (Table 3). Thus, the clinical characteristics listed above of cervical tumors showed no correlation with the additional ICBT given in this study.
Table 2.
Number of additional intracavitary brachytherapy (ICBT) each FIGO stage
| Additional ICRT | 0 | 1 | 2 | Total (%) |
|---|---|---|---|---|
| Stage | ||||
| I | 9 | 3 | 0 | 12 (16) |
| II | 19 | 6 | 2 | 27 (36) |
| III | 15 | 4 | 3 | 22 (29) |
| IV | 9 | 5 | 0 | 14 (19) |
| Total (%) | 52 (69) | 18 (24) | 5 (7) | 75 (100) |
χ2 test; p = 0.6
Table 3.
Percentage of performing additional intracavitary radiotherapy (ICRT)
| Characteristics | Additional ICRT | p value |
|---|---|---|
| Treatment | ||
| Chemoradiotherapy | 33% (19/58) | 0.47 |
| Radiation therapy – alone | 24% (4/17) | |
| FIGO Stage | ||
| I/II | 28% (11/39) | 0.63 |
| III/IV | 33% (12/36) | |
| Hb | ||
| < 12 | 44% (14/32) | 0.1 |
| ≥ 12 | 24% (8/33) | |
| Age | ||
| < 60 | 30% (12/40) | 0.89 |
| ≥ 60 | 31% (11/35) | |
| Pathological type | ||
| Squamous cell carcinoma | 29% (19/65) | 0.67 |
| Adenocarcinoma | 50% (2/4) | |
| Adenosquamous cell carcinoma | 33% (2/6) | |
Late complications
Late complications were defined as complications that persisted or occurred more than 60 days after treatment. Non-hematological toxicities greater than grade 2 occurred in 12 patients (16%), and were comprised of the following: intestinal obstruction (n = 1, Grade 3), bloody stool (n = 7, Grade 3), cystitis (n = 1, Grade 4), small-intestinal perforation (n = 3, Grade 4) (Table 4). Of these, only two patients received one additional ICBT (one grade 3 intestinal obstruction and one grade 3 bloody stool) (Table 5). One patient received five ICBT treatments because of local recurrence. The difference in the additional ICBT between > grade 3 late toxicity was not significant (p = 0.8).
Table 4.
Non-hematological toxicity
| Grade | 1 | 2 | 3 | 4 | Total (%) |
|---|---|---|---|---|---|
| Intestinal obstruction | 0 | 2 | 1 | 0 | 3 (4) |
| Bloody stool | 3 | 3 | 7 | 0 | 13 (17) |
| Cystitis | 1 | 0 | 0 | 1 | 2 (3) |
| Small-intestinal perforation | – | – | – | 3 | 3 (4) |
| Total (%) | 4 (5) | 5 (7) | 8 (11) | 5 (7) | 22 (29) |
Table 5.
Details of severe (> grade 2) complications
| No. | Toxicity | Grade | Onset (months) | No. of ICRT | Risk |
|---|---|---|---|---|---|
| 1 | Intestinal obstruction | 3 | 4 | 4 | None |
| 2 | Bloody stool | 3 | 8 | 4 | Steroid for rheumatoid arthritis |
| 3 | Bloody stool | 3 | 59 | 4 | None |
| 4 | Bloody stool | 3 | 8 | 4 | None |
| 5 | Bloody stool | 3 | 10 | 4 | None |
| 6 | Bloody stool | 3 | 9 | 5 | None |
| 7 | Bloody stool | 3 | 14 | 4 + α | Re-ICBT for local recurrence |
| 8 | Bloody stool | 3 | 10 | 4 | None |
| 9 | Small-intestinal perforation | 4 | 7 | 5 | FEC regimen for breast cancer |
| 10 | Small-intestinal perforation | 4 | 16 | 4 | Diabetes mellitus (type 1) |
| 11 | Small-intestinal perforation | 4 | 9 | 4 | Severe constipation |
| 12 | Cystitis | 4 | 20 | 4 | Severe constipation |
FEC regimen – 5-fluorouracil/epirubicin/cyclophosphamide
Discussion
In 1997, a randomized controlled trial demonstrated no difference in the survival rates between definitive radiotherapy and surgery for cervical cancer patients in early stages of IB and IIA [5]. Therefore, radiation therapy may have been considered one of the good options in the treatment of cervical cancer. In the USA, the American Brachytherapy Society issued radiotherapy guidelines for uterine cervical cancer [6, 7], the GEC-ESTRO working group recommended 3D dose-volume parameters for brachytherapy [8, 9], and in Japan, the General Rules for Clinical and Pathological Study of Uterine Cervical Cancer provided treatment guidelines, including the standard treatment schedule of radiotherapy [10]. Since the Japanese have a smaller physique than Westerners, a local irradiated dose is lower in Japan than in the USA. The total biologically effective dose delivered to the primary lesion in Japan was reported to be approximately 80% of that in USA (85.5 Gy and 103 Gy, respectively) [1].
However, our treatment planning centered on increasing radiation dose with the aim of improving local control rate and survival rate. Therefore, ICBT was added only for cases, in which a poor early treatment reaction was revealed by biopsy when the treatment was concluded in our institution. The maximum consisted of performing two additional ICBT treatments. Nevertheless, when the central block was inserted after 40 Gy, BED (a/b = 10 Gy) was 98.7 Gy (EQD2 = 82.9 Gy), which was not a very high dose when compared with procedures in the USA.
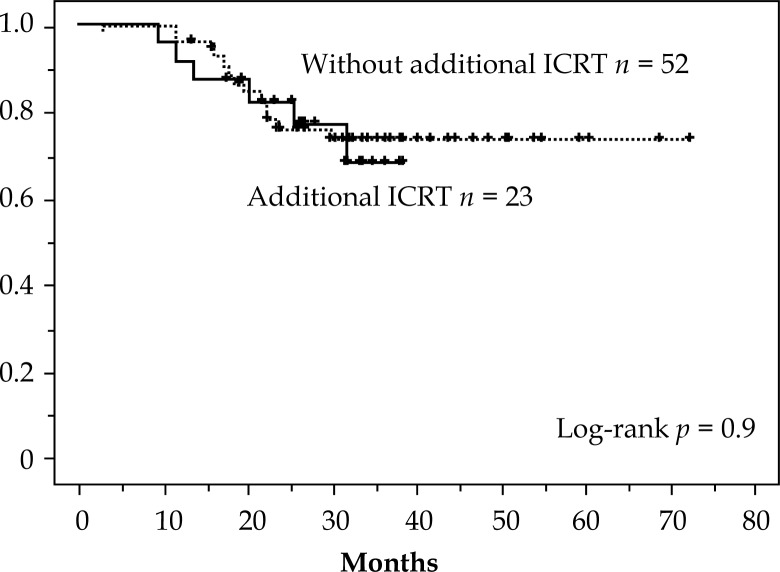
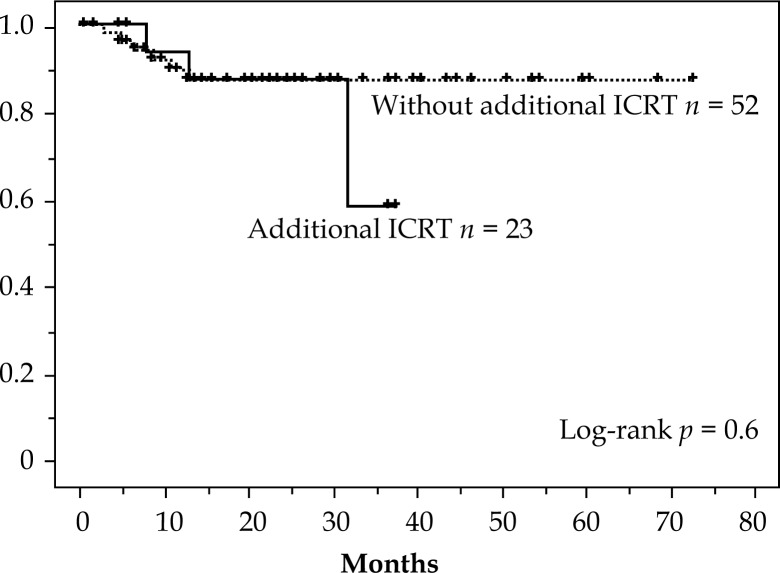
The 3 year OS for each of the four stages showed the following results: I, 100%; II, 73%; III, 57%, and IV, 59%. Considering that half of the cases were in stages IIB & IIIB and the small number of patients in the other stages, the outcome was encouraging. Notably, for 12 cases in stage IVB with para-aortic lymph node metastases, OS at 3 years was 59% and compared favorably with other previous reports (Table 6). Local disease-free survival (LDFS) showed an effective result of 83% at 5 years (Figure 2). There was no difference in CSS (p = 0.9) and in LDFS (p = 0.6) in treating with or without additional intracavitary radiotherapy (ICRT) (Figs. 3 and 4). This result suggests that additional ICRT treatment did not exert a deleterious effect on prognosis. It is also clear that additional ICRT should not be performed for all patients.
Table 6.
Survival outcomes due to stage
| Current study | Foundation for promotion of cancer research in Japan 2005 | Guideline of radiation therapy in Japan 2008 | The committee of gynecologic oncology of Japan 2004 | |
|---|---|---|---|---|
| 3y OS | 3y OS | 5y OS | 5y OS | |
| All stage | 69% | 81% | ||
| Stage I | 100% | 90% | 80-90% | 82.3% |
| Stage II | 73% | 83% | 60-80% | 61.4% |
| Stage III | 57% | 65% | 40-60% | 37.6% |
| Stage IV | 59% | 36% | (stage IVA) 10-40% | 12.6% |
OS – overall survival
Fig. 3.
Cause specific survival: with and without additional intracavitary radiotherapy (ICRT)
Fig. 4.
Local disease free survival: with and without additional intracavitary radiotherapy (ICRT)
In this study, boost ICBT was planned by using CT scan and was performed for the uterine cervix. As the GEC-ESTRO recommendations of 2005 [8] described the concepts of GTV, and CTV for brachytherapy, magnetic resonance imaging (MRI) will decide the residual-GTV for boost ICBT on pathological residual tumors. However, not all cases with residual malignant tumors will be detected by MRI, especially in those with a small number of residual tumor cells. It is recommended that the combination of image-based therapy and pathological diagnosis be used.
The GEC-ESTRO recommendations of 2006 [9] reported the data set for 3D image-based cervical cancer brachytherapy for use as a minimum requirement to determine which parameters provide clinically useful information for boost ICBT. On the other hand, it was reported that interstitial techniques used in addition to ICBT for advanced uterine cervix improved cancer specific survival (CSS) and OS [11]. At the three-year point of our study, OS showed good outcomes. That study reported 74% for IB, 79% for IIB, 45% for IIIB, while in our study the results were 100% for stage I, 73% for stage II, 57% for stage III, and 59% for stage IV. Both studies tried the dose up for local tumors, and that resulted in a better outcome. However, it suggests the local dose up will be able to improve the treatment of advanced cervical carcinoma, this boost treatment has a limited ability to reach the residual tumor extension in cases with asymmetric topography and/or unfavorable topography of the organs at risk. Currently accepted optimal or gold-standard method is based on MRI guided optimization of application technique and dose distribution and typically includes insertion and loading of interstitial parametrial needles [12–14].
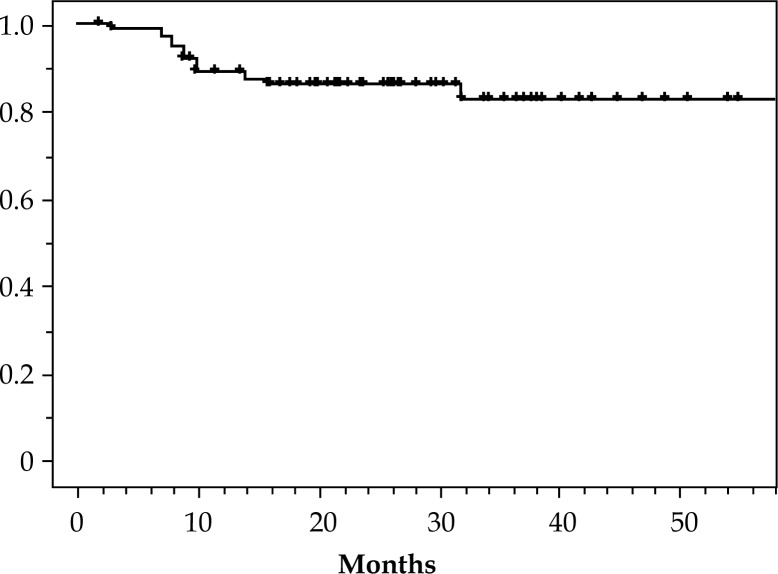
The accumulated incidence of complications at 3 years for ≥ grade 3 was 18% (Fig. 5). Additionally, no difference was seen in the complication incidences in those patients with and without additional ICRT (p = 0.8). The decision on performing additional treatment was made according to the biopsy result at the end of ICRT treatment. Although residual tumor cells may have been recognized in the biopsy, it is also possible that they would have succumbed to the radiation effect without additional treatment. In such a scenario, additional treatment would have constituted an “over treatment” for such cases. Moreover, if additional ICRT had resulted in good results for the cases with poor prognosis, the lack of differences in CSS and LDFS when treating with or without additional ICRT was not a disappointment. Of course the rate of response by the tumor might indicate the need for higher dosage.
Fig. 5.
Overall rate without non-hematological complications ≥ grade 2
The reason for the good three year OS for FIGO stage IVB cases in this study as well as the overall good DFS, LDFS, and CSS, is not clear. It might have been due to the aggressive treatment for advanced stage of cervical carcinoma. The standard therapy for FIGO stage IVB is chemotherapy. In this study, all detected tumors were included the field of radiotherapy. This recalls the fact that EBRT with midline block was often used previously in Japan, and that radiotherapy protocol had shown favorable results [15].
We acknowledge several limitations. For example, case number was low, resulting statistical insufficiencies and the CT based technique, utilizing the point A prescription approach, which is somehow outdated in the modern state-of-the-art ICRT approaches.
Conclusions
Additional CT based ICRT not using MRI guided was performed for cases, in which early local treatment reaction was presumed inadequate. Although the local control rate was encouraging, the results for OS and CSS were not significant to support the decision for additional biopsy at the end of treatment. However, the method to perform dose escalation should be examined depending on the treatment response. This boost ICRT was not the currently recommended gold standard. Several problems raised here will be studied in a future prospective study.
Disclosure
Authors report no conflict of interest.
References
- 1.Toita T, Kodaira T, Shinoda A, et al. Patterns of radiotherapy practice for patients with cervical cancer (1999-2001): patterns of case study in Japan. Int J Radiat Oncol Biol Phys. 2012;83:1506–1513. [Google Scholar]
- 2.Rose PG, Bundy BN, Watkins J, et al. Concurrent cisplatin based chemotherapy and radiotherapy for locally advanced cervical cancer. N Engl J Med. 1999;340:1144–1153. doi: 10.1056/NEJM199904153401502. [DOI] [PubMed] [Google Scholar]
- 3.Whitney CW, Sause W, Bundy BN, et al. A randomized comparison of fluorouracil plus cisplatin versus hydroxyurea as an adjunct to radiation therapy in stages IIB-IVA carcinoma of the cervix with negative para-aortic lymph nodes: a Gynecologic Oncology Group and Southwest Oncology Group study. J Clin Oncol. 1999;17:1339–1348. doi: 10.1200/JCO.1999.17.5.1339. [DOI] [PubMed] [Google Scholar]
- 4.Morris M, Eifel PJ, Lu J, et al. Pelvic radiation with concurrent chemotherapy compared with pelvic and paraaortic radiation for high-risk cervical cancer. N Engl J Med. 1999;340:1137–1143. doi: 10.1056/NEJM199904153401501. [DOI] [PubMed] [Google Scholar]
- 5.Landoni F, Maneo A, Colombo A, et al. Randomized study of radical surgery versus radiotherapy for stage Ib-IIa cervical cancer. Lancet. 1997;350:535–540. doi: 10.1016/S0140-6736(97)02250-2. [DOI] [PubMed] [Google Scholar]
- 6.Nag S, Erickson B, Thomadsen B, et al. The American Brachytherapy Society recommendations for high-dose-rate brachytherapy for carcinoma of the cervix. Int J Radiat Oncol Biol Phys. 2000;48:201–211. doi: 10.1016/s0360-3016(00)00497-1. [DOI] [PubMed] [Google Scholar]
- 7.Nag S, Chao C, Erickson B, et al. The American Brachytherapy Society recommendations for low-dose-rate brachytherapy for carcinoma of the cervix. Int J Radiat Oncol Biol Phys. 2002;52:33–48. doi: 10.1016/s0360-3016(01)01755-2. [DOI] [PubMed] [Google Scholar]
- 8.Haie-Meder C, Pötter R, Van Limbergen E, et al. Recommendations from Gynaecological (GYN) GEC-ESTRO Working Group (I): concepts and terms in 3D image based 3D treatment planning in cervix cancer brachytherapy with emphasis on MRI assessment of GTV and CTV. Gynaecological (GYN) GEC-ESTRO Working Group. Radiother Oncol. 2005;74:235–245. doi: 10.1016/j.radonc.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 9.Pötter R, Haie-Meder C, Van Limbergen E, GEC ESTRO Working Group Recommendations from gynaecological (GYN) GEC ESTRO working group (II): concepts and terms in 3D image-based treatment planning in cervix cancer brachytherapy-3D dose volume parameters and aspects of 3D image-based anatomy, radiation physics, radiobiology. Radiother Oncol. 2006;78:67–77. doi: 10.1016/j.radonc.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 10.Kinbara Shuppan. Tokyo: 1997. The general rules for clinical and pathological management of uterine cervical cancer; pp. 14–17. [Google Scholar]
- 11.Pötter R, Georg P, Dimopoulos J, et al. Clinical outcome of protocol based image (MRI) guided adaptive brachytherapy combined with 3D conformal radiotherapy with or without chemotherapy in patients with locally advanced cervical cancer. Radiother Oncol. 2011;100:116–123. doi: 10.1016/j.radonc.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dimopoulos JC, Kirisits C, Petric P, et al. The Vienna applicator for combined intracavitary and interstitial brachytherapy of cervical cancer: clinical feasibility and preliminary results. Int J Radiat Oncol Biol Phys. 2006;66:83–90. doi: 10.1016/j.ijrobp.2006.04.041. [DOI] [PubMed] [Google Scholar]
- 13.Pötter R, Dimopoulos J, Georg P, et al. Clinical impact of MRI assisted dose volume adaptation and dose escalation in brachytherapy of locally advanced cervix cancer. Radiother Oncol. 2007;83:148–155. doi: 10.1016/j.radonc.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 14.Pötter R, Georg P, Dimopoulos JC, et al. Clinical outcome of protocol based image (MRI) guided adaptive brachytherapy combined with 3D conformal radiotherapy with or without chemotherapy in patients with locally advanced cervical cancer. Radiother Oncol. 2011;100:116–123. doi: 10.1016/j.radonc.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Toita T, Kitagawa R, Hamano T, et al. Feasibility and acute toxicity of Concurrent Chemoradiotherapy (CCRT) with high-dose rate intracavitary brachytherapy (HDR-ICBT) and 40-mg/m2 weekly cisplatin for Japanese patients with cervical cancer: results of a Multi-Institutional Phase 2 Study (JGOG1066) Int J Gynecol Cancer. 2012;22:1420–1426. doi: 10.1097/IGC.0b013e3182647265. [DOI] [PubMed] [Google Scholar]