Abstract
Thyroid antibodies are frequently observed in urticaria patients, but their roles in urticaria are not clearly elucidated. We investigated the role of serum specific IgE to thyroid peroxidase (TPO) in patients with aspirin intolerant acute urticaria (AIAU) and aspirin intolerant chronic urticaria (AICU). We recruited 59 AIAU and 96 AICU patients with 69 normal controls (NC). Serum specific IgE to TPO was measured by manual direct ELISA, and CD203c expressions on basophil with additions of TPO were measured to prove a direct role of TPO in effector cells. The prevalences of serum specific IgE to TPO were significantly higher in AIAU (15.2%) and AICU groups (7.5%) compared to NC (0%, P=0.018: P=0.013, respectively). Flow cytometry showed CD203c induction in a dose dependent manner with serial additions of TPO in some AIAU and AICU patients having high specific IgE to TPO. Our findings show that the prevalence of serum specific IgE to TPO was significantly higher in both AIAU and AICU patients than in NC. It is suggested that specific IgE to TPO play a pathogenic role in AIAU and AICU.
Graphical Abstract
Keywords: Urticaria, Autoimmunity, Thyroid Peroxidase Antibody, Aspirin Intolerance, Basophil Activation Test
INTRODUCTION
Urticaria is a heterogeneous group of diseases characterized by wheals and flares accompanied with or without angioedema. Aspirin/NSAIDs are the major drugs to provoke acute urticaria called aspirin intolerant acute urticaria (AIAU) and exacerbate urticaria in patients with chronic urticaria, called aspirin intolerant chronic urticaria (AICU). Usually, aspirin induced urticaria patients could have a more severe and chronic disease course. Three have been some cases of aspirin intolerant acute urticaria progressed to aspirin intolerant chronic urticaria (1). Although the dysregulation of arachidonic acid metabolism which are a suppression of prostaglandin and a relative overproduction of leukotrienes caused by aspirin/NSAIDs is suggested in the pathogenesis of aspirin hypersensitivity, the exact mechanisms of AIAU and AICU are not understood. Mast cell activation is involved in this mechanism since tryptase levels are significantly elevated in sera and anti-histamines are considered a drug of choice for urticaria patients. Successful results of anti-IgE therapy in severe urticaria patients are other evidence of the involvement of mast cell in urticaria pathophysiology (2, 3) as well as atopy is a predisposing factor for both AIAU and AICU (4).
There is solid evidences that patients suffering from urticaria have been reported to frequently associate with autoimmune disease (5, 6, 7), and the prevalence of thyroid auto-antibodies such as IgG to thyroid peroxidase (TPO) have been detected in 12%-29% of urticaria patients (8). Patients with coexistent thyroid autoimmunity and chronic urticaria have an even more severe and prolonged course of urticaria than those without thyroid autoimmunity (9). However so far, all studies have been evaluated for IgG to thyroid autoantibodies in chronic urticaria.
A recent study demonstrated the presence of highly cytokinergic IgE antibodies with polyreactivity to autoantigens including TPO in sera of atopic dermatitis patients, which could induce mast cell activation (10), and Altrichter et al. (11) reported that some of chronic spontaneous urticaria patients expressed IgE antibodies against TPO as a novel pathomechanism of chronic urticaria. However, they could not confirm whether these antibodies directly activate effector cells in chronic urticaria.
In this study, we hypothesized that specific IgE response to TPO may involve mast cell activation in AIAU and AICU and detected serum specific IgE to TPO with a basophil activation test.
MATERIALS AND METHODS
Patients
We recruited 59 AIAU patients, 96 AICU patients and 69 normal controls (NC). The diagnosis of aspirin intolerance was confirmed based on clear clinical histories of recurrent urticaria after aspirin/NSAIDs ingestions or positive results of oral challenge test to aspirin. Chronic urticaria was defined as daily urticaria that lasted more than 6 weeks. Urticaria severity in this study was determined by urticarial activity score (UAS) system which combines pruritus and four characteristics of wheals, including number, duration, distribution range, and mean diameter in total score range, 0-15. UAS score more than 13 is considered as a severe urticaria in this system (12).
Detection of serum specific IgE and IgG to TPO
Serum specific IgE to TPO was measured by our manual direct ELISA system. Briefly, dissolved commercial recombinant TPO (RayBiotech, Norcross, GA) in 0.1 M of carbonate buffer was coated at 96 well micro plate (Corning, New York, USA) for 12 hr at room temperature and then, each well was blocked with 10% FCS in PBS for two hours. After blocking, micro plates were incubated with 1:2 diluted serum for two hours at room temperature, followed by biotinylated human anti-IgE antibody (diluted 1:1,000 in 10% FBS in PBS, Vector, Burlingame, CA, USA) for one hour, and streptavidine-HRP (diluted 1:5,000) for thirty minutes. Finally, the enzymatic dye reaction was stopped with 2 N H2SO4 solution and the optical density was measured at 450 nm. Between each step intensive washings with 0.05% Tween 20 in PBS were performed more than three times. In this method, the cut-off value for serum specific IgE to TPO level was defined as mean and three times standard deviation of absorbance values from 69 healthy controls. This definition was commonly used in manual ELISA which has no standard sample (13). Serum IgG to TPO was measured by radioimmunoassay (BRAHMS Aktiengesellschaft, Hennigsdorf, Germany).
CD203c expression on basophil by flow cytometry
Peripheral blood basophils were obtained from three subjects with high levels of serum specific IgE to TPO and one healthy controls (NC). Basophil activation tests with the additions of TPO were then performed to prove a direct role of specific IgE to TPO on effector cells of urticaria as previously described (14). Briefly, whole blood was collected in ethylene diamine tetra-acetic acid (EDTA) tubes and red blood cells (RBCs) were lysed with RBC lysis buffer (0.154 M NH4Cl, 10 mM KHCO3, 0.1 mM EDTA, pH 7.2-7.4), and then aliquots of 50 µL of each stimulator were added to 100 µL of the leukocyte suspension. Stimulators included calcium ionophore A2319 (3 µM; Sigma-Aldrich, St. Louis, MO, USA), anti-human goat IgE antibodies (1:100 vol/vol; KPL, Gaithersburg, MD, USA), and anti-IgG4 (1 and 10 µg/mL; Sigma-Aldrich), and different concentration of TPO antigens (0, 0.01, 0.1, and 1 µg/mL). After stimulation, basophil CD203c expression was measured by flow cytometry. After washing with phosphate-buffered saline (PBS), the resuspended cells were stained with phycoerythrin (PE)-conjugated antihuman CD203c (Beckman Coulter, Marseille, France), fluorescein isothiocyanate (FITC)-conjugated antihuman CD123 (BD PharMingen, San Jose, CA, USA), and allophycocyanin (APC)-conjugated antihuman human leukocyte antigen (HLA)-DR (BD PharMingen), or isotype-matched controls on ice in the dark for 30 min. After washing once with PBS, the cells were analyzed on a FACS Canto II flow cytometer (Becton Dickinson, San Jose, CA, USA). The cells were gated initially based on the dot plot defined by the forward and side scatter, and then a second gate of high FITC-CD123+ cells and low APC-HLA-DR was defined to select the basophil population, analyzing at least 500 basophils. The percent CD203c expression was defined as the percentage of basophils expressing more CD203c than the critical point located at 103<×<104 in the histogram, which was about 10% of the basophils incubated with buffer only in the NC (19).
Statistical analysis
Data was presented by descriptive analysis. A Mann-Whitney U-test were used to compare the clinical characteristics of chronic urticaria, including the positive rate of atopy, UAS, total IgE and serum specific IgE to TPO level. Pearson's Chi-square tested the differences between AIAU and AICU groups. Differences were regarded as statistically significant for P<0.05. All statistical analyses were performed using SPSS for Windows (SPSS 12.0, Chicago, IL, USA).
Ethics statement
This study was approved by the ethics committee of Ajou University Hospital (IRB No. GEN-09-140) and done in accordance with the Declaration of Helsinki. Written informed consent was obtained from each subject, and confirmed by the IRB.
RESULTS
Clinical and laboratory characteristics of the study population
The clinical and laboratory characteristics of the study population are summarized in Table 1. There were no significant differences in age, gender, atopic status, and association with angioedema between AIAU and AICU groups except disease duration, and total IgE and eosinophilic cationic protein levels in serum were not significant differences between AIAU and AICU groups.
Table 1. Clinical and laboratory characteristics of the study subjects.
| Parameters | AIAU (n = 59) | AICU (n = 96) | NC (n = 69) | P value | ||
|---|---|---|---|---|---|---|
| AIAU vs. AICU | AIAU vs. NC | AICU vs. NC | ||||
| Age (yr)* | 40.44 ± 12.4 | 40.17 ± 12.1 | 27.36 ± 4.9 | 0.892 | < 0.001 | < 0.001 |
| Gender (female)† | 33 (55.9) | 59 (61.5) | 39 (54.2) | 0.496 | 0.979 | 0.491 |
| Atopy† | 23/41 (56.1) | 35/70 (50.0) | 0 (0.0) | 0.539 | < 0.001 | < 0.001 |
| Angioedema† | 27/43 (62.8) | 53/76 (69.7) | 0 (0.0) | 0.407 | < 0.001 | < 0.001 |
| UAS ≥ 13† | ND | 6/33 (26.1) | ND | NA | NA | NA |
| Total IgE (IU/mL)* | 366.45 ± 458.1 | 178.41 ± 464.13 | ND | 0.265 | NA | NA |
| ECP (ug/L)* | 23.26 ± 17.87 | 25.77 ± 24.62 | ND | 0.644 | NA | NA |
| Prevalence of IgG to TPO (%) | 14.8 | 7.7 | ND | 0.277 | NA | NA |
| Absolute value of IgE to TPO | 0.218 ± 0.446 | 0.157 ± 0.344 | 0.111 ± 0.050 | 0.338 | 0.071 | 0.204 |
| Prevalence of elevated IgE to TPO | 9/59 (15.3) | 8/96 (8.3) | 0 (0.0) | 0.188 | NA | NA |
P values were calculated using t-test. *Data are expressed as mean±standard deviation; †Data are expressed as number of positive patients with percentage given in parentheses. P values were calculated using Pearson's chi-squre test. Total IgE>114 IU/mL was regarded to elevated level, ECP>13.5 µg/L was regarded to elevated level, the cut-off value for serum specific IgE to TPO level was defined as mean and three times standard deviation of absorbance values from 69 healthy controls. AIAU, aspirin intolerant acute urticaria; AICU, aspirin intolerant chronic urticaria; NC, normal control; UAS, urticarial activity score; ECP, eosinophilic cationic protein; ND, not done; NA, not applicable.
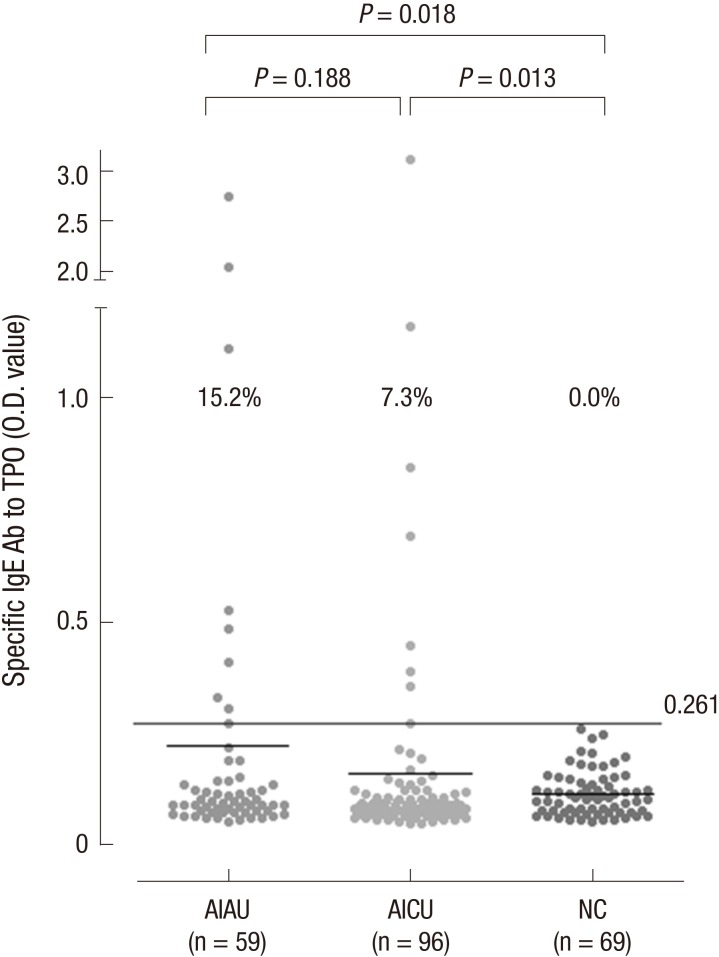
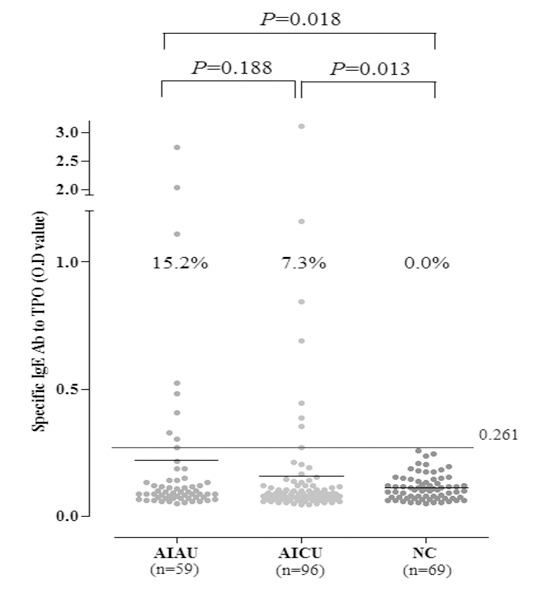
The prevalence of serum specific IgE to TPO in AIAU and AICU patients
Levels of serum specific IgE to TPO were assessed by direct ELISA. The absolute values of serum specific IgE to TPO were 0.218±0.446 in AIAU, 0.157±0.344 in AICU and 0.111±0.050 in NC. These have a tendency to be increased in AIAU and AICU compared to NC with no significant difference. However, the prevalence of serum specific IgE to TPO above the cutoff were significantly higher in AIAU (15.2%) and AICU (7.3%) groups compared to NCs (0%, P=0.018 ; P=0.013, respectively) (Fig. 1). AIAU patients showed a high tendency to have above cutoff IgE level to TPO compared with AICU patients although it was not statistically significant (Table 1). The prevalence of serum specific IgG to TPO was 14.8% in AIAU and 7.7% in AICU groups (P=0.277), and there was no significant association between the prevalence of specific IgE and IgG to TPO. When analyzed clinical and laboratory characteristics according to the presence of serum specific IgE to TPO, there were significant increase of total IgE level in patients with IgE to TPO compared to the other group (723.562 ±12.937 vs. 262.353±409.401, P=0.019) (Table 2).
Fig. 1. The prevalence of serum specific IgE to TPO. Serum specific IgE to TPO was measured by manual direct ELISA system from AIAU (n = 59), AICU (n = 96), and NC (n = 69) groups. The cut-off value for serum specific IgE to TPO level was defined as mean and three standard deviation of healthy controls. AIAU, aspirin intolerant acute urticaria; AICU, aspirin intolerant chronic urticaria; NC, normal control; TPO, thyroid peroxidase.
Table 2. Characteristics of study population according to the presence of serum specific IgE to TPO in aspirin induced urticaria.
| Parameters | Serum specific IgE to TPO | P value | |
|---|---|---|---|
| Presence (n = 16) | Absence (n = 139) | ||
| Age (yr)* | 39.00 ± 12.93 | 40.15 ± 12.19 | 0.896 |
| Gender (female)† | 8/17 (47.1%) | 86/137 (62.8%) | 0.210 |
| Atopy† | 9/13 (69.2%) | 49/97 (50.5%) | 0.204 |
| Angioedema† | 7/15 (46.7%) | 63/109 (57.8%) | 0.415 |
| UAS ≥ 13† | 5/15 (33.3%) | 11/30 (36.7%) | 0.909 |
| Total IgE (IU/mL)* | 723.56 ± 12.93 | 262.35 ± 409.40 | 0.019 |
| ECP (µg/L)* | 25.60 ± 12.49 | 24.24 ± 23.41 | 0.456 |
P values were calculated using t-test. *Data are expressed as mean±standard deviation; †Data are expressed as number of positive patients with percentage given in parentheses. P values were calculated using Pearson's chi-squre test. AIAU, aspirin intolerant acute urticaria; AICU, aspirin intolerant chronic urticaria; NC, normal control; UAS, urticarial activity score; ECP, eosinophilic cationic protein; ND, not done; NA, not applicable.
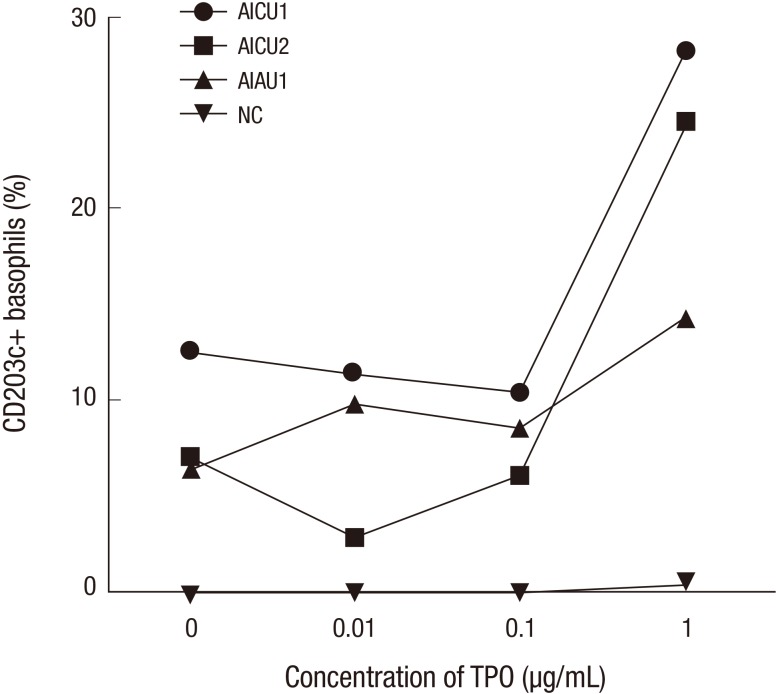
The level of CD203c expression on basophil after TPO stimulation
Next, we selected the basophil donors from patients who have high levels of serum specific IgE to TPO and NC, and a basophil activation test was done to show a direct role of specific IgE to TPO on effector cells of urticaria. The result showed that the expression of activation marker, CD203c, increased remarkably in dose dependent manners in representative two AICU and one AIAU patients with the addition of TPO compared to NC (Fig. 2).
Fig. 2. Expression level of CD203c on basophils. Peripheral blood basophils were obtained from two subjects with high serum specific IgE to TPO and healthy control. The expression of CD203c on basophil were measured with the additions of TPO to prove a direct role of specific IgE to TPO on effector cells of urticaria. AIAU, aspirin intolerant acute urticaria; AICU, aspirin intolerant chronic urticaria; NC, normal control; TPO, thyroid peroxidase.
DISCUSSION
Mast cell activation has been known to play a key role in urticarial reaction, and many studies investigated the association between autoimmune disease and this mast cell activation (15, 16, 17). However, majority of them were about to IgG autoantibodies not IgE autoantibodies in urticaria. In fact, IgG autoantibodies is one of the mechanism to activate effector cells such as mast cell or basophil in urticaria with binding to FcεRI or IgE antibodies, but these reactions were different from well-known allergic reaction between allergens, IgE antibodies, and IgE receptors on mast cell or basophil.
In 2002, Sabroe et al. (18) reported different kind of autoantibodies in autoimmune urticaria which consisted of histamine-releasing anti-FcεRI autoantibodies, anti-FcεRI autoantibodies without histamine-releasing activity, and anti-IgE like autoantibodies, but they couldn't identify almost half of autoantibodies. This finding means that another kind of autoantibodies other than IgG may play a role in urticaria. Autologous serum skin test is the only available in vivo test to detect histamine releasing autoimmune status with a prevalence of 42% to 68% in urticaria patients (15, 16, 17, 18); however, it is impossible to characterize the kind of autoantibodies in CSU patients through ASST.
Recently, Altrichter et al. (11) found that 54% of CSU patients had a higher serum specific IgE to TPO with their site-directed ELISA method. They concluded that subgroup of CSU patients expresses IgE antibodies against TPO, and these autoantibodies could be auto-allergic pathomechanism in CSU. However, they could not show direct activation of effector cells to these autoantibodies. In this study, we confirmed the presence of circulating serum specific IgE to TPO using our manual direct ELISA in both AIAU and AICU patients in line with previous study, and the most important part of this paper is to prove the direct role of serum specific IgE to TPO in effector cell with basophil activation test for the first time. The expression of CD203c on basophils is well-known activation marker and can be used as a potential marker of severe urticaria. The mean basophil CD203c expression was significantly higher in severe urticaria patients than in non-severe urticaria and NC (19).
However, there was quite big difference in the prevalence of serum specific IgE to TPO between previous and our results (11). This difference might be caused by ELISA method to detect TPO specific IgE. We used direct ELISA method instead of site directed IgE capture ELISA which was used in previous study. Direct ELISA is more specific method to detect serum specific IgE to TPO, although sensitivity is lower compared to other method. Further study will be needed to compare the sensitivity and specificity of both methods in detecting IgE to TPO.
Another finding of our study is significant high level of total IgE in presence of IgE to TPO compared to the absence of IgE to TPO in aspirin intolerant urticaria patients. Total IgE is relatively non-specific marker in various allergic diseases, but recent study found that it could be a potential marker for urticarial severity (20). Whereas 93% of patients with increased level of total IgE suffered from moderate-to-severe chronic urticaria, this was observed in only 69% of patients with normal IgE. Especially, patients with higher level of IgE had significant higher percentage of anti-thyroid antibodies compared to patients with normal level of IgE (31% vs. 7%, P<0.001), although these antibodies were all IgG against thyroglobulin and thyroid microsomal antigens (20, 21). Total IgE is not attributed to a specific trigger, but the result of the B cell polyclonal activation. The elevated expression of CD40L and Bcl-2 proved polyclonal activation of both B and T cell in CU (22). Further study may clarify the effect of this polyclonal activation on production of IgE to thyroid antigen such as TPO.
We also found relatively high prevalence of serum specific IgG and IgE to TPO in AIAU patients compared to AICU patients. Actually, there is no significant difference between levels of TPO-specific IgE in patients with AICU and aspirin tolerant chronic urticaria in previous our study (unpublished data). The reason to choose only aspirin intolerant urticaria was to evaluate the difference between AIAU and AICU. You can see some difference (15.2% vs. 7.3%) of TPO specific IgE level as shown in Fig. 1, although statistical significance was not noted. Viral infections such as hepatitis C virus are known triggers for acute urticaria that can induce serum TPO formation. HCV antibodies were found in 11.6% of the patients with autoimmune thyroid diseases and 38.4% of them had circulating TPO antibodies (23). Further studies are required to investigate possible interactions between autoantibody and viral infections in association with aspirin/NSAID exposure that induces mast cell activation in AIAU and AICU.
In this study, we found that the prevalence of serum specific IgE to TPO was significantly higher in both AIAU and AICU patients than in NC and that the presence of circulating serum specific IgE to TPO may contribute to mast cell activation in AIAU and AICU.
Footnotes
This study was supported by a grant of the Korean Health Technology R&D project, Ministry of Health & Welfare, Korea (HI14C0065).
DISCLOSURE: The authors have no conflict of interest to disclose.
AUTHOR CONTRIBUTION: Study design: Suh DH, Yang EM, Park HS. Data collection: Suh DH, Yang EM. Data analysis and writing: Shin YS, Ye YM. Revision: Park HS. Agreement of final manuscript and submission: all authors.
References
- 1.Asero R. Intolerance to nonsteroidal anti-inflammatory drugs might precede by years the onset of chronic urticaria. J Allergy Clin Immunol. 2003;111:1095–1098. doi: 10.1067/mai.2003.1444. [DOI] [PubMed] [Google Scholar]
- 2.Saini S, Rosen KE, Hsieh HJ, Wong DA, Conner E, Kaplan A, Spector S, Maurer M. A randomized, placebo-controlled, dose-ranging study of single-dose omalizumab in patients with H1-antihistamine-refractory chronic idiopathic urticaria. J Allergy Clin Immunol. 2011;128:567–573.e1. doi: 10.1016/j.jaci.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 3.Maurer M, Rosén K, Hsieh HJ, Saini S, Grattan C, Gimenéz-Arnau A, Agarwal S, Doyle R, Canvin J, Kaplan A, et al. Omalizumab for the treatment of chronic idiopathic or spontaneous urticaria. N Engl J Med. 2013;368:924–935. doi: 10.1056/NEJMoa1215372. [DOI] [PubMed] [Google Scholar]
- 4.Asero R. Risk factors for acetaminophen and nimesulide intolerance in patients with NSAID-induced skin disorders. Ann Allergy Asthma Immunol. 1999;82:554–558. doi: 10.1016/S1081-1206(10)63166-3. [DOI] [PubMed] [Google Scholar]
- 5.Di Lorenzo G, Leto-Barone MS, La Piana S, Seidita A, Rini GB. Chronic spontaneous urticaria: an autoimmune disease? A revision of the literature. Clin Exp Med. 2013;13:159–164. doi: 10.1007/s10238-012-0188-3. [DOI] [PubMed] [Google Scholar]
- 6.Confino-Cohen R, Chodick G, Shalev V, Leshno M, Kimhi O, Goldberg A. Chronic urticaria and autoimmunity: associations found in a large population study. J Allergy Clin Immunol. 2012;129:1307–1313. doi: 10.1016/j.jaci.2012.01.043. [DOI] [PubMed] [Google Scholar]
- 7.Leznoff A, Josse RG, Denburg J, Dolovich J. Association of chronic urticaria and angioedema with thyroid autoimmunity. Arch Dermatol. 1983;119:636–640. [PubMed] [Google Scholar]
- 8.Doutre MS. Chronic urticaria and thyroid auto-immunity. Clin Rev Allergy Immunol. 2006;30:31–37. doi: 10.1385/CRIAI:30:1:031. [DOI] [PubMed] [Google Scholar]
- 9.Dreskin SC, Andrews KY. The thyroid and urticaria. Curr Opin Allergy Clin Immunol. 2005;5:408–412. doi: 10.1097/01.all.0000182546.83465.5a. [DOI] [PubMed] [Google Scholar]
- 10.Kim MA, Park HS. Highly cytokinergic IgE antibodies and autoimmune mechanisms. Allergy Asthma Immunol Res. 2012;4:311–312. doi: 10.4168/aair.2012.4.6.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Altrichter S, Peter HJ, Pisarevskaja D, Metz M, Martus P, Maurer M. IgE mediated autoallergy against thyroid peroxidase--a novel pathomechanism of chronic spontaneous urticaria? PLoS One. 2011;6:e14794. doi: 10.1371/journal.pone.0014794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ye YM, Jin HJ, Hwang EK, Nam YH, Kim JH, Shin YS, Park HS. Co-existence of chronic urticaria and metabolic syndrome: clinical implications. Acta Derm Venereol. 2013;93:156–160. doi: 10.2340/00015555-1443. [DOI] [PubMed] [Google Scholar]
- 13.Gershwin LJ, Netherwood KA, Norris MS, Behrens NE, Shao MX. Equine IgE responses to non-viral vaccine components. Vaccine. 2012;30:7615–7620. doi: 10.1016/j.vaccine.2012.10.029. [DOI] [PubMed] [Google Scholar]
- 14.Nam YH, Hwang EK, Ban GY, Jin HJ, Yoo HS, Shin YS, Ye YM, Nahm DH, Park HS, Lee SK. Immunologic evaluation of patients with cefotetan-induced anaphylaxis. Allergy Asthma Immunol Res. 2015;7:301–303. doi: 10.4168/aair.2015.7.3.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Altrich ML, Halsey JF, Altman LC. Comparison of the in vivo autologous skin test with in vitro diagnostic tests for diagnosis of chronic autoimmune urticaria. Allergy Asthma Proc. 2009;30:28–34. doi: 10.2500/aap.2009.30.3185. [DOI] [PubMed] [Google Scholar]
- 16.Sabroe RA, Grattan CE, Francis DM, Barr RM, Kobza Black A, Greaves MW. The autologous serum skin test: a screening test for autoantibodies in chronic idiopathic urticaria. Br J Dermatol. 1999;140:446–452. doi: 10.1046/j.1365-2133.1999.02707.x. [DOI] [PubMed] [Google Scholar]
- 17.Grattan CE. Autoimmune urticaria. Immunol Allergy Clin North Am. 2004;24:163–181. v. doi: 10.1016/j.iac.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 18.Sabroe RA, Fiebiger E, Francis DM, Maurer D, Seed PT, Grattan CE, Black AK, Stingl G, Greaves MW, Barr RM. Classification of anti-FcepsilonRI and anti-IgE autoantibodies in chronic idiopathic urticaria and correlation with disease severity. J Allergy Clin Immunol. 2002;110:492–499. doi: 10.1067/mai.2002.126782. [DOI] [PubMed] [Google Scholar]
- 19.Ye YM, Yang EM, Yoo HS, Shin YS, Kim SH, Park HS. Increased level of basophil CD203c expression predicts severe chronic urticaria. J Korean Med Sci. 2014;29:43–47. doi: 10.3346/jkms.2014.29.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kessel A, Helou W, Bamberger E, Sabo E, Nusem D, Panassof J, Toubi E. Elevated serum total IgE: a potential marker for severe chronic urticaria. Int Arch Allergy Immunol. 2010;153:288–293. doi: 10.1159/000314370. [DOI] [PubMed] [Google Scholar]
- 21.Toubi E, Kessel A, Avshovich N, Bamberger E, Sabo E, Nusem D, Panasoff J. Clinical and laboratory parameters in predicting chronic urticaria duration: a prospective study of 139 patients. Allergy. 2004;59:869–873. doi: 10.1111/j.1398-9995.2004.00473.x. [DOI] [PubMed] [Google Scholar]
- 22.Toubi E, Adir-Shani A, Kessel A, Shmuel Z, Sabo E, Hacham H. Immune aberrations in B and T lymphocytes derived from chronic urticaria patients. J Clin Immunol. 2000;20:371–378. doi: 10.1023/a:1006624331022. [DOI] [PubMed] [Google Scholar]
- 23.Testa A, Castaldi P, Fant V, Fiore GF, Grieco V, De Rosa A, Pazardjiklian MG, De Rosa G. Prevalence of HCV antibodies in autoimmune thyroid disease. Eur Rev Med Pharmacol Sci. 2006;10:183–186. [PubMed] [Google Scholar]