Abstract
The aim of the present study was to evaluate the efficacy and toxicity of stereotactic body radiation therapy (SBRT) for low- to intermediate-risk prostate adenocarcinoma. Thirty-nine patients were retrospectively reviewed. The SBRT was delivered using the CyberKnife with the fiducial tracking method combined with In-tempo imaging. The gross target volume, which included the prostate only, was delineated on the fused CT/MRI scans. The prescription dose was delivered every other day as 5 fractions of 7.5 Gy. Venous blood was obtained before and after SBRT to assess the prostate-specific antigen (PSA) level. Toxicity was evaluated using the CTCAE, v4.03. The median follow-up time was 30.0 months. The median initial PSA level was 7.7 ng/mL. PSA levels decreased in all patients treated with SBRT, and after 5 months, the median PSA was less than 2 ng/mL. The rate of overall 3-yr actuarial biochemical failure free survival was 93.9%. Acute side effects were generally comparable with those of previous studies. The PSA change and toxicity after SBRT for low- to intermediate-risk prostate adenocarcinoma indicates favorable biochemical responses and tolerable levels of toxicity. Additionally short course treatment may produce cost benefit and convenience to patients.
Keywords: Prostate Neoplasms, Stereotactic Body Radiation Therapy, Cyberknife Radiosurgery, Prostate-Specific Antigen
INTRODUCTION
Prostate adenocarcinoma is the 2nd most frequently diagnosed cancer and the 6th leading cause of cancer death in males, accounting for 14% of the total new cancer cases and 6% of the total cancer deaths in 2008 worldwide (1).
A variety of treatment options for patients with low- to intermediate- risk prostate adenocarcinoma such as surgery, external beam radiation therapy (EBRT), interstitial brachytherapy, hormonal therapy, watchful waiting, and active surveillance have been adopted singly or in combination (2). Although EBRT provokes normal tissue toxicity and requires treatment course, EBRT is one of the preferred treatments modality for prostate adenocarcinoma.
Hypofractionation is a method for reducing the duration of radiation therapy. Additionally, several studies have suggested a therapeutic gain associated with hypofractionated courses of radiation therapy for prostate adenocarcinoma because of a low α/β ratio (3, 4, 5, 6). This implies that biologic response of the prostate adenocarcinomas to radiation therapy is more sensitive to a dose per fraction than the conventional value. This is the basis for the widespread use of hypofractionated radiation therapy for prostate adenocarcinoma. The therapeutic advantages of the hypofractionated EBRT have indeed reported in the several clinical studies with doses per fraction ranging from 2.5 to 3.1 Gy (7, 8). Other studies that used a linear accelerator-based stereotactic body radiation therapy (SBRT) technique to deliver 5 fractions of 6.7 Gy over 5 consecutive days also showed promising results (9).
It is imperative to distribute high doses along the prostate gland without causing serious adverse side effects in surrounding normal organs in radiation therapy, especially hypofractionation radiation therapy. The CyberKnife (CK) is an excellent system for this purpose (10). Its high accuracy and image-guided tracking technology enables the delivery of a higher dose to the target, creating steep dose gradients. Several phase I/II feasibility studies of the CK to treat prostate adenocarcinoma have demonstrated that stereotactic, extreme hypofractionation regimens have both acceptable toxicity profiles and excellent rates of biochemical control (10, 11, 12).
Presented here are results of biochemical response and toxicity of the single institute experiments using CK-based SBRT for patients with low- to intermediate-risk prostate adenocarcinoma.
MATERIALS AND METHODS
Patient selection
From June 2010 through November 2013, 39 patients underwent SBRT using the CK radiosurgical system (Accuray Inc., Sunnyvale, CA) as the primary treatment for their biopsy proven low- to intermediate-risk prostate adenocarcinoma, diagnosed according to the National Comprehensive Cancer Network (NCCN) criteria (Gleason score ≤7; clinical state T1c-T2c; prostate specific antigen (PSA) level ≤20 ng/mL). All patients had an Eastern Cooperative Oncology Group performance status of 0-2, normal bone marrow, liver, and renal functions, and no serious diseases that could affect treatment outcome. Patients who had undergone chemotherapy or radiation therapy for any tumor were excluded from this study.
Target volume delineation
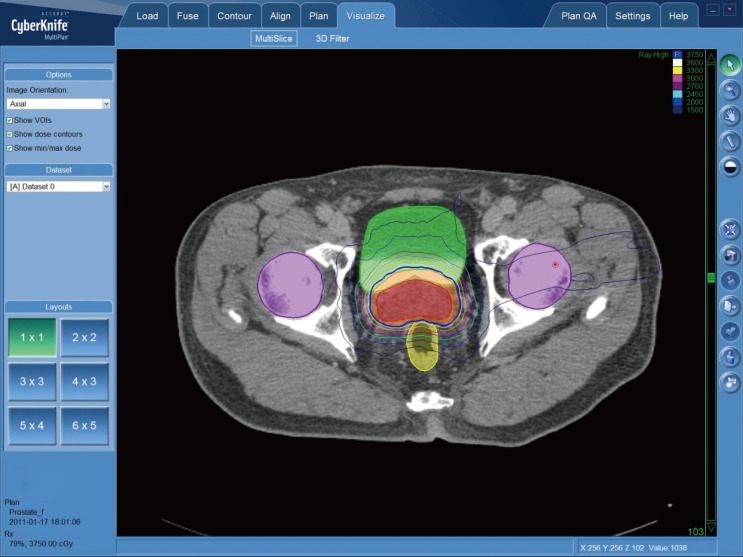
For each patient, computed tomography (CT) and magnetic resonance imaging (MRI) scans of the prostate region were obtained on the same day in the supine position with the bladder filling. The prostate delineation was guided by MRI. Three-dimensional coregisteration was applied to the MRI and CT images. Gross target volume (GTV) was defined as prostate only and the clinical target volume (CTV) was equal to the GTV. CTV with a 3 mm posterior expansion and 5 mm expansion in all other directions was defined as the planning target volume (PTV). The rectum, bladder, and penile bulb were contoured as organs at risk. Fig. 1 shows an axial view of a typical CK SBRT treatment plan.
Fig. 1. Example of CyberKnife SBRT treatment plan showing an axial view in a 70-yr old man who was diagnosed with adenocarcinoma of the prostate (cT1c; initial PSA: 3.01; GS: 7).
Treatment planning
Inverse treatment planning was performed for all patients using Multiplan version 3.5.4 (Accuray Inc., Sunnyvale, CA). The treatment course was in total 37.5 Gy, consisting of 5 fractions of 7.5 Gy corresponds to a tumor equivalent dose in 2 Gy per fraction of approximately 89 Gy delivered on alternate days. The PTV was used as a reference for the prescribed dose, which was normalized to 78%-84% of Dmax. Coverage requirements for GTV and PTV by the prescription iso-dose surface were 100% and 95%, respectively. Our dose constraints for normal tissues were basically based on the widely accepted recommendations for SBRT (13, 14), but allowed for some minor deviations, when not possible to fully satisfy the recommendations; The maximum rectal wall dose was required to be ≤100% of the prescribed dose; 1 mL or less volume receiving at least 36 Gy (V36 Gy), but V36 Gy ≤3 mL was accepted. Maximum bladder dose was required to be ≤100% of the prescribed dose; V37.5 Gy was ≤5 mL of the bladder, but V37.5 Gy ≤10 mL was accepted. The image-guided tumor tracking technique was applied using the fiducial tracking method combined with In-tempo system (Accuray Inc., Sunnyvale, CA).
Evaluation of SBRT
All patient data of PSA level and clinical symptoms of gastrointestinal (GI) and genitourinary (GU) were checked at just before SBRT and every month after SBRT. Endpoints of the study were PSA response and toxicity. The Phoenix definition of PSA, nadir + 2 ng/mL, was used to determine biochemical failure (15), PSA bounce was defined as a PSA increase of ≥0.4 ng/mL between any two consecutive measurements followed by a decline to or below the previous nadir (16). GI and GU toxicity were evaluated by the physician using the Common Toxicity Criteria for Adverse Events version 4.03 (CTCAEv4.03). Evaluated toxicities were categorized as early (before 6 months) and as late (6 months and later). Biochemical failure free survival (BFFS) was estimated using the Kaplan-Meier test and descriptive statistics were used to summarize the clinical characteristics of the patients. Statistical software SPSS ver. 21 (SPSS Inc., Chicago, IL) was used in performance of all statistical analyses.
Ethics statements
The current study was reviewed and approved by the institutional review board (IRB) of the Gyeongsang National University Hospital (IRB No. 2013-12-029-001). Informed consent was waived by the board.
RESULTS
Patients characteristics
The median age of patient was 70.0 yr (range: 47-80 yr) and proportion of patients was evenly distributed both side based on median age. More than half of patients were stage Ic. The Gleason score was 7 in one quarter of the patients. The median initial PSA level was 7.7 ng/mL (range: 2.7-19.5 ng/mL). Based on NCCN criteria (T stage, initial PSA level, and Gleason score), low-risk patients were 16 patients (41.0%), and intermediate-risk patients were 23 patients (59.0%). The median volume of GTV and PTV were 49.5 mL (range: 25.0-94.8) and 96.8 mL (range: 51.2-154.0), respectively. None were subjected to androgen deprivation therapy prior to SBRT. Patient characteristics are summarized in Table 1.
Table 1. Patient characteristics.
| Characteristics | No. (%) of patients |
|---|---|
| Total patients | 39 |
| Age (median) | |
| <70 yr | 19 |
| ≥ 70 yr | 20 (47-80) |
| Performance state (ECOG) | |
| 0 | 30 (76.9) |
| 1 | 8 (20.5) |
| 2 | 1 (2.6) |
| Comorbidity | |
| No | 17 (43.6) |
| Yes | 22 (56.4) |
| Stage | |
| T1c | 26 (66.7) |
| T2a | 4 (10.3) |
| T2b | 5 (12.8) |
| T2c | 4 (10.3) |
| Gleason score | |
| 2-6 | 29 (74.4) |
| 7 | 10 (25.6) |
| Initial PSA (median) | 7.7 ng/mL (2.7-19.5) |
| Risk group | |
| Low | 16 (41.0) |
| Intermediate | 23 (59.0) |
| GTV (median) | 49.5 mL (25.0-94.8) |
| PTV (median) | 96.8 mL (51.2-154.0) |
| Isodose line (median) | 80 (78-84) |
| Dose of radiotherapy (fraction) | 37.5 Gy (5) |
ECOG, eastern cooperative oncology group; PSA, prostate specific antigen; GTV, gross target volume; PTV, planning target volume.
PSA change after SBRT
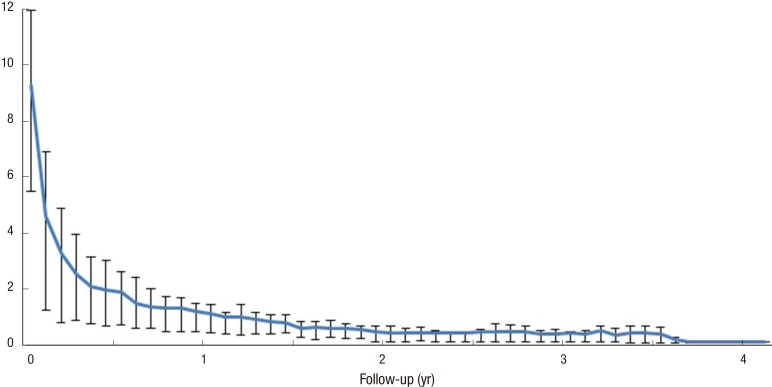
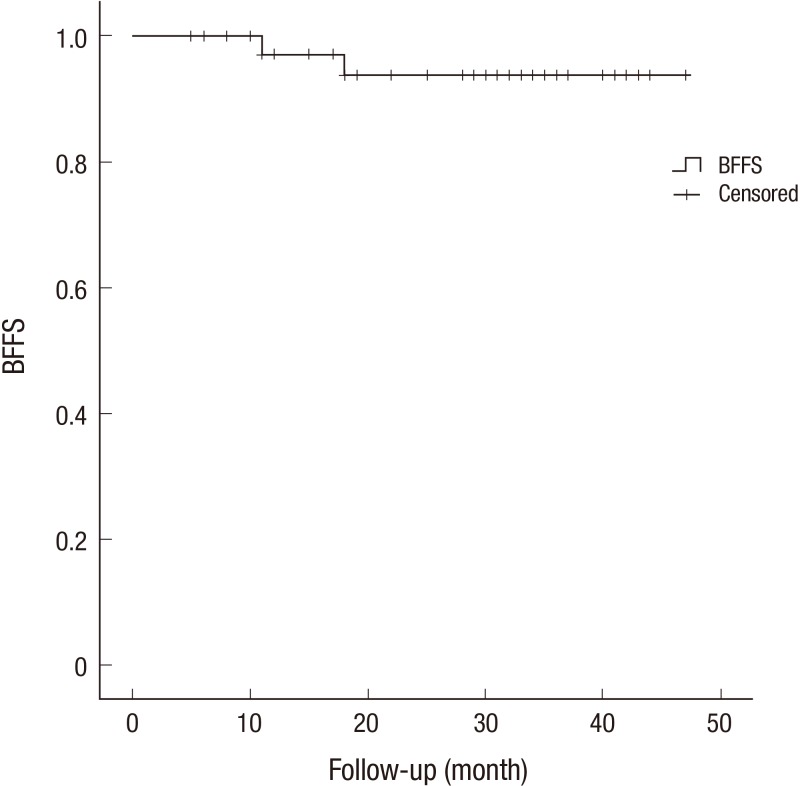
The median follow-up time in this study was 30.0 months (range: 5-48 months). The median PSA level declined from the initial value of 7.7 ng/mL to a final value of 0.1 ng/mL at the time of patient follow-up. Median PSA values at 3, 6, 12, 24, and 36 months were 2.5, 1.9, 1.1, 0.4, and 0.4 ng/mL, respectively. After 5 months, the median PSA level was less than 2 ng/mL (1.9 ng/mL), representing a decline of approximately 76.3% relative to the initial value. The change in median PSA level as a function of the time elapsed after completion of treatment is illustrated in Fig. 2. There were 2 cases of biochemical failure (at 11 and 18 months), and there were 3 cases of PSA bounce. The patients with biochemical recurrence received salvage therapy with radical prostatectomy or hormonal therapy, and PSA has been successfully controlled up to the present time. The overall 3-yr actuarial BFFS rates were 93.9% (Fig. 3).
Fig. 2. Change of median PSA value with IQR after SBRT (25%-75%).
Fig. 3. BFFS for the low- to intermediate-risk group patients with prostate adenocarcinoma.
Side effects of SBRT
For side effects following the SBRT, among the total of 39 patients, GI toxicity was experienced by 23 patients [15 (38.5%) with grade I, 7 (17.9%) with grade II, and 1 (2.5%) with grade III]. GU toxicity was experienced by 23 patients [17 patients (43.6%) with grade I, 6 patients (15.3%) with grade II]. Four patients (10.3%) experienced grade I impotence. More detailed information for radiation-induced toxicity is summarized in Table 2 and the comparison of the toxicities between the present and previous studies is given in Table 3.
Table 2. Treatment related adverse events (CTCAEv4.03).
| Organs | Symptoms | Grades | Acute toxicity (<6 months) | Late toxicity (≥ 6 months) |
|---|---|---|---|---|
| Gastrointestinal | Frequency (%) | 0 | 30 (76.9) | 37 (94.9) |
| 1 | 8 (20.5) | 1 (2.6) | ||
| 2 | 1 (2.6) | 1 (2.6) | ||
| Proctitis (%) | 0 | 20 (51.3) | 32 (82.1) | |
| 1 | 11 (28.2) | 4 (10.3) | ||
| 2 | 8 (20.5) | 2 (5.1) | ||
| 3 | 0 (0) | 1 (2.6) | ||
| Bleeding (%) | 0 | 32 (82.1) | 36 (92.3) | |
| 1 | 4 (10.3) | 2 (5.1) | ||
| 2 | 3 (7.7) | 0 (0) | ||
| 3 | 0 (0) | 1 (2.6) | ||
| Genitourinary | Hematuria (%) | 0 | 39 (100) | 38 (97.4) |
| 1 | 0 (0) | 1 (2.6) | ||
| Dysuria (%) | 0 | 27 (69.2) | 37 (94.9) | |
| 1 | 7 (17.9) | 2 (5.1) | ||
| 2 | 5 (12.8) | 0 (0) | ||
| Incontinence (%) | 0 | 37 (94.9) | 36 (92.3) | |
| 1 | 0 (0) | 0 (0) | ||
| 2 | 2 (5.1) | 3 (7.7) | ||
| Frequency (%) | 0 | 32 (82.1) | 34 (87.2) | |
| 1 | 4 (10.3) | 4 (10.3) | ||
| 2 | 3 (7.7) | 1 (2.6) | ||
| Impotence | 0 | 38 (97.4) | 36 (92.3) | |
| 1 | 1 (2.6) | 3 (7.7) |
CTCAE, common toxicity criteria for adverse events.
Table 3. Comparison of GI and GU toxicities on the CTCAE scale from the SBRT studies.
| Studies | Radiotherapy | Gastrointestinal (%) | Genitourinary (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | I | II | III | 0 | I | II | III | ||
| King et al., 2009 (20) | 36.25 Gy/5fx | 51 | 33 | 15 | - | 30 | 41 | 24 | 5 |
| King et al., 2012 (12) | 36.25 Gy/5fx | 95 | 5 | - | 5 | 80 | 12 | 5 | 2 |
| McBride et al., 2011 (19) | 36.25 Gy/5fx | 66 | 17 | 17 | - | 20 | 59 | 19 | 2 |
| 37.5 Gy/5fx | |||||||||
| Our study | 37.5 Gy/5fx | 26 | 38.5 | 17.9 | 2.6 | 26 | 44 | 15.3 | - |
GI, gastrointestinal; GU, genitourinary; CTCAE, common toxicity criteria for adverse events; SBRT, stereotactic body radiation therapy.
DISCUSSION
Although intensity-modulated radiation therapy is recognized as the standard treatment for localized prostate adenocarcinoma (17, 18), CK-based SBRT, which is safety and convenience radiation therapy, is considered as an alternative treatment method for patients with low- and intermediate-risk prostate adenocarcinoma (10, 11, 12). The aim of the present investigation was to assess the efficacy and toxicity of CK-based SBRT for patients with low- to intermediate-risk prostate adenocarcinoma and to compare the results obtained to those of previous studies.
The 3-yr actuarial BFFS rate of 93.9% for all patients is comparable to that reported by King et al. (12) (94.0%) and lower than that reported by McBride et al. (19) (97.7%). However, considering that the 3-yr BFFS reported by McBride et al. was only for patients with low risk. The median PSA value at 5 months after SBRT was 1.97 ng/mL (Fig. 2); this decline of 74.6%, from the initial value was similar to the previously reported results (12, 19, 20). In this study, the median initial and final PSA values were 7.77 ng/mL and 0.01 ng/mL, respectively (Table 1); previously reported values were 10 ng/mL and 1.5 ng/mL, respectively, and the PSA did not decrease below 2 ng/mL until 12 months (21). Therefore, the PSA values obtained here show a greater rate of decline and lower final value (i.e., 0.01 ng/mL vs. 1.5 ng/mL). Additionally, several previous studies have showed longer times until the decline of PSA to less than 2 ng/mL (11, 19, 20). However, it is not clear whether the differences of rapidity of PSA change have prognostic significance in this study.
Two patients experienced biochemical failure at 11 (cT2a; initial PSA: 15.09; Gleason score: 2) and 18 months (cTc; initial PSA: 5.18 ng/mL; Gleason score: 6). No significant risk factor for recurrence was identified for these patients than the others, and the recurrences were successfully managed by salvage therapy with radical prostatectomy and hormonal therapy, respectively. PSA bounce may lead to unnecessary interventions and significant patient anxiety; some studies have shown that PSA bounce occurs more frequently in younger patients, and that patient age is a predictor for its occurrence (19, 22), although this point is still controversial. In this study, PSA bounce was observed in 2 patients, but they were not younger than other patients (63 and 75 yr old). Therefore, the argument that PSA bounce is more frequent in younger patient could not be conjectured from this study.
GI toxicity following SBRT, only one patient in the study experienced grade III rectal ulcer at 7 months and confirmed recto-urethral fistula by colonoscopy and MRI scan at 17 months following the prostate SBRT and then managed by colostomy. We suppose the inter-fractional or intra-fractional anatomical change of the rectum could be the origin of the complication. This case led to a modification of our treatment protocol: rectal volume and shape should be checked before each treatment.
Grade I and II GU toxicities in our study are comparable with those in several previous studies (12, 19, 20), but more common than in James' (23) study comparing the toxicity outcomes among patients receiving IMRT or SBRT for prostate adenocarcinoma. However, there were no cases of grade III or higher GU toxicity. In current study, bladder may be partially overlapped with the targeted PTV. We did not apply the above dose constraint for this overlapped bladder volume to prevent the under-dosage of targeted PTV, implying the priority to disease control over bladder sparing, while may increase the GU toxicity. However, as for the present result with no grade III or higher GU toxicity, it may be acceptable in the clinical practices. Although the patient population in this study was relatively small (N=24), the overall toxicity was relatively mild in these patients. This is likely due to the high degree of accuracy of the image-guided CK SBRT method, which allows the targeted delivery of radiation, thereby minimizing damage to normal adjacent tissues.
The results of our study indicate that the CK-based SBRT for patients with low- to intermediate-risk prostate adenocarcinoma, employing a stereotactic, hypofractionated regimen, could be relatively well-tolerated by patients, as indicated by acceptable toxicity profiles and the low rate of biochemical recurrence. The data from this study are also in accordance with those of several previous Phase I/II feasibility studies (9, 11, 12, 21).
In conclusion, due to the small study population and relatively short follow-up period, broad conclusions about treatment efficacy cannot be made based solely on these results; however, the PSA change and toxicity after CK-based SBRT for low- to intermediate-risk prostate adenocarcinoma indicates favorable biochemical responses and tolerable levels of toxicity. In addition to short course of radiation therapy schedule may produce cost benefit and convenience to patients. Examining more patients and having longer follow-up times would provide robust evidence for the efficacy of CK-based SBRT in prostate adenocarcinoma. Future studies should more closely examine the significance of PSA values and bounce in predicting later GI and GU toxicity and also in disease recurrence.
Footnotes
DISCLOSURE: The authors have no conflicts of interest to disclose.
AUTHOR CONTRIBUTION: Conception and design: Kang KM, Jeong BK. Performed the experiments: Jeong BK, Jeong H, Ha IB, Choi HS. Data analysis: Jeong BK, Jeong H. Collection of materials: Ha IB, Choi HS. Writing the manuscript: Jeong BK, Kang KM. Revision: Jeong BK, Jeong H, Ha IB, Choi HS, Kang KM. Agree with results and conclusions and manuscript submission: All authors.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Thompson I, Thrasher JB, Aus G, Burnett AL, Canby-Hagino ED, Cookson MS, D'Amico AV, Dmochowski RR, Eton DT, Forman JD, et al. AUA Prostate Cancer Clinical Guideline Update Panel. Guideline for the management of clinically localized prostate cancer: 2007 update. J Urol. 2007;177:2106–2131. doi: 10.1016/j.juro.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 3.Brenner DJ, Hall EJ. Fractionation and protraction for radiotherapy of prostate carcinoma. Int J Radiat Oncol Biol Phys. 1999;43:1095–1101. doi: 10.1016/s0360-3016(98)00438-6. [DOI] [PubMed] [Google Scholar]
- 4.D'Souza WD, Thames HD. Is the alpha/beta ratio for prostate cancer low? Int J Radiat Oncol Biol Phys. 2001;51:1–3. doi: 10.1016/s0360-3016(01)01650-9. [DOI] [PubMed] [Google Scholar]
- 5.King CR, Fowler JF. A simple analytic derivation suggests that prostate cancer alpha/beta ratio is low. Int J Radiat Oncol Biol Phys. 2001;51:213–214. doi: 10.1016/s0360-3016(01)01651-0. [DOI] [PubMed] [Google Scholar]
- 6.Miralbell R, Roberts SA, Zubizarreta E, Hendry JH. Dose-fractionation sensitivity of prostate cancer deduced from radiotherapy outcomes of 5,969 patients in seven international institutional datasets: alpha/beta = 1.4 (0.9-2.2) Gy. Int J Radiat Oncol Biol Phys. 2012;82:e17–e24. doi: 10.1016/j.ijrobp.2010.10.075. [DOI] [PubMed] [Google Scholar]
- 7.Lukka H, Hayter C, Julian JA, Warde P, Morris WJ, Gospodarowicz M, Levine M, Sathya J, Choo R, Prichard H, et al. Randomized trial comparing two fractionation schedules for patients with localized prostate cancer. J Clin Oncol. 2005;23:6132–6138. doi: 10.1200/JCO.2005.06.153. [DOI] [PubMed] [Google Scholar]
- 8.Yeoh EE, Botten RJ, Butters J, Di Matteo AC, Holloway RH, Fowler J. Hypofractionated versus conventionally fractionated radiotherapy for prostate carcinoma: final results of phase III randomized trial. Int J Radiat Oncol Biol Phys. 2011;81:1271–1278. doi: 10.1016/j.ijrobp.2010.07.1984. [DOI] [PubMed] [Google Scholar]
- 9.Madsen BL, Hsi RA, Pham HT, Fowler JF, Esagui L, Corman J. Stereotactic hypofractionated accurate radiotherapy of the prostate (SHARP), 33.5 Gy in five fractions for localized disease: first clinical trial results. Int J Radiat Oncol Biol Phys. 2007;67:1099–1105. doi: 10.1016/j.ijrobp.2006.10.050. [DOI] [PubMed] [Google Scholar]
- 10.King CR, Lehmann J, Adler JR, Hai J. CyberKnife radiotherapy for localized prostate cancer: rationale and technical feasibility. Technol Cancer Res Treat. 2003;2:25–30. doi: 10.1177/153303460300200104. [DOI] [PubMed] [Google Scholar]
- 11.Aluwini S, van Rooij P, Hoogeman M, Bangma C, Kirkels WJ, Incrocci L, Kolkman-Deurloo IK. CyberKnife stereotactic radiotherapy as monotherapy for low- to intermediate-stage prostate cancer: early experience, feasibility, and tolerance. J Endourol. 2010;24:865–869. doi: 10.1089/end.2009.0438. [DOI] [PubMed] [Google Scholar]
- 12.King CR, Brooks JD, Gill H, Presti JC., Jr Long-term outcomes from a prospective trial of stereotactic body radiotherapy for low-risk prostate cancer. Int J Radiat Oncol Biol Phys. 2012;82:877–882. doi: 10.1016/j.ijrobp.2010.11.054. [DOI] [PubMed] [Google Scholar]
- 13.Freeman DE, King CR. Stereotactic body radiotherapy for low-risk prostate cancer: five-year outcomes. Radiat Oncol. 2011;6:3. doi: 10.1186/1748-717X-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Michalski JM, Moughan J, Purdy JA, Bosch WR, Bahary J, Lau H, Duclos M, Parliament M, Morton G, Hamstra DA, et al. Initial Results of a Phase 3 Randomized Study of High Dose 3DCRT/IMRT versus Standard Dose 3D-CRT/IMRT in Patients Treated for Localized Prostate Cancer (RTOG 0126) Int J Radiat Oncol Biol Phys. 2014;90:1263. [Google Scholar]
- 15.Abramowitz MC, Li T, Buyyounouski MK, Ross E, Uzzo RG, Pollack A, Horwitz EM. The Phoenix definition of biochemical failure predicts for overall survival in patients with prostate cancer. Cancer. 2008;112:55–60. doi: 10.1002/cncr.23139. [DOI] [PubMed] [Google Scholar]
- 16.Stock RG, Stone NN, Cesaretti JA. Prostate-specific antigen bounce after prostate seed implantation for localized prostate cancer: descriptions and implications. Int J Radiat Oncol Biol Phys. 2003;56:448–453. doi: 10.1016/s0360-3016(02)04470-x. [DOI] [PubMed] [Google Scholar]
- 17.Nakamura K, Akimoto T, Mizowaki T, Hatano K, Kodaira T, Nakamura N, Kozuka T, Shikama N, Kagami Y. Patterns of practice in intensity-modulated radiation therapy and image-guided radiation therapy for prostate cancer in Japan. Jpn J Clin Oncol. 2012;42:53–57. doi: 10.1093/jjco/hyr175. [DOI] [PubMed] [Google Scholar]
- 18.Namiki S, Ishidoya S, Ito A, Tochigi T, Numata I, Narazaki K, Yamada S, Takai Y, Arai Y. Five-year follow-up of health-related quality of life after intensity-modulated radiation therapy for prostate cancer. Jpn J Clin Oncol. 2009;39:732–738. doi: 10.1093/jjco/hyp086. [DOI] [PubMed] [Google Scholar]
- 19.McBride SM, Wong DS, Dombrowski JJ, Harkins B, Tapella P, Hanscom HN, Collins SP, Kaplan ID. Hypofractionated stereotactic body radiotherapy in low-risk prostate adenocarcinoma: preliminary results of a multi-institutional phase 1 feasibility trial. Cancer. 2012;118:3681–3690. doi: 10.1002/cncr.26699. [DOI] [PubMed] [Google Scholar]
- 20.King CR, Brooks JD, Gill H, Pawlicki T, Cotrutz C, Presti JC., Jr Stereotactic body radiotherapy for localized prostate cancer: interim results of a prospective phase II clinical trial. Int J Radiat Oncol Biol Phys. 2009;73:1043–1048. doi: 10.1016/j.ijrobp.2008.05.059. [DOI] [PubMed] [Google Scholar]
- 21.Boike TP, Lotan Y, Cho LC, Brindle J, DeRose P, Xie XJ, Yan J, Foster R, Pistenmaa D, Perkins A, et al. Phase I dose-escalation study of stereotactic body radiation therapy for low- and intermediate-risk prostate cancer. J Clin Oncol. 2011;29:2020–2026. doi: 10.1200/JCO.2010.31.4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hinnen KA, Monninkhof EM, Battermann JJ, van Roermund JG, Frank SJ, van Vulpen M. Prostate specific antigen bounce is related to overall survival in prostate brachytherapy. Int J Radiat Oncol Biol Phys. 2012;82:883–888. doi: 10.1016/j.ijrobp.2010.11.049. [DOI] [PubMed] [Google Scholar]
- 23.Yu JB, Cramer LD, Herrin J, Soulos PR, Potosky AL, Gross CP. Stereotactic body radiation therapy versus intensity-modulated radiation therapy for prostate cancer: comparison of toxicity. J Clin Oncol. 2014;32:1195–1201. doi: 10.1200/JCO.2013.53.8652. [DOI] [PMC free article] [PubMed] [Google Scholar]