Abstract
Conventional coronary angiography (CAG) has limitations in evaluating lesions producing ischemia. Three dimensional quantitative coronary angiography (3D-QCA) shows reconstructed images of CAG using computer based algorithm, the Cardio-op B system (Paieon Medical, Rosh Ha'ayin, Israel). The aim of this study was to evaluate whether 3D-QCA can reliably predict ischemia assessed by myocardial fractional flow reserve (FFR) < 0.80. 3D-QCA images were reconstructed from CAG which also were evaluated with FFR to assess ischemia. Minimal luminal diameter (MLD), percent diameter stenosis (%DS), minimal luminal area (MLA), and percent area stenosis (%AS) were obtained. The results of 3D-QCA and FFR were compared. A total of 266 patients was enrolled for the present study. FFR for all lesions ranged from 0.57 to 1.00 (0.85 ± 0.09). Measurement of MLD, %DS, MLA, and %AS all were significantly correlated with FFR (r = 0.569, 0609, 0.569, 0.670, respectively, all P < 0.001). In lesions with MLA < 4.0 mm2, %AS of more than 65.5% had a 80% sensitivity and a 83% specificity to predict FFR < 0.80 (area under curve, AUC was 0.878). 3D-QCA can reliably predict coronary lesions producing ischemia and may be used to guide therapeutic approach for coronary artery disease.
Graphical Abstract
Keywords: Coronary Angiography, Myocardial Ischemia, Coronary Artery Disease
INTRODUCTION
Conventional coronary angiography has some limitations in the evaluation of lesions because it provides two dimensional images for a three dimensional structure. And it does not adequately define lesions that are angulated, eccentric, or obscured by overlapped vessels (1, 2). In addition, conventional angiography cannot measure ischemia.
Myocardial fractional flow reserve (FFR) is currently a standard method to assess whether lesions are associated with myocardial ischemia. Earlier studies showed that FFR < 0.75 had diagnostic value for ischemia as well as prognostic value regarding cardiovascular outcomes (3, 4). More recently, FFR < 0.80 has become used as a cutoff value based on several studies (5, 6). Besides two dimensional conventional angiography often does not show stenosis clearly, two-dimensional severity which is considered as significant with more than 50% diameter stenosis, is poorly correlated with findings measured with FFR (7).
Although FFR is accurate for evaluating stenosis, it is more invasive than coronary angiography and require additional cost, time and efforts. Three-dimensional quantitative coronary angiography (3D-QCA) uses multiple images acquired from conventional coronary angiography to reconstruct three-dimensional images by its own algorithm. We and others have demonstrated the accuracy to display coronary anatomy has been documented compared with conventional angiography and intravascular ultrasound (IVUS) (8, 9, 10, 11, 12). In contrast to two-dimensional angiography, 3D-QCA could potentially be able to evaluate stenoses and predict lesions producing ischemia more accurately because it analyzes and measures lesions from 3 dimensional views. The current study was designed to test the hypothesis that 3D-QCA can predict lesions producing ischemia as compared to FFR.
MATERIALS AND METHODS
Study population
Patients who underwent both coronary angiography and FFR measurement between February, 2008 and January, 2011 for lesions with intermediate functional significance were screened. Exclusion criteria were lesions 1) in left main stem, 2) bypass graft, 3) instent restenosis, 4) a vessel protected by bypass graft, 5) poorly visualized, 6) related with myocardial infarction, 7) in patients presented in shock, and 8) located in tandem.
FFR measurement
Diagnostic coronary angiography was performed with 5 Fr catheter prior to coronary pressure measurement through femoral or radial percutaneous approach. Heparin (5,000-7,000 U intravenous bolus), nitroglycerin (200 µg intracoronary bolus) were administered and then FFR measurement was done as previously described (13, 14). Catheter changed to 6 Fr guiding catheter, a 0.014-in. pressure guidewire (St. Jude Medical, Inc., St. Paul, MN, USA or Volcano, Rancho Cordova, CA, USA) was calibrated, advanced through the guiding catheter into the coronary artery, and positioned distal to the stenosis.
The pressure proximal to the stenosis was recorded through the guiding catheter using a pressure transducer (Edwards Life Sciences, Irvine, CA, USA). Maximal coronary blood flow was achieved by incremental doses of intracoronary adenosine (18-72 µg for the right coronary artery and 24-72 µg for the left coronary artery) or intravenous adenosine (140 µg/kg/min continuous infusion via antecubital vein) until a plateau response in FFR was achieved. FFR was automatically calculated by the ratio of distal mean coronary pressure to mean aortic pressure during maximal hyperemia. The minimum FFR value achieved during the administration of the highest dose of adenosine was used for data analysis.
Three dimensional quantitative coronary angiography
Conventional coronary angiographic cine images were acquired at 15 frames per second. Among them two images at least 30° apart from each other showing index lesion with the least foreshortening and the best depiction were selected offline. After calibrating pixel size with contrast-filled catheter on one image in the ECG-gated end-diastolic frame, the sites with minimum luminal diameter, its proximal and distal reference coronary artery segments were manually identified on respective images. Assigning these three points, lumen was lineated by automatic edge detection function. Edge detection correction was manually performed if required.
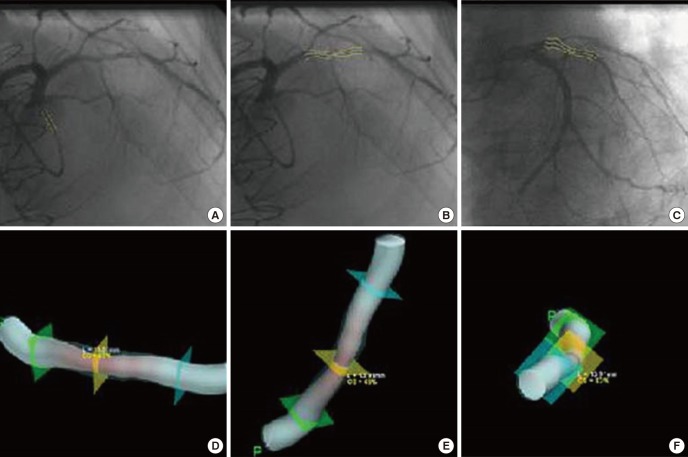
3D reconstruction images were automatically obtained from selected two dimensional (2D) angiography images using the Cardio-op B system (Paieon Medical, Rosh Ha'ayin, Israel) installed in workstation (Fig. 1). This software outlined minimal luminal diameter (MLD), proximal and distal reference diameter (PRD, DRD respectively), minimal luminal area (MLA), proximal and distal reference luminal area, percent area stenosis (%AS), percent diameter stenosis (%DS), lesion length as well as 3D representation of the arterial lumen. This measurement was undertaken by a experienced cardiologist blinded to FFR values. Interobserver and intraobserver error was reported to be acceptable in previous study (15, 16).
Fig. 1. Reconstruction of three dimensional images. (A) Calibration is done with catheter seen on the same image. (B, C) Clicking the narrowest point, proximal and distal reference segment make the lesion lineated automatically. It should be done on two images at least 30 degree apart from each other. (D-F), After finishing (A-C), computer based algorithm is activated and shows three dimensional images and quantitative data.
To assess the superiority of 3D-QCA to preexisting 2D-QCA in predicting FFR <0.80, we measured 2D-QCA variables for lesions in randomly selected 39 patients and analysed correlation with their corresponding FFR, diagnostic value predicting FFR <0.80.
Intra- and Inter-observer variation analysis
For intraobserver variation analysis, a single observer reconstructed 3D-QCA image and measured one index lesion twice in 10 patients. For interobserver variation analysis, two independent observer did the same procedure for 3D-QCA measurement separately in 20 patients. The variations were assessed by Tukey method and the difference between two measurements was regarded as not different if P value is more then 0.05, meaning the difference is located within 95% confidence interval.
Statistical analysis
An independent statistician performed the statistical analysis. Continuous variables were expressed as mean±standard deviation and categorical variables as percentage and counts. Each 3D-QCA variable was examined by bivariate correlation analysis and Pearson's correlation coefficients were obtained to determine if they have linear relation with FFR. Receiver operating characteristic (ROC) curve was drawn and area under curve (AUC) calculated to analyse and quantify the discriminatory ability of individual morphologic variable in predicting FFR <0.80. For a variable with acceptable AUC, the value with the best sensitivity and specificity was suggested as a diagnostic cutoff for significant ischemia. A two-sided P value of <0.05 is considered significant. All of statistical analyses were performed using JMP 9.0. (SAS Institute, Cary, NC, USA).
Ethics statement
Each patient gave written informed consent for all procedures and the present study was approved by institutional review board (Mayo Clinic, Rochester Minnesota, IRB approval number 11-005843).
RESULTS
Patients and angiographic characteristics
Only one lesion per patient was screened. Thirteen lesions (4.9%) of screened 279 lesions were excluded. They were two lesions protected by bypass graft, one a instent restenotic lesion, five poorly visualized, and another five in tandem. Two hundred sixty-six lesions of 266 patients were enrolled for the present study finally. Patients' mean age was sixty seven and 67% of all were males. 73.7% of all patients had hypertension, 23.4% diabetes mellitus, 88.2% dyslipidemia, and 15.7% were current smokers. Target lesions for comparing FFR and 3D-QCA parameter were located mainly in left anterior descending artery (59.4%), 19.2% in left circumflex artery and 21.4% in right coronary artery.
FFR for all target lesions ranged from 0.57 to 1.00. The mean was 0.85 and standard deviation was 0.09. Two dimensional angiographic characteristics of lesions are summarized in Table 1. Reference diameter was 2.66±0.54 mm proximally, 2.45±0.52 mm distally. Lesions were moderately narrowed as percent diameter stenosis (40.9%±10.7%), percent area stenosis (60.6%±11.0%) has indicated.
Table 1. Demographic and angiographic characteristics.
| Mean ± SD | |
|---|---|
| Age (yr) | 66.6 ± 11.0 |
| Sex (Male, %) | 66.5 |
| Lesion location (%) | |
| LAD | 158/266 (59.4) |
| LCX | 51/266 (19.2) |
| RCA | 57/266 (21.4) |
| Proximal:Nonproximal (%) | 32.3:67.7 |
| Lesion length (mm) | 12.2 ± 5.8 |
| Proximal reference diameter (mm) | 2.66 ± 0.54 |
| Distal reference diameter (mm) | 2.45 ± 0.52 |
| Minimal luminal diameter (mm) | 1.52 ± 0.41 |
| Percent diameter stenosis (%) | 40.9 ± 10.7 |
| Minimal luminal area (mm2) | 2.14 ± 1.13 |
| Percent area stenosis (%) | 60.6 ± 11.0 |
LAD, left anterior descending artery; LCX, left circumflex artery; RCA, right coronary artery.
Intra- and Inter-observer variation
We assessed intraobserver variation of MLD, %DS, MLA, %AS from 3D-QCA measurement. The difference between two measurement by a single observer existed within 95% confidence interval so that P value had more than 0.05. Similar statistical results were obtained in interobserver variation analysis (data not shown).
Correlation of 3D-QCA variables with FFR
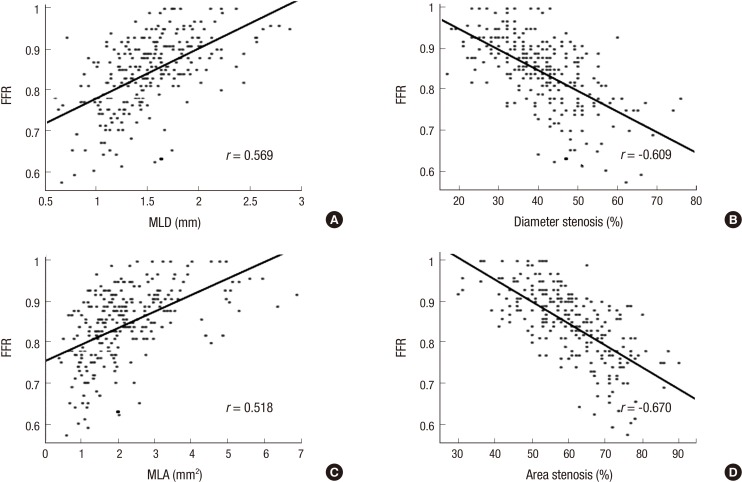
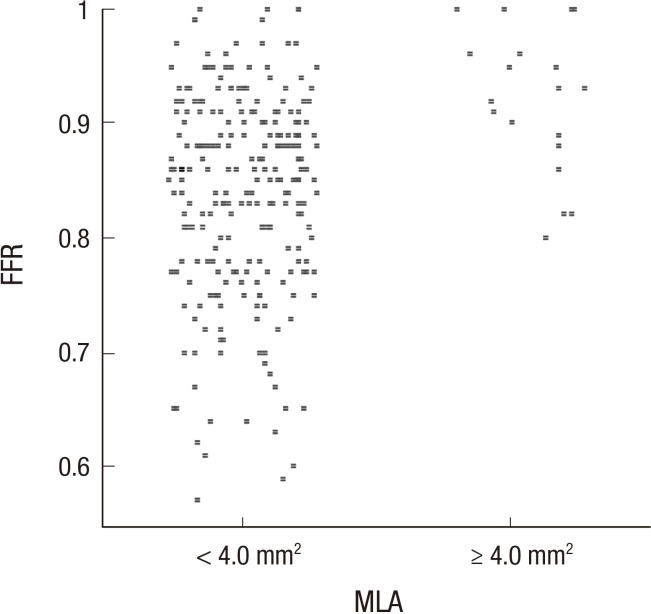
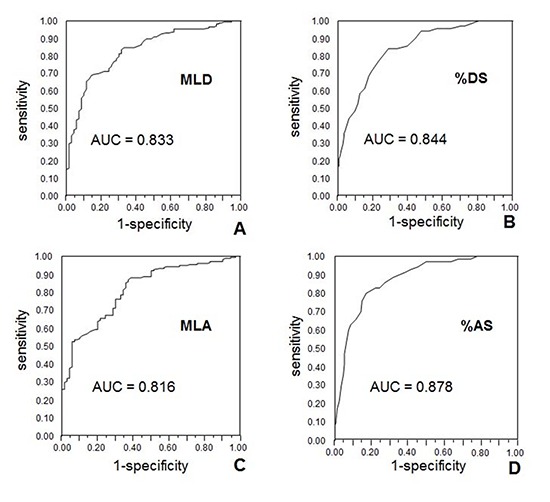
%AS were strongly correlated with FFR (Pearson correlation coefficient of -0.670, P<0.01) (Fig. 2). %DS and MLD, MLA also showed moderate correlations ranging in absolute value from 0.518-0.609. As shown in Fig. 3, all of lesions with MLA more than 4.0 mm2 have FFR ≥0.80, which indicates lack of significant ischemia. Excluding these lesions ≥4.0 mm2 in MLA, MLA's correlation coefficient became better to be 0.542 compared with previous 0.518.
Fig. 2. Bivariate linear correlation of 3D-QCA variables with FFR. (A) MLD, (B) %DS, (C) MLA, and (D) %AS. 3D-QCA, three-dimensional quantitative coronary angiography; FFR, fractional flow reserve; MLD, minimal luminal diameter; %DS, percent diameter stenosis; MLA, minimal luminal area; %AS, percent area stenosis; r, Pearson's correlation coefficient.
Fig. 3. Difference in FFR according to MLA divided by 4.0 mm2 on 3D-QCA. 3D-QCA, three-dimensional quantitative coronary angiography; MLA, minimal luminal area; FFR, fractional flow reserve.
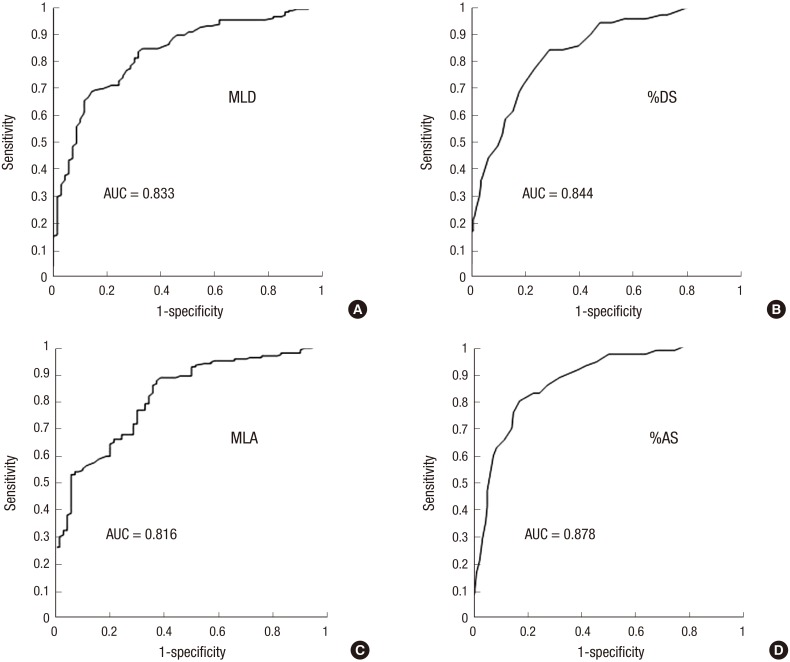
Predicting value of 3D-QCA for FFR
As a next step, 247 lesions less than 4.0 mm2 in MLA, with a wide range of FFR values, were further analysed to investigate if 3D-QCA can predict their hemodynamic significance as compared to FFR. To evaluate diagnostic value of each 3D-QCA variable, the ROC curve and its AUC were obtained. Relative values such as %AS, %DS had better AUC predicting FFR <0.80 than absolute values such as MLA, MLD. The AUC were 0.878, and 0.844, respectively for %AS and %DS (95% confidence interval was 0.832-0.924 for %AS, 0.792-0.896 for %DS, P<0.001 for both), and 0.816 and 0.833, respectively for MLA and MLD (95% confidence interval was 0.759-0.872 for MLA, 0.779-0.887 for MLD, P<0.001 for both) (Fig. 4).
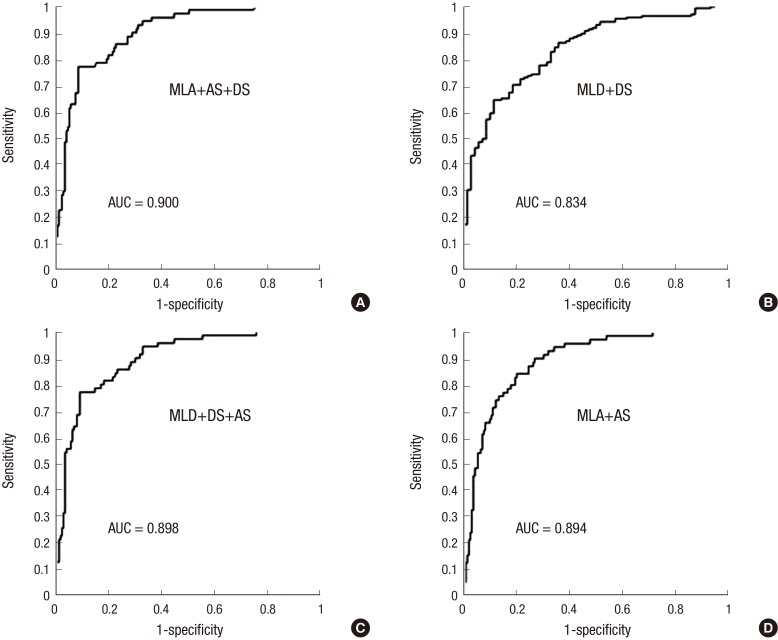
Fig. 4. Receiver operating characteristic curve of 3D-QCA variables in lesions with MLA < 4.0 mm2 for FFR < 0.80. (A) Minimal luminal diameter. (B) percent area stenosis. (C) minimal luminal area. (D) percent area stenosis. 3D-QCA, three-dimensional quantitative coronary angiography; FFR, fractional flow reserve; MLD, minimal luminal diameter; %DS, percent diameter stenosis; MLA, minimal luminal area; %AS, percent area stenosis; AUC, area under curve.
A cutoff value of %AS of 65.5% can predict FFR <0.80 with 80.0% of sensitivity and 83.1% of specificity. In terms of MLA, 1.6 mm2 can predict FFR <0.80 with sensitivity, specificity of 72.9%, 70.0%. For the %DS, MLD, sensitivity was 77.1%, 74.6%, specificity was 76.8%, 74.3% with %DS of 43.5%, MLD of 1.35 mm, respectively (Table 2).
Table 2. Diagnostic value of 3D-QCA variables predicting fractional flow reserve < 0.80 as gold standard of myocardial ischemia.
| Cutoff | Sens (%) | Spec (%) | PPV (%) | NPV (%) | Acc (%) | |
|---|---|---|---|---|---|---|
| MLD | 1.35 mm | 74.6 | 74.3 | 88.0 | 53.6 | 74.5 |
| % DS | 43.5% | 77.1 | 76.8 | 56.8 | 89.4 | 76.9 |
| MLA | 1.6 mm2 | 72.9 | 70.0 | 86.0 | 50.5 | 72.1 |
| % AS | 65.5% | 80.0 | 83.1 | 65.1 | 91.3 | 82.8 |
MLD, minimal luminal diameter; %DS, percent diameter stenosis; MLA, minimal luminal area; %AS, percent area stenosis; Sens, sensitivity; Spec, specificity; PPV, positive predictive value; NPV, negative predictive value; Acc, accuracy.
With %AS and MLA simultaneously as lumen area related variables, AUC increased to be 0.894. Combining %DS and MLD which were lumen diameter related variables, AUC was 0.868. If MLA, %DS, %AS which were composed of two area related variables and one diameter related one, were combined to be analysed for AUC, the AUC increased to 0.900. With all of MLA <4.0 mm2, %DS >43.5, %AS >65, this condition has 71.2% positive predictability for FFR (FFR <0.8). AUC could also be elevated to be 0.898 with %DS, MLD, %AS, if two diameter related variables were combined with %AS (Fig. 5).
Fig. 5. Receiver operating characteristic curve of combined 3D-QCA variables in lesions with MLA < 4.0 mm2 for FFR < 0.80. (A) MLA plus %AS plus %DS. (B) MLD plus %DS. (C) MLD plus %DS plus %AS. (D) MLA plus %AS. 3D-QCA, three-dimensional quantitative coronary angiography; FFR, fractional flow reserve; MLD, minimal luminal diameter; %DS, percent diameter stenosis; MLA, minimal luminal area; %AS, percent area stenosis; AUC, area under curve.
However, MLD and %DS obtained by 2D-QCA showed poor correlation with FFR (r=0.11 for both). Their diagnostic values to detect FFR <0.80 were also low so that AUC was 0.550, 0.526 for MLD, %DS, respectively.
DISCUSSION
Current study demonstrates that combining several parameters derived from 3D-QCA has high sensitivity and specificity to detect hemodynamically significant lesions as assessed by FFR. The current study suggests the use of 3D-QCA may help to guide coronary intervention.
Since introduction by Dr. Sones in the 1960s, contrast coronary angiography has been a mainstay in diagnosis of coronary artery disease. It visualizes the lumen of the coronary arteries, quantifies diameter stenosis and can evaluate its flow semiquantitatively by frame count (17). Traditionally more than 50% diameter stenosis has been regarded as significant because it compromises arterial lumen by more than 75%. This angiographical criteria was a long standing cutoff value of functional diagnosis, i.e., ischemia as well as anatomical diagnosis because of the lack of proper and accurate method measuring ischemia or blood flow.
Recently FFR, an invasive quantitative method for coronary blood flow, was developed for assessment of the hemodynamic significance of specific coronary lesions (4). Although FFR does not measure flow directly, it is pressure derived flow index and consistently validated for its accuracy (4, 14). The value of 0.80 was suggested as a cutoff with diagnostic and prognostic significance (5, 18, 19, 20). This cutoff is relatively specific for ischemia (3, 4, 21). Conventional coronary angiography alone cannot predict FFR based ischemia. Tonino et al. have demonstrated that coronary angiography based decisions were incorrect in 47% in predicting ischemia. According to their analysis, in patients with angiographically triple vessel disease, only 14% had functional triple vessel disease and surprisingly 9% had normal perfusion even if they had multiple stenoses in their coronary trees (7).
However, there are some limitations in performing FFR measurement. The use of FFR requires instrumentation within the coronary artery that may increase the risk of diagnostic procedure. Moreover, it requires additional procedural time and expense due to equipment, personnel and the use of pharmacological intervention such such as adenosine, and heparin.
Thus, there is a need for more accurate and less invasive imaging modalities based on coronary angiography. Three dimensional QCA could be an acceptable method as an alternative to assess the significance of coronary lesions compared to FFR. This new imaging technique is a computer based algorithm that uses two 2D images to create a three dimensional rendering. It takes a short time to reconstruct images per patient and can be done immediately or at a later period of time with little additional cost (22, 23).
Yong et al. has investigated Cardio-op B system as our study did. They showed MLA had the best AUC value, that is 0.86 to predict FFR <0.75 and FFR <0.75 permitted larger AUC to 3D-QCA than FFR <0.80. They also have revealed excellent reproducibility. However, the study included relatively small number of lesions and FFR <0.80 is currently more widely accepted as an ischemic cut-off value. The present study serves as an extension of previous observations and demonstrated that the combination of several 3D parameters improves the accuracy and predictive values of 3D-QCA.
IVUS is another invasive imaging modality that is well validated. 3D-QCA is similar to IVUS as both can measure luminal area and % area stenosis. Although IVUS does not yield functional data, MLA <4.0 mm2 measured by IVUS was known to have a diagnostic and prognostic value (24, 25). Interestingly, in the present study, all of lesions with MLA more than 4.0 mm2 by 3D-QCA actually have FFR ≥0.80. This finding is therefore consistent with IVUS studies, indicating that lesions with more than 4.0 mm2 of MLA may not need further evaluation for ischemia.
For lesions with MLD <4.0 mm2, the current study demonstrated that %AS revealed the best AUC among variables, and had 65.5% as a cutoff value with acceptable sensitivity and specificity. Similarly Briguori et al. have shown previously that 70% %AS by IVUS is appropriate for predicting ischemia (24). Our cutoff value of MLA, 1.6 mm2 is quite smaller than IVUS's. However, 4.0 mm2 of MLA on IVUS study had poor specificity 56% although sensitivity was 92%. Recently, Kang et al. suggested that 2.4 mm2 as a new IVUS cutoff value (26). However, it should be notable their AUC was 0.80, specificity 60% which were both inferior to our combined parameters. Previously, there was a debate about which parameter might predict better FFR based ischemia between MLA and %AS (15, 23). Both studies had a small number of patients. In our current study which included larger number of lesions, we have demonstrated that %AS is the best predictor as a single variable. It is reasonable to consider that MLA cannot be uniformly applied to different sized coronary artery because it is an absolute value. It becomes evident that FFR values are diffusely distributed for lesions with MLA <4.0 mm2 (Fig. 3). Because MLA >4.0 mm2 is sufficient to exclude a lesion mediating myocardial ischemia (100% negative predictability), we focused more on lesions with MLA <4.0 mm2. Finally we evaluated additional variables for a cut-off other than MLA <4.0 mm2 to enhance the sensitivity and specificity.
The ability to predict FFR associated with myocardial ischemia with 3D-QCA was enhanced if variables were considered in combination. Among many possible combinations, area related values such as %AS and MLA produced the best AUC. If %DS were considered along with %AS and MLA, AUC reached 0.900. In real practice, we can experience the situation in which 3D-QCA derived %AS or MLA alone is debatable. At that time, integrative decision of %AS, MLA, %DS would increase diagnostic yield.
As the first step applying using 3D-QCA for intermediate lesions in real world practice, we can exclude the lesions if MLA >4.0 mm2. Next, we can exclude the lesions again if %AS <65.5 having the highest AUC with high negative predictability and specificity. For the remained lesion, we can conclude based on MLA <1.6 mm2 and %DS of 43.5% comprehensively if they would have FFR <0.80 although it is uncertain to decide when MLA and %DS values are contradictory.
There are some limitations in our study. Left main lesions were excluded, and thus the results can not be extended to left main lesions. Second, as 3D-QCA depends on the quality of conventional angiography, the appropriate 3D-QCA images and variables may not be obtained, thereby limit its clinical application. Third, this study was done in a single center and has small study population. It prevents from modeling well structured diagnostic algorithm suggesting 3D-QCA variables step by step to be more useful in clinical practice. Larger sized study would solve this limitation.
In conclusion, parameters derived from 3D-QCA may reliably predict the functional significance of lesions based on FFR and may become a useful less invasive method to guide decision making in cardiac catheterization laboratory. It is reasonable that in future practice, the combination of lesion specific parameter such as MLA, %AS and %DS will be calculated and presented simultaneously during angiogram and may create 3D-QCA derived specific parameter of the target lesion to assess its functional significance.
ACKNOWLEDGEMENTS
We, all authors, thank Robert Cole and Rebecca E. Nelson for their executive works and efforts to proceed with this study and maintain 3D-QCA software.
Footnotes
DISCLOSURE: The authors have no conflicts of interest to disclose.
AUTHOR CONTRIBUTION: Study concepts and design: Chung WY, Lerman A, Lim SH. Study coordination: Chung WY, Lerman A, Lim SH. Acquisition of data: Chung WY, Lim SH, Choi BJ, Matsuo Y. Data review: Chung WY, Lim SH, Choi BJ, Matsuo Y. Statistical analysis: Chung WY, Lim SH, Lennon RJ. Manuscript preparation: Chung WY, Lerman A, Gulati R, Sandhu GS, Holmes DR Jr, Rihal CS. Manuscript approval: all authors.
References
- 1.Gottsauner-Wolf M, Sochor H, Moertl D, Gwechenberger M, Stockenhuber F, Probst P. Assessing coronary stenosis. Quantitative coronary angiography versus visual estimation from cine-film or pharmacological stress perfusion images. Eur Heart J. 1996;17:1167–1174. doi: 10.1093/oxfordjournals.eurheartj.a015033. [DOI] [PubMed] [Google Scholar]
- 2.Topol EJ, Nissen SE. Our preoccupation with coronary luminology. The dissociation between clinical and angiographic findings in ischemic heart disease. Circulation. 1995;92:2333–2342. doi: 10.1161/01.cir.92.8.2333. [DOI] [PubMed] [Google Scholar]
- 3.Pijls NH, van Schaardenburgh P, Manoharan G, Boersma E, Bech JW, van't Veer M, Bär F, Hoorntje J, Koolen J, Wijns W, et al. Percutaneous coronary intervention of functionally nonsignificant stenosis: 5-year follow-up of the DEFER Study. J Am Coll Cardiol. 2007;49:2105–2111. doi: 10.1016/j.jacc.2007.01.087. [DOI] [PubMed] [Google Scholar]
- 4.Pijls NH, De Bruyne B, Peels K, Van Der Voort PH, Bonnier HJ, Bartunek JKJJ, Koolen JJ. Measurement of fractional flow reserve to assess the functional severity of coronary-artery stenoses. N Engl J Med. 1996;334:1703–1708. doi: 10.1056/NEJM199606273342604. [DOI] [PubMed] [Google Scholar]
- 5.Tonino PA, De Bruyne B, Pijls NH, Siebert U, Ikeno F, van't Veer M, Klauss V, Manoharan G, Engstrøm T, Oldroyd KG, et al. FAME Study Investigators. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009;360:213–224. doi: 10.1056/NEJMoa0807611. [DOI] [PubMed] [Google Scholar]
- 6.Hamilos M, Muller O, Cuisset T, Ntalianis A, Chlouverakis G, Sarno G, Nelis O, Bartunek J, Vanderheyden M, Wyffels E, et al. Long-term clinical outcome after fractional flow reserve-guided treatment in patients with angiographically equivocal left main coronary artery stenosis. Circulation. 2009;120:1505–1512. doi: 10.1161/CIRCULATIONAHA.109.850073. [DOI] [PubMed] [Google Scholar]
- 7.Tonino PA, Fearon WF, De Bruyne B, Oldroyd KG, Leesar MA, Ver Lee PN, Maccarthy PA, Van't Veer M, Pijls NH. Angiographic versus functional severity of coronary artery stenoses in the FAME study fractional flow reserve versus angiography in multivessel evaluation. J Am Coll Cardiol. 2010;55:2816–2821. doi: 10.1016/j.jacc.2009.11.096. [DOI] [PubMed] [Google Scholar]
- 8.Agostoni P, Biondi-Zoccai G, Van Langenhove G, Cornelis K, Vermeersch P, Convens C, Vassanelli C, Van Den Heuvel P, Van Den Branden F, Verheye S. Comparison of assessment of native coronary arteries by standard versus three-dimensional coronary angiography. Am J Cardiol. 2008;102:272–279. doi: 10.1016/j.amjcard.2008.03.048. [DOI] [PubMed] [Google Scholar]
- 9.Dvir D, Marom H, Guetta V, Kornowski R. Three-dimensional coronary reconstruction from routine single-plane coronary angiograms: in vivo quantitative validation. Int J Cardiovasc Intervent. 2005;7:141–145. doi: 10.1080/14628840500193398. [DOI] [PubMed] [Google Scholar]
- 10.Gollapudi RR, Valencia R, Lee SS, Wong GB, Teirstein PS, Price MJ. Utility of three-dimensional reconstruction of coronary angiography to guide percutaneous coronary intervention. Catheter Cardiovasc Interv. 2007;69:479–482. doi: 10.1002/ccd.20955. [DOI] [PubMed] [Google Scholar]
- 11.Rubinshtein R, Lerman A, Spoon DB, Rihal CS. Anatomic features of the left main coronary artery and factors associated with its bifurcation angle: a 3-dimensional quantitative coronary angiographic study. Catheter Cardiovasc Interv. 2012;80:304–309. doi: 10.1002/ccd.23425. [DOI] [PubMed] [Google Scholar]
- 12.Tu S, Huang Z, Koning G, Cui K, Reiber JH. A novel three-dimensional quantitative coronary angiography system: in-vivo comparison with intravascular ultrasound for assessing arterial segment length. Catheter Cardiovasc Interv. 2010;76:291–298. doi: 10.1002/ccd.22502. [DOI] [PubMed] [Google Scholar]
- 13.Pijls NH, van Son JA, Kirkeeide RL, De Bruyne B, Gould KL. Experimental basis of determining maximum coronary, myocardial, and collateral blood flow by pressure measurements for assessing functional stenosis severity before and after percutaneous transluminal coronary angioplasty. Circulation. 1993;87:1354–1367. doi: 10.1161/01.cir.87.4.1354. [DOI] [PubMed] [Google Scholar]
- 14.Pijls NH, Van Gelder B, Van der Voort P, Peels K, Bracke FA, Bonnier HJ, el Gamal MI. Fractional flow reserve. A useful index to evaluate the influence of an epicardial coronary stenosis on myocardial blood flow. Circulation. 1995;92:3183–3193. doi: 10.1161/01.cir.92.11.3183. [DOI] [PubMed] [Google Scholar]
- 15.Yong AS, Ng AC, Brieger D, Lowe HC, Ng MK, Kritharides L. Three-dimensional and two-dimensional quantitative coronary angiography, and their prediction of reduced fractional flow reserve. Eur Heart J. 2011;32:345–353. doi: 10.1093/eurheartj/ehq259. [DOI] [PubMed] [Google Scholar]
- 16.Schuurbiers JC, Lopez NG, Ligthart J, Gijsen FJ, Dijkstra J, Serruys PW, Van der Steen AF, Wentzel JJ. In vivo validation of CAAS QCA-3D coronary reconstruction using fusion of angiography and intravascular ultrasound (ANGUS) Catheter Cardiovasc Interv. 2009;73:620–626. doi: 10.1002/ccd.21872. [DOI] [PubMed] [Google Scholar]
- 17.Gibson CM, Cannon CP, Daley WL, Dodge JT, Jr, Alexander B, Jr, Marble SJ, McCabe CH, Raymond L, Fortin T, Poole WK, et al. TIMI frame count: a quantitative method of assessing coronary artery flow. Circulation. 1996;93:879–888. doi: 10.1161/01.cir.93.5.879. [DOI] [PubMed] [Google Scholar]
- 18.Pijls NH, Fearon WF, Tonino PA, Siebert U, Ikeno F, Bornschein B, van't Veer M, Klauss V, Manoharan G, Engstrøm T, et al. FAME Study Investigators. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention in patients with multivessel coronary artery disease: 2-year follow-up of the FAME (Fractional Flow Reserve Versus Angiography for Multivessel Evaluation) study. J Am Coll Cardiol. 2010;56:177–184. doi: 10.1016/j.jacc.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 19.Bech GJ, De Bruyne B, Akasaka T, Liistro F, Bonnier HJ, Koolen JJ, Pijls NH. Coronary pressure and FFR predict long-term outcome after PTCA. Int J Cardiovasc Intervent. 2001;4:67–76. doi: 10.1080/146288401753258303. [DOI] [PubMed] [Google Scholar]
- 20.Legalery P, Schiele F, Seronde MF, Meneveau N, Wei H, Didier K, Blonde MC, Caulfield F, Bassand JP. One-year outcome of patients submitted to routine fractional flow reserve assessment to determine the need for angioplasty. Eur Heart J. 2005;26:2623–2629. doi: 10.1093/eurheartj/ehi484. [DOI] [PubMed] [Google Scholar]
- 21.Berger A, Botman KJ, MacCarthy PA, Wijns W, Bartunek J, Heyndrickx GR, Pijls NH, De Bruyne B. Long-term clinical outcome after fractional flow reserve-guided percutaneous coronary intervention in patients with multivessel disease. J Am Coll Cardiol. 2005;46:438–442. doi: 10.1016/j.jacc.2005.04.041. [DOI] [PubMed] [Google Scholar]
- 22.Rittger H, Schertel B, Schmidt M, Justiz J, Brachmann J, Sinha AM. Three-dimensional reconstruction allows accurate quantification and length measurements of coronary artery stenoses. EuroIntervention. 2009;5:127–132. doi: 10.4244/eijv5i1a20. [DOI] [PubMed] [Google Scholar]
- 23.Saad M, Toelg R, Khattab AA, Kassner G, Abdel-Wahab M, Richardt G. Determination of haemodynamic significance of intermediate coronary lesions using three-dimensional coronary reconstruction. EuroIntervention. 2009;5:573–579. doi: 10.4244/eijv5i5a93. [DOI] [PubMed] [Google Scholar]
- 24.Briguori C, Anzuini A, Airoldi F, Gimelli G, Nishida T, Adamian M, Corvaja N, Di Mario C, Colombo A. Intravascular ultrasound criteria for the assessment of the functional significance of intermediate coronary artery stenoses and comparison with fractional flow reserve. Am J Cardiol. 2001;87:136–141. doi: 10.1016/s0002-9149(00)01304-7. [DOI] [PubMed] [Google Scholar]
- 25.Abizaid AS, Mintz GS, Mehran R, Abizaid A, Lansky AJ, Pichard AD, Satler LF, Wu H, Pappas C, Kent KM, et al. Long-term follow-up after percutaneous transluminal coronary angioplasty was not performed based on intravascular ultrasound findings: importance of lumen dimensions. Circulation. 1999;100:256–261. doi: 10.1161/01.cir.100.3.256. [DOI] [PubMed] [Google Scholar]
- 26.Kang SJ, Lee JY, Ahn JM, Mintz GS, Kim WJ, Park DW, Yun SC, Lee SW, Kim YH, Lee CW, et al. Validation of intravascular ultrasound-derived parameters with fractional flow reserve for assessment of coronary stenosis severity. Circ Cardiovasc Interv. 2011;4:65–71. doi: 10.1161/CIRCINTERVENTIONS.110.959148. [DOI] [PubMed] [Google Scholar]