Abstract
The evaluation of the quality of a sputum specimen prior to bacterial culture has been an accepted practice. However, optimal sputum criteria for pulmonary tuberculosis (TB) are not well established. We investigated indicators for sputum acceptability in tuberculosis cultures and acid-fast bacilli (AFB) smear. A post-hoc analysis of a randomized trial with 228 sputum specimens from 77 patients was conducted. In the trial, pulmonary TB suspects were requested for collecting three sputum specimens. We performed both TB study (AFB smear and M. tuberculosis culture) and Gram staining in each specimen. By using generalized estimating equations, the association between sputum characteristics and positive TB testings were analyzed. Although acceptable specimens for bacterial pneumonia showed higher TB-culture positive rates than unacceptable specimens (adjusted odds ratio [aOR]=1.66; 95% confidence interval [CI]=1.11-2.49), a specimen with ≥25 white blood cells/low-power field was the better predictor for positive M. tuberculosis cultures (aOR=2.30; 95% CI=1.48-3.58) and acid-fast bacilli smears (aOR=1.85; 95% CI=1.05-3.25). Sputum leukocytosis could be an indicator of sputum acceptability for diagnosing pulmonary tuberculosis.
Graphical Abstract
Keywords: Acceptable Sputum; Tuberculosis, Pulmonary; Sputum WBC
INTRODUCTION
Sputum specimens containing ≥10 leukocytes with mucus, but <25 squamous epithelial cells per low-power field (LPF, ×100), are unlikely to be contaminated by oropharyngeal flora and considered suitable for bacterial culture in patients with suspected bacterial pneumonia (1, 2).
According to the pulmonary tuberculosis (TB) guidelines, patients require instructions regarding the proper method of sputum collection (3). Patients need to be informed that a desired sputum specimen consists of material brought up from the lungs after a productive cough and not nasopharyngeal discharge or saliva. However, there is no clear consensus regarding the indicators for appropriate sputum specimens in diagnosing pulmonary TB. Furthermore, it has not been fully evaluated whether the sputum criteria for bacterial pneumonia is helpful for determining acceptable sputum specimens for diagnosing pulmonary TB. However, saliva-containing sputum specimens contribute to the diagnosis of TB (4, 5). Good indicators for the 'acceptable' specimen that can predict microbiological diagnosis of TB has not been fully evaluated.
We previously conducted a randomized trial to evaluate whether educating patients with a simple brochure was beneficial for obtaining an appropriate sputum sample for effectively diagnosing pulmonary TB (6). Herein, we investigated indicators for sputum acceptability in M. tuberculosis cultures.
MATERIALS AND METHODS
The randomized trial was conducted from January 2009 to July 2010 at a single center. Patients with suspected pulmonary TB were randomly allocated to 2 groups on the basis of whether they received the simple brochure with instructions on sputum collection: brochure group vs. non-brochure group. Patients were requested to collect 3 sputum specimens on consecutive days (6). Each sputum specimen was divided into 2 for the TB study (AFB smear and M. tuberculosis culture) and Gram staining. According to the Gram staining results, the specimens were graded using the Murray and Washington's system. Sputum specimens of grade 4 or 5 were considered acceptable for diagnosing bacterial pneumonia (1, 7, 8, 9).
Statistical analysis
We performed repeated measures logistic regression analyses using generalized-estimating equation (GEE) models (Stata command: xtgee) adjusted by age, sex, and brochure group (vs. the non-brochure group) to elucidate whether the sputum criteria are independently associated with positive M. tuberculosis cultures and positive AFB smears. The identification number of each participant was defined as the variable of repetitions. Statistical significance was determined at P<0.05. All analyses were performed using Stata 13.1 software (StataCorp, Texas, USA).
Ethics statement
The original study protocol (6) was approved by the institutional review board of Seoul Metropolitan Government Seoul National University Boramae Medical Center (number: 20090602/06-2009-73/84) and conducted in compliance with the Declaration of Helsinki. Written informed consent was obtained from all participants.
RESULTS
In total, 228 sputum specimens from 77 patients were analyzed. Approximately 34.2% of sputum specimens and 40.3% of patients were diagnosed with culture positive pulmonary TB; 25.4% of sputum specimens and 32.5% of patients showed positive AFB smears (Table 1).
Table 1. Characteristics of included patients and sputum specimen results for diagnosing pulmonary tuberculosis.
| Variables | Values |
|---|---|
| Patients, No. | 77 |
| Sputum specimens, No. | 228 |
| mean (±SD) | 2.96 (±0.49) |
| Age, median (range) | 55 (18-88) |
| Male, No. (%) | 54 (70.1) |
| History of tuberculosis, No. (%) | 27 (35.1) |
| History of smoking, No. (%) | |
| Non-smoker | 48 (62.3) |
| Current smoker | 18 (23.4) |
| Ex-smoker | 11 (14.3) |
| Cavitary lesion in X-ray/CT | 20(26.0) |
| Positive TB culture | |
| Sputum, No. (%) | 78 (34.2) |
| Patients, No. (%) | 31 (40.3) |
| Positive AFB smear | |
| Sputum, No. (%) | 58 (25.4) |
| Patients, No. (%) | 25 (32.5) |
TB, tuberculosis; AFB, acid-fact bacilli; SD, standard deviation.
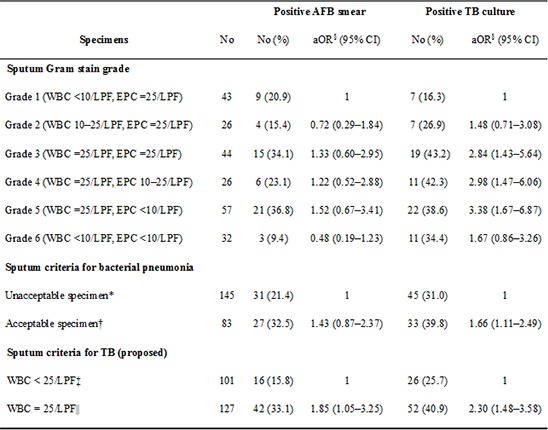
Sputum specimens with grade 3-5 were independent predictors of positive M. tuberculosis cultures in GEE models (grade 3: adjusted adds ratio [aOR], 2.84; 95% confidence interval [CI], 1.43-5.64; grade 4: aOR, 2.98; 95% CI, 1.47-6.06; grade 5: aOR, 3.38; 95% CI, 1.67-6.87). Therefore, we defined the sputum criteria for TB as specimens with grade 3-5 and a white blood cell (WBC) count of ≥25 cells/LPF.
The sputum criteria for bacterial pneumonia (grade 4 or 5) were significantly associated with positive M. tuberculosis cultures (aOR, 1.66; 95% CI, 1.11-2.49), but not positive AFB smears (aOR, 1.43; 95% CI, 0.87-2.37). The sputum criteria for TB that we newly proposed were independently associated with both positive M. tuberculosis cultures (aOR, 2.30; 95% CI, 1.48-3.58) and positive AFB smears (aOR, 1.85; 95% CI, 1.05-3.25; Table 2).
Table 2. Multivariate logistic regression model for the prediction of AFB smear and tuberculosis culture positivity.
| Specimens | No. | Positive AFB smear | Positive TB culture | ||
|---|---|---|---|---|---|
| No. (%) | aOR§ (95% CI) | No. (%) | aOR§ (95% CI) | ||
| Sputum gram stain grade | |||||
| Grade 1 (WBC<10/LPF, EPC≥25/LPF) | 43 | 9 (20.9) | 1 | 7 (16.3) | 1 |
| Grade 2 (WBC 10-25/LPF, EPC≥25/LPF) | 26 | 4 (15.4) | 0.72 (0.29-1.84) | 7 (26.9) | 1.48 (0.71-3.08) |
| Grade 3 (WBC≥25/LPF, EPC≥25/LPF) | 44 | 15 (34.1) | 1.33 (0.60-2.95) | 19 (43.2) | 2.84 (1.43-5.64) |
| Grade 4 (WBC≥25/LPF, EPC 10-25/LPF) | 26 | 6 (23.1) | 1.22 (0.52-2.88) | 11 (42.3) | 2.98 (1.47-6.06) |
| Grade 5 (WBC≥25/LPF, EPC<10/LPF) | 57 | 21 (36.8) | 1.52 (0.67-3.41) | 22 (38.6) | 3.38 (1.67-6.87) |
| Grade 6 (WBC<10/LPF, EPC<10/LPF) | 32 | 3 (9.4) | 0.48 (0.19-1.23) | 11 (34.4) | 1.67 (0.86-3.26) |
| Sputum criteria for bacterial pneumonia | |||||
| Unacceptable specimen* | 145 | 31 (21.4) | 1 | 45 (31.0) | 1 |
| Acceptable specimen† | 83 | 27 (32.5) | 1.43 (0.87-2.37) | 33 (39.8) | 1.66 (1.11-2.49) |
| Sputum criteria for TB (proposed) | |||||
| WBC<25/LPF‡ | 101 | 16 (15.8) | 1 | 26 (25.7) | 1 |
| WBC≥25/LPF∥ | 127 | 42 (33.1) | 1.85 (1.05-3.25) | 52 (40.9) | 2.30 (1.48-3.58) |
*Unacceptable specimen (Gram stain grade 1, 2, 3, and 6); †Acceptable specimen (Gram stain grade 4 and 5); ‡WBC<25/LPF (Gram stain grade 1, 2, and 6); §adjusted for age, sex, and education group; ∥WBC≥25/LPF (Gram stain grade 3,4, and 5). aOR, adjusted odds ratio; CI, confidence interval; AFB, acid-fast bacilli; TB, tuberculosis; WBC, white blood cell; LPF, low-power field; EPC, squamous epithelial cells.
When all sputum specimens (n=3) showed a WBC count of ≥25 cells/LPF, the percentages of positive M. tuberculosis cultures and positive AFB smears were both 48.1%. However, when all specimens showed a WBC count of <25 cells/LPF, only 30.0% of the specimens had positive M. tuberculosis cultures and 30.0% of the specimens had positive AFB smears (Table 3).
Table 3. Positive rate of acid-fast bacilli smears and tuberculosis cultures in patients with ≥ 3 consecutive sputum specimens according to the WBC count.
| WBC counts | Patients n |
Positive AFB smear n (%) |
Positive TB culture n (%) |
|---|---|---|---|
| All specimens with WBC≥25/LPF | 27 | 13 (48.1) | 13 (48.1) |
| 1 or 2 specimens with WBC≥25/LPF | 25 | 5 (20.0) | 9 (36.0) |
| All specimens with WBC<25/LPF | 20 | 6 (30.0) | 6 (30.0) |
WBC, white blood cell; LPF, low-power field.
In a sensitivity analysis using 91 specimens from 31 patients with microscopically confirmed TB (Table 4), the specimens with WBC >25 showed statistically higher positive AFB-stain (64.3% vs. 37.1% in the group with WBC>25/LPF, P=0.012) and M. tuberculosis culture rates (92.9% vs. 74.3%, P=0.014). The multiple regression model adjusted for age, gender, and education showed similar results. However, sputum criteria for bacterial pneumonia failed to show a statistical significance in this sensitivity analysis (Table 4). Specimen with a WBC count of ≥25 cells/LPF was not significantly associated with the extent of lesion and the presence of cavity in chest X-ray.
Table 4. Sensitivity analysis for AFB smear and TB culture positivity in microscopically confirmed patients.
| Specimens | n | Positive AFB smear | Positive TB culture | ||
|---|---|---|---|---|---|
| n (%) | aOR§ (95% CI) | n (%) | aOR§ (95% CI) | ||
| Sputum criteria for bacterial pneumonia | |||||
| Unacceptable specimen* | 56 | 27 (48.2) | 1 | 45 (80.4) | 1 |
| Acceptable specimen† | 35 | 22 (62.9) | 1.9 (0.79-4.56) | 33 (94.3) | 4.6(0.93-22.97) |
| Sputum criteria for TB (proposed) | |||||
| WBC<25/LPF‡ | 35 | 13 (37.1) | 1 | 26 (74.3) | 1 |
| WBC≥25/LPF∥ | 56 | 36 (64.3) | 3.46 (1.38-8.69) | 52 (92.9) | 5.30 (1.40-20.4) |
*Unacceptable specimen (Gram stain grade 1, 2, 3, and 6); †Acceptable specimen (Gram stain grade 4 and 5); ‡WBC<25/LPF (Gram stain grade 1, 2, and 6); §adjusted for age, sex, and education group; ∥WBC≥25/LPF (Gram stain grade 3,4, and 5). aOR, adjusted odds ratio; CI, confidence interval; AFB, acid-fast bacilli; TB, tuberculosis; WBC, white blood cell; LPF, low-power field; EPC, squamous epithelial cells.
DISCUSSION
Sputum leukocytosis was a good indicator for positive M. tuberculosis cultures and positive AFB smears in patents with suspected pulmonary TB. We proposed a new sputum criterion for TB, defined as a sputum specimen with a WBC count of ≥25 cells/LPF, which was a good predictor of positive M. tuberculosis cultures and positive AFB smears; the criteria for bacterial pneumonia were also able to predict positive M. tuberculosis culture.
True pathogens and oropharyngeal (i.e., saliva) contamination should be differentiated when diagnosing bacterial pneumonia. Therefore, an acceptable sputum specimen with a high WBC and low epithelial cell count would be a good indicator of sputum quality for diagnosing bacterial pneumonia (1).
We identified an association between sputum leukocytosis and mycobacterial positivity, which corresponds with findings from a previous retrospective study. McCarter et al. (10) evaluated 665 sputum specimens and showed that a total of 51 (7.0%) primary smears and 121 (16.7%) bacterial cultures were positive for mycobacteria. Approximately 92.2% of the positive smears and 90.1% of the positive cultures from these specimens contained neutrophils. Although we did not analyze the sputum specimens according to WBC counts, all specimens with positive AFB staining (n=58) and positive M. tuberculosis culture (n=77) had a WBC count of >10 cells/LPF. No specimens with a positive AFB stain and positive M. tuberculosis culture had a WBC count of <10 cells/LPF.
Purulent sputum is associated with more neutrophils, a higher degree of inflammation, and bacterial isolation (11, 12, 13). Furthermore, the gross appearance of sputum in patients with suspected TB may contribute to the increase in smear positivity (14, 15, 16, 17). Therefore, sputum specimens with higher WBC counts might reflect severe inflammation and greater mycobacterial counts.
According to our results, sputum leukocytosis might be an effective indicator for evaluating sputum acceptability for pulmonary TB diagnosis. We cautiously suggest that the instructions for patients indicate "purulent sputum" instead of "no saliva" (3).
However, this study does not mean that sputum specimens with WBC <25/LPF should be discarded to diagnose tuberculosis. M. tuberculosis in sputum is rarely contaminated (13). In a study by Isaac-Renton et al. (4), salivary specimens yielded 30.9% of the total 42 M. tuberculosis isolates and 19% of the 21 positive smear and culture specimens. The concept of 'acceptability' in this study is 'an indicator related to positive tuberculosis' and sputum WBC count could be the indicator. It is not easy to perform Gram staining to evaluate acceptability of sputum for TB in clinical practice. Therefore, further investigations regarding this are needed.
In conclusion, a WBC count of >25 cells/LPF with a positive Gram stain could be an indicator for appropriate sputum specimens for diagnosing pulmonary TB and further large study is needed.
Footnotes
DISCLOSURE: The authors declare that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
AUTHOR CONTRIBUTION: Conception and design: Lee YJ, Lee CH, Kim DK, Chung HS. Acquisition of data, drafting, revision: Lee YJ, Lee CH, Roh EY, Yoon JH. Interpretation of data: Kim DK, Chung HS. Approval of manuscript and submission: All authors.
References
- 1.Murray PR, Washington JA. Microscopic and baceriologic analysis of expectorated sputum. Mayo Clin Proc. 1975;50:339–344. [PubMed] [Google Scholar]
- 2.Rosón B, Carratalà J, Verdaguer R, Dorca J, Manresa F, Gudiol F. Prospective study of the usefulness of sputum Gram stain in the initial approach to community-acquired pneumonia requiring hospitalization. Clin Infect Dis. 2000;31:869–874. doi: 10.1086/318151. [DOI] [PubMed] [Google Scholar]
- 3.Diagnostic Standards and Classification of Tuberculosis in Adults and Children. This official statement of the American Thoracic Society and the Centers for Disease Control and Prevention was adopted by the ATS Board of Directors, July 1999. This statement was endorsed by the Council of the Infectious Disease Society of America, September 1999. Am J Respir Crit Care Med. 2000;161:1376–1395. doi: 10.1164/ajrccm.161.4.16141. [DOI] [PubMed] [Google Scholar]
- 4.Isaac-Renton JL, Puselja BB, Allen EA, Grzybowski S, Black WA. Microscopic evaluation of sputum specimens submitted for Mycobacterium tuberculosis culture. Am J Clin Pathol. 1985;84:361–363. doi: 10.1093/ajcp/84.3.361. [DOI] [PubMed] [Google Scholar]
- 5.Curione CJ, Jr, Kaneko GS, Voss JL, Hesse F, Smith RF. Gram stain evaluation of the quality of sputum specimens for mycobacterial culture. J Clin Microbiol. 1977;5:381–382. doi: 10.1128/jcm.5.3.381-382.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee YJ, Shin S, Roh EY, Yoon JH, Kim DK, Chung HS, Lee CH. The effectiveness of a brochure describing an acceptable method of sputum collection for tuberculosis testing. Int J Tuberc Lung Dis. 2013;17:1587–1589. doi: 10.5588/ijtld.13.0336. [DOI] [PubMed] [Google Scholar]
- 7.Vieira MO, Pizzichini E, Steidle LJ, da Silva JK, Pizzichini MM. Sputum induction in severe exacerbations of asthma: safety of a modified method. Eur Respir J. 2011;38:979–980. doi: 10.1183/09031936.00029511. [DOI] [PubMed] [Google Scholar]
- 8.Veras TN, Pizzichini E, Steidle LJ, Rocha CC, Moritz P, Pizzichini MM. Cellular composition of induced sputum in healthy adults. J Bras Pneumol. 2011;37:348–353. doi: 10.1590/s1806-37132011000300011. [DOI] [PubMed] [Google Scholar]
- 9.Singh D, Edwards L, Tal-Singer R, Rennard S. Sputum neutrophils as a biomarker in COPD: findings from the ECLIPSE study. Respir Res. 2010;11:77. doi: 10.1186/1465-9921-11-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCarter YS, Robinson A. Quality evaluation of sputum specimens for mycobacterial culture. Am J Clin Pathol. 1996;105:769–773. doi: 10.1093/ajcp/105.6.769. [DOI] [PubMed] [Google Scholar]
- 11.Anil N, Singh M, Rajwanshi A, Vohra H. Induced sputum nitrites correlate with FEV1 in children with cystic fibrosis. Acta Paediatr. 2010;99:711–714. doi: 10.1111/j.1651-2227.2010.01682.x. [DOI] [PubMed] [Google Scholar]
- 12.Liesker JJ, Bathoorn E, Postma DS, Vonk JM, Timens W, Kerstjens HA. Sputum inflammation predicts exacerbations after cessation of inhaled corticosteroids in COPD. Respir Med. 2011;105:1853–1860. doi: 10.1016/j.rmed.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 13.Burman WJ, Reves RR. Review of false-positive cultures for Mycobacterium tuberculosis and recommendations for avoiding unnecessary treatment. Clin Infect Dis. 2000;31:1390–1395. doi: 10.1086/317504. [DOI] [PubMed] [Google Scholar]
- 14.Alisjahbana B, van Crevel R, Danusantoso H, Gartinah T, Soemantri ES, Nelwan RH, van der Meer JW. Better patient instruction for sputum sampling can improve microscopic tuberculosis diagnosis. Int J Tuberc Lung Dis. 2005;9:814–817. [PubMed] [Google Scholar]
- 15.Khan MS, Dar O, Sismanidis C, Shah K, Godfrey-Faussett P. Improvement of tuberculosis case detection and reduction of discrepancies between men and women by simple sputum-submission instructions: a pragmatic randomised controlled trial. Lancet. 2007;369:1955–1960. doi: 10.1016/S0140-6736(07)60916-7. [DOI] [PubMed] [Google Scholar]
- 16.Hirooka T, Higuchi T, Tanaka N, Ogura T. The value of proper sputum collection instruction in detection of acid-fast bacillus. Kekkaku. 2004;79:33–37. [PubMed] [Google Scholar]
- 17.Yoon SH, Lee NK, Yim JJ. Impact of sputum gross appearance and volume on smear positivity of pulmonary tuberculosis: a prospective cohort study. BMC Infect Dis. 2012;12:172. doi: 10.1186/1471-2334-12-172. [DOI] [PMC free article] [PubMed] [Google Scholar]


