Abstract
IgG4-related disease (IgG4-RD) is a potentially multiorgan disorder. In this study, clinical and serological features from 132 IgG4-RD patients were compared about organ correlations. Underlying pathologies comprised autoimmune pancreatitis (AIP) in 85 cases, IgG4-related sclerosing cholangitis (IgG4-SC) in 12, IgG4-related sialadenitis (IgG4-SIA) in 56, IgG4-related dacryoadenitis (IgG4-DAC) in 38, IgG4-related lymphadenopathy (IgG4-LYM) in 20, IgG4-related retroperitoneal fibrosis (IgG4-RF) in 19, IgG4-related kidney disease (IgG4-KD) in 6, IgG4-related pseudotumor (IgG4-PT) in 3. Sixty-five patients (49%) had multiple IgG4-RD (two affected organs in 36 patients, three in 19, four in 8, five in 1, and six in 1). Serum IgG4 levels were significantly higher with multiple lesions than with a single lesion (P<0.001). The proportion of association with other IgG4-RD was 42% in AIP, the lowest of all IgG4-RDs. Serum IgG4 level was lower in AIP than in other IgG4-RDs. Frequently associated IgG4-RDs were SIA (25%) and DAC (12%) for AIP; AIP (75%) for IgG4-SC; DAC (57%), AIP (38%) and LYM (27%) for IgG4-SIA; AIP (26%) and LYM (26%) for IgG4-DAC; SIA (75%), DAC (50%) and AIP (45%) for IgG4-LYM; SIA (58%), AIP (42%) and LYM (32%) for IgG4-RF; AIP (100%) and SIA (67%) for IgG4-KID; and DAC (67%) and SIA (67%) for IgG4-PT. Most associated IgG4-RD lesions were diagnosed simultaneously, but IgG4-SIA and IgG4-DAC were sometimes identified before other lesions. About half of IgG4-RD patients had multiple IgG4-RD lesions, and some associations were seen between specific organs.
Graphical Abstract
Keywords: IgG4; Pancreatitis; Cholangitis, Sclerosing
INTRODUCTION
IgG4-related disease (IgG4-RD) is a newly recognized concept presenting a potentially multiorgan disorder. IgG4-RD is a fibroinflammatory condition characterized by a tendency toward the formation of tumefactive lesions, elevated serum IgG4 levels, abundant infiltration of IgG4-positive plasma cells and lymphocytes with fibrosis, and steroid responsiveness (1, 2, 3). The pancreas was the first organ in which IgG4-RD was identified, but the disease has now been described in virtually every organ system, including the biliary tract, salivary glands, lacrimal glands, kidneys, lungs, lymph nodes, and retroperitoneum. Clinical manifestations are identified in single organ in some cases, whereas others show effects on two or more organs simultaneously or metachronously (4, 5).
Although many reports have described the clinical and pathological characteristics of individual IgG4-RDs, no investigations appear to have discussed correlations between organs involved in IgG4-RD. In this study, clinical and serological features of IgG4-RD were retrospectively examined with a focus on correlations between affected organs.
MATERIALS AND METHODS
Participants in this retrospective study were 132 patients diagnosed with and managed for IgG4-RD at Tokyo Metropolitan Komagome Hospital from 1991 to 2013. The underlying pathologies were autoimmune pancreatitis (AIP) in 85 cases, IgG4-related sclerosing cholangitis (IgG4-SC) in 12, IgG4-related sialadenitis (IgG4-SIA) in 56, IgG4-related dacryoadenitis (IgG4-DAC) in 38, IgG4-related lymphadenopathy (IgG4-LYM) in 20, IgG4-related retroperitoneal fibrosis (IgG4-RF) in 19, IgG4-related kidney disease (IgG4-KD) in 6, IgG4-related pseudotumor (IgG4-PT) in 3 (pleura, n=1; breast, n=1; and dura, n=1). Diagnosis was established by a multidisciplinary approach including imaging, serological, and pathological tests. When the patient was diagnosed as having one IgG4-RD, systemic examination by ultrasonography and computed tomography (CT) and/or magnetic resonance imaging were prospectively performed in each patient. Although whole-body 18F-fluorodeoxyglucose (FDG)-PET was performed in 10 AIP patients, findings from FDG-PET were not included in this study. Histopathological examinations of resected or biopsy specimens were performed for 64 organs.
All patients met definite, probable or possible criteria in the following diagnostic criteria. AIP type 1 was diagnosed using international consensus diagnostic criteria (6). IgG4-related SC was diagnosed using diagnostic criteria for IgG4-related SC 2012 (7), when the stenosis was located in the hilar and/or intrahepatic bile duct and limited intrapancreatic bile duct stricture associated with AIP was excluded. IgG4-related RF was diagnosed using our previously proposed criteria (8). IgG4-related KD was diagnosed using the diagnostic criteria for IgG4-related KD (9). All other IgG4-RDs were diagnosed using comprehensive diagnostic criteria for IgG4-RD (2). IgG4-LYM was diagnosed when lymph node swellings occurred at multiple sites, such as the neck, mediastinum, axilla and abdomen. Clinical and serological features of each IgG4-RD were compared to clarify organ correlations.
For statistical analysis, the Mann-Whitney U test and unpaired t-test were used. When repeated comparisons were made, P values were corrected using the Bonferroni method. Values of P<0.05 were considered statistically significant.
Ethics statement
This study was approved by the institutional review board of Tokyo Metropolitan Komagome Hospital (IRB No. 7299). Informed consent for invasive modalities and comprehensive informed consent for the study had been obtained.
RESULTS
Of the 132 IgG4-RD patients, diagnoses in 111 patients were classified as definite, 3 as probable, and 18 as possible (Table 1).
Table 1. Diagnosis of IgG4-related disease.
| Diseases | No. of cases | Definite (%) | Probable (%) | Possible (%) |
|---|---|---|---|---|
| IgG4-RD | 132 | 111 (84) | 3 (2) | 18 (14) |
| AIP | 85 | 72 (85) | 3 (3) | 10 (12) |
| SC | 12 | 11 (92) | 0 | 1 (8) |
| DAC | 38 | 36 (95) | 0 | 2 (5) |
| SIA | 56 | 50 (89) | 0 | 6 (11) |
| LYM | 20 | 19 (95) | 0 | 1 (5) |
| RF | 19 | 17 (89) | 0 | 2 (11) |
| KD | 6 | 6 (100) | 0 | 0 |
| PT | 3 | 3 (100) | 0 | 0 |
AIP, autoimmune pancreatitis; SC, IgG4-related sclerosing cholangitis; DAC, IgG4-related dacryoadenitis; SIA, IgG4-related sialadenitis; LYM, IgG4-related lymphadenopathy; RF, IgG4-related retroperitoneal fibrosis; KD, IgG4-related kidney disease; PT, IgG4-related pseudotumor.
Sixty-seven patients had only one IgG4-RD, while the remaining 65 (49%) showed multiple IgG4-RD (two diseases in 36 patients, three diseases in 19, four diseases in 8, five diseases in 1, and six diseases in 1). Serum IgG4 levels were significantly higher in patients with multiple lesions (mean, 783.2 mg/dL) than in those with a single lesion (307.4 mg/dL, P<0.001) (Table 2).
Table 2. Comparison of clinical and serological features between single and multiple lesions.
| Parameters | Single lesion | Double lesions | Triple lesions | Four or more lesions |
|---|---|---|---|---|
| No. | 67 | 36 | 19 | 10 |
| Male:Female | 46:21 | 22:14 | 11:8 | 8:2 |
| IgG4 (mg/dL) | 307.4 | 655.2* | 930.9* | 976.6* |
| IgG (mg/dL) | 2,003.6 | 2,190.9 | 2,432.8 | 2,644.2 |
*P<0.001 compared to single lesion. Serum IgG4 levels were significantly higher in patients with multiple lesions (783.2 mg/dL) than in those with a single lesion (P<0.01).
The proportion of association with other IgG4-RDs was 42% for AIP, the lowest for all IgG4-RDs. Serum IgG4 level was lower in AIP (491 mg/dL) than in other IgG4-RDs. Steroid therapy was performed mainly for symptomatic IgG4-RD patients (62%) (Table 3).
Table 3. Clinical feature of each organ for IgG4-related disease.
| Features | IgG4-RD | AIP | SC | DAC | SIA | LYM | RF | KID | PT |
|---|---|---|---|---|---|---|---|---|---|
| No. association | 132 | 85 | 12 | 38 | 56 | 20 | 19 | 6 | 3 |
| Single lesion | 67 | 49 | 3 | 3 | 7 | 2 | 2 | 0 | 1 |
| Multiple lesion | 65 | 36 | 9 | 35 | 49 | 18 | 17 | 6 | 2 |
| Double lesions | 36 | 17 | 6 | 16 | 24 | 2 | 6 | 1 | 0 |
| Triple lesions | 19 | 13 | 2 | 10 | 15 | 9 | 6 | 2 | 0 |
| Four lesions | 8 | 4 | 1 | 8 | 8 | 5 | 3 | 2 | 1 |
| Five lesions | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 |
| Six lesions | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 |
| Rate (%) | 0 | 42 | 75 | 92 | 88 | 90 | 89 | 100 | 67 |
| Serum levels | |||||||||
| IgG4 (mg/dL) | 548.3 | 491.0 | 568.0 | 724.8 | 821.3 | 979.0 | 769.1 | 524.5 | 600.0 |
| IgG (mg/dL) | 2,094.2 | 2,082.0 | 2,402.0 | 2,107.8 | 2,432.8 | 2,687.4 | 2,526.8 | 1,838.3 | 1,967.0 |
| Treatment | |||||||||
| Steroid | 82 | 60 (71%) | 10 (83%) | 21 (55%) | 30 (54%) | 7 (35%) | 13 (68%) | 5 (83%) | 1 (33%) |
| Observation | 36 | 15 | 1 | 17 | 22 | 12 | 6 | 1 | 0 |
| Operation | 14 | 10 | 1 | 0 | 4 | 1 | 0 | 0 | 2 |
AIP, autoimmune pancreatitis; SC, IgG4-related sclerosing cholangitis; DAC, IgG4-related dacryoadenitis; SIA, IgG4-related sialadenitis; LYM, IgG4-related lymphadenopathy; RF, IgG4-related retroperitoneal fibrosis; KD, IgG4-related kidney disease; PT, IgG4-related pseudotumor.
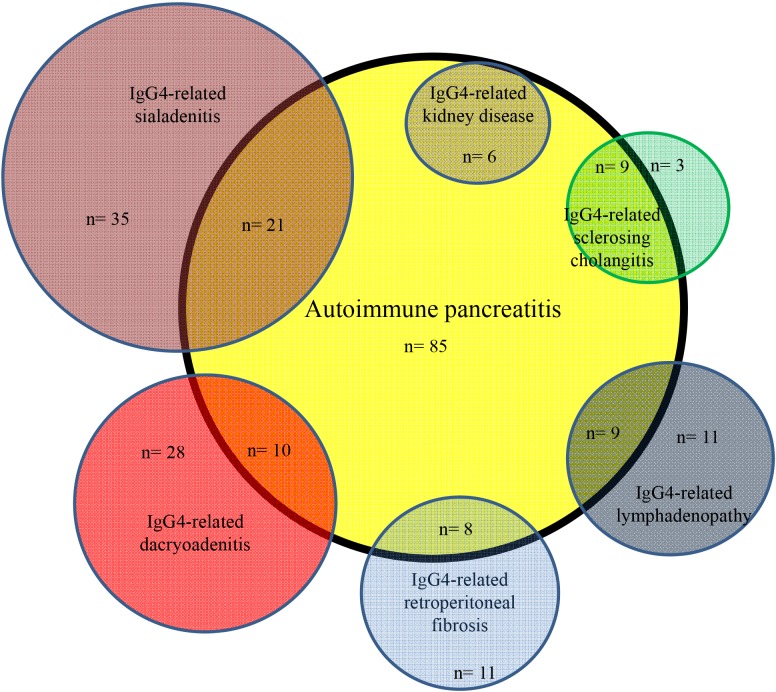
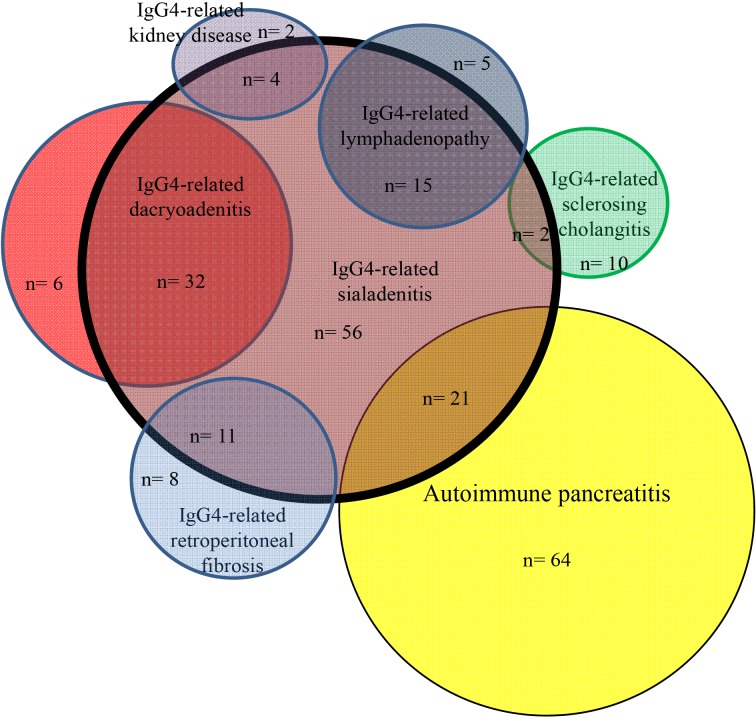
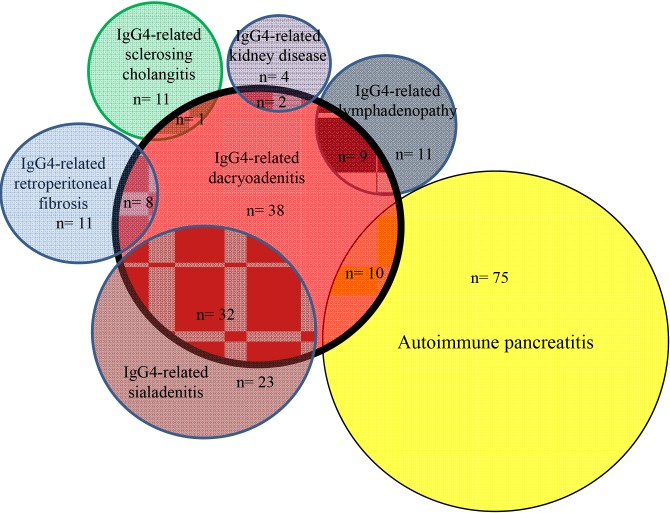
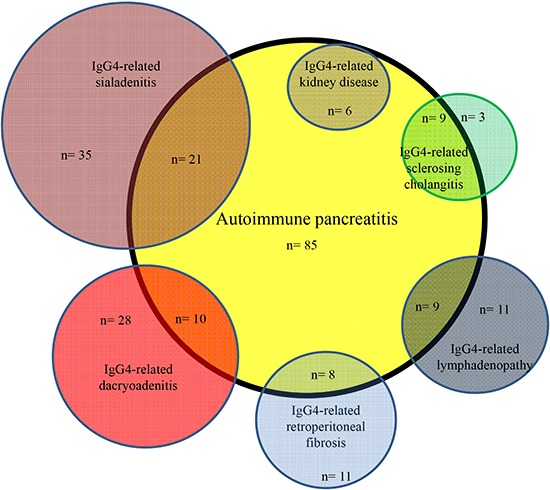
Frequently associated IgG4-RDs were: SIA (25%) and DAC (12%) in AIP; AIP (75%) in IgG4-SC; DAC (57%), AIP (38%) and LYM (27%) in IgG4-SIA; SIA (84%), AIP (26%) and LYM (26%) in IgG4-DAC; SIA (75%), DAC (50%) and AIP (45%) in IgG4-LYM; SIA (58%), AIP (42%) and LYM (32%) in IgG4-RF; AIP (100%) and SIA (67%) in IgG4-KID; and DAC (67%) and SIA (67%) in IgG4-PT (Fig. 1, 2, 3). Most associated IgG4-RD lesions were diagnosed simultaneously, but IgG4-DAC and IgG4-SIA were sometimes identified before other lesions (Table 4).
Fig. 1. Venn diagram showing correlation between autoimmune pancreatitis and associated other IgG4-related diseases.
Fig. 2. Venn diagram showing correlation between IgG4-related sialadenitis and associated other IgG4-related diseases.
Fig. 3. Venn diagram showing correlation between IgG4-related dacryoadenitis and associated other IgG4-related diseases.
Table 4. Synchronous and metachronous association of other IgG4-related diseases.
| Associated IgG4-RD | AIP (n=85) | SC (n=12) | DAC (n=38) | SIA (n=56) | LYM (n=20) | RF (n=19) | KID (n=6) | PT (n=3) | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n (%) | B | S | A | n (%) | B | S | A | n (%) | B | S | A | n (%) | B | S | A | n (%) | B | S | A | n (%) | B | S | A | n (%) | B | S | A | n (%) | B | S | A | |
| AIP | 9 (75) | 0 | 9 | 0 | 10 (26) | 1 | 5 | 4 | 21 (38) | 2 | 14 | 5 | 9 (45) | 0 | 7 | 2 | 8 (42) | 1 | 6 | 1 | 6 (100) | 0 | 6 | 0 | 1 (33) | 0 | 1 | 0 | ||||
| SC | 9 (11) | 0 | 9 | 0 | 1 (3) | 0 | 1 | 0 | 2 (4) | 0 | 1 | 1 | 1 (5) | 0 | 1 | 0 | 0 (0) | 0 | 0 | 0 | 0 (0) | 0 | 0 | 0 | 0 (0) | 0 | 0 | 0 | ||||
| DAC | 10 (12) | 4 | 5 | 1 | 1 (8) | 0 | 1 | 0 | 32 (57) | 3 | 27 | 2 | 10 (50) | 0 | 9 | 1 | 8 (43) | 3 | 3 | 0 | 2 (33) | 0 | 2 | 0 | 1 (33) | 1 | 0 | 0 | ||||
| SIA | 21 (25) | 5 | 14 | 2 | 2 (17) | 0 | 1 | 0 | 32 (84) | 2 | 27 | 3 | 15 (75) | 2 | 12 | 1 | 11 (58) | 3 | 8 | 0 | 4 (67) | 0 | 4 | 0 | 2 (67) | 0 | 2 | 0 | ||||
| LYM | 9 (10) | 2 | 7 | 0 | 1 (8) | 0 | 1 | 0 | 10 (26) | 1 | 9 | 0 | 15 (27) | 1 | 12 | 2 | 6 (32) | 1 | 5 | 0 | 1 (17) | 0 | 1 | 0 | 2 (67) | 0 | 2 | 0 | ||||
| RF | 8 (9) | 1 | 6 | 1 | 0 (0) | 0 | 0 | 0 | 8 (21) | 0 | 5 | 3 | 11 (20) | 0 | 8 | 3 | 6 (3) | 0 | 5 | 1 | 2 (33) | 0 | 2 | 0 | 1 (33) | 0 | 1 | 0 | ||||
| KID | 6 (7) | 0 | 6 | 0 | 0 (0) | 0 | 0 | 0 | 2 (5) | 0 | 2 | 0 | 4 (7) | 0 | 4 | 0 | 1 (5) | 0 | 1 | 0 | 2 (11) | 0 | 2 | 0 | 1 (33) | 0 | 1 | 0 | ||||
| PT | 1 (1) | 0 | 1 | 0 | 0 (0) | 0 | 0 | 0 | 1 (3) | 0 | 0 | 1 | 2 (4) | 0 | 2 | 0 | 2 (10) | 0 | 2 | 0 | 1 (5) | 0 | 1 | 0 | 1 (17) | 0 | 1 | 0 | ||||
B, before; S, synchronous; A, after; AIP, autoimmune pancreatitis; SC, IgG4-related sclerosing cholangitis; DAC, IgG4-related dacryoadenitis; SIA, IgG4-related sialadenitis; LYM, IgG4-related lymphadenopathy; RF, IgG4-related retroperitoneal fibrosis; KD, IgG4-related kidney disease; PT, IgG4-related pseudotumor.
DISCUSSION
IgG4-RD can occur, either synchronously or metachronously, in a variety of organs throughout the body. Clinical symptoms of IgG4-RD depend on the location of the lesion (1, 2, 3, 4, 5). However, the prevalence, distribution and correlation of each IgG4-RD are unknown.
This is the first study to clarify correlations between affected organs in IgG4-RD patients. A total of 49% of the 132 IgG4-RD patients had multiple IgG4-RDs. The proportion of association with another IgG4-RD was 42% in AIP, but ≥75% in other IgG4-RDs. Proportions of association with other IgG4-RD in AIP have been reported as 23% (10) and 63% (11), but the latter study included diabetes and rheumatoid arthritis as other organ involvements. According to the report by Hamano et al. (12), the proportion of association among AIP patients was as high as 91%, but they included hilar lymph node swelling (80%) examined by gallium scintigraphy as extrapancreatic lesions of AIP. In our study, the other IgG4-RD pathology most commonly associated with AIP was IgG4-SIA (25%), followed by IgG4-DAC (12%), IgG4-SC (11%), IgG4-LYM (10%), IgG4-RF (9%), and IgG4-KD (7%). According to a nationwide survey of AIP in Japan, prevalence of other organ involvements in AIP patients was 53% for SC, 14% for SIA, 13% for LYM, 8% for RF, and 7% for DAC, but SC restricted to the lower bile duct was included among extrapancreatic lesions in that survey (13). The present study excluded SC restricted to the lower bile duct from IgG4-RD associated with AIP, as this pathology is sometimes affected by inflammation of the pancreatic head in AIP. The other prevalence is broadly comparable to our own results.
AIP was present in 75% of IgG4-SC, and isolated IgG4-SC was rare. IgG4-DAC and AIP were associated in 57% and 38% of patients with IgG4-SIA, respectively, and IgG4-SIA and AIP were present in 84% and 26% of patients with IgG4-DAC, respectively. Symmetrical swelling of the salivary and/or lacrimal glands has been recognized as Mikulicz's disease, and swelling of salivary glands and lacrimal glands is thus known to frequently occur together. In the study by Moriyama et al. (14) into Mikulicz's disease, swelling of the salivary glands was detected in 83% of patients with swelling of the lacrimal glands, and swelling of the lacrimal glands was detected in 28% of patients with swelling of the salivary glands. Swelling of both salivary and lacrimal glands was also reportedly detected in 14% and 7% of AIP patients (13). IgG4-SIA and IgG4-DAC were associated in 75% and 50% of patients with IgG4-LYM. Salivary and lacrimal gland swelling were detected in 33% of patients with systemic IgG4-related lymphadenopathy (15), and swelling of the lacrimal glands was detected in all 3 patients with systemic IgG4-related lymphadenopathy (16). Although IgG4-SIA and AIP were associated in 58% and 42% of our patients with IgG4-related RF, respectively, IgG4-SIA, IgG4-DAC and AIP were reportedly associated only in 6% of 17 patients with IgG4-RF, respectively (17). AIP and IgG4-SIA were seen in 100% and 67% of our IgG4-KD patients. According to the report by Saeki et al. (18), IgG4-SIA, IgG4-DAC and AIP were present in 74%, 26%, and 35% of 43 patients with IgG4-KD. Although each IgG4-RD is frequently associated with other IgG4-RD, some patterns of association seem to exist between specific organs (Fig. 1, 2, 3). IgG4-SC is likely to be present with AIP, IgG4-SIA and IgG4-DAC are likely to coexist, and IgG4-LYM is likely to be associated with IgG4-DAC and IgG4-SIA.
Whole-body 18F-FDG PET is useful for detecting IgG4-RD lesions (19, 20). According to a study examining the utility of FDG-PET/CT for diagnosing IgG4-RD, 97% of 35 patients with IgG4-RD had more than one organ and 68% showed the involvement of three or more organs (20). In that study, IgG4-LYM, IgG4-SIA and AIP were involved in 86%, 66%, and 51% of the 35 patients. Our study did not include FDG-PET findings, due to the small number of cases examined. The detection rate for IgG4-RD lesions appears rather different according to imaging modalities used to screen for other IgG4-RD lesions.
Most associated IgG4-RD lesions were diagnosed simultaneously with screening of associated other IgG4-RDs, but IgG4-DAC, and IgG4-SIA were sometimes identified before other lesions. Whether the onset periods for each lesion differ between IgG4-RDs is unclear. However, compared with AIP or RF, swelling of the salivary or lacrimal glands is easily noticed even in the absence of symptoms. When IgG4-SIA or IgG4-DAC is diagnosed, other IgG4-RDs such as AIP or RF might be present at a subclinical level.
Mean serum IgG4 level in AIP patients was 491 mg/dL, the lowest of all the IgG4-RDs. Mean serum IgG4 level in patients with Mikulicz's disease has been reported as 1,110.0 mg/dL (21), and AIP patients with salivary or lacrimal gland involvement show higher serum IgG4 levels than those without (12, 22). However, at this time, it is difficult to determine which organ involvement led to the elevation of serum IgG4 because there are cases with many complications. AIP patients with other IgG4-RD are also reported to have higher serum IgG4 levels than those without, reflecting higher disease activity (12).
There are some limitations of this study that must be considered when interpreting the results. First limitation is that the study was retrospective and we could not include FDG-PET findings. Second is that bias might be exist due to our special area of gastroenterology. Third is that identical screening method was not applied to all the patients due to the long duration of enrolment period. However, this represents the first study to assess organ correlation in IgG4-RDs.
In conclusion, about half of IgG4-RD patients displayed multiple IgG4-RD lesions, and some patterns of association appear to exist between specific affected organs.
Footnotes
DISCLOSURE: The authors declare that they have no conflicts of interest to disclose.
AUTHOR CONTRIBUTION: All the authors contributed to conception and design, acquisition of data, analysis and interpretation of data, drafting the article, and revising it critically. All have approved the submission of this version.
References
- 1.Stone JH, Zen Y, Deshpande V. IgG4-related disease. N Engl J Med. 2012;366:539–551. doi: 10.1056/NEJMra1104650. [DOI] [PubMed] [Google Scholar]
- 2.Umehara H, Okazaki K, Masaki Y, Kawano M, Yamamoto M, Saeki T, Matsui S, Yoshino T, Nakamura S, Kawa S, et al. Comprehensive diagnostic criteria for IgG4-related disease (IgG4-RD), 2011. Mod Rheumatol. 2012;22:21–30. doi: 10.1007/s10165-011-0571-z. [DOI] [PubMed] [Google Scholar]
- 3.Kamisawa T, Zen Y, Pillai S, Stone JH. IgG4-related disease. Lancet. 2015;385:1460–1471. doi: 10.1016/S0140-6736(14)60720-0. [DOI] [PubMed] [Google Scholar]
- 4.Kamisawa T, Funata N, Hayashi Y, Eishi Y, Koike M, Tsuruta K, Okamoto A, Egawa N, Nakajima H. A new clinicopathological entity of IgG4-related autoimmune disease. J Gastroenterol. 2003;38:982–984. doi: 10.1007/s00535-003-1175-y. [DOI] [PubMed] [Google Scholar]
- 5.Kamisawa T, Takuma K, Egawa N, Tsuruta K, Sasaki T. Autoimmune pancreatitis and IgG4-related sclerosing disease. Nat Rev Gastroenterol Hepatol. 2010;7:401–409. doi: 10.1038/nrgastro.2010.81. [DOI] [PubMed] [Google Scholar]
- 6.Shimosegawa T, Chari ST, Frulloni L, Kamisawa T, Kawa S, Mino-Kenudson M, Kim MH, Klöppel G, Lerch MM, Löhr M, et al. International Association of Pancreatology. International consensus diagnostic criteria for autoimmune pancreatitis: guidelines of the International Association of Pancreatology. Pancreas. 2011;40:352–358. doi: 10.1097/MPA.0b013e3182142fd2. [DOI] [PubMed] [Google Scholar]
- 7.Ohara H, Okazaki K, Tsubouchi H, Inui K, Kawa S, Kamisawa T, Tazuma S, Uchida K, Hirano K, Yoshida H, et al. Research Committee of IgG4-related Diseases; Research Committee of Intractable Diseases of Liver and Biliary Tract; Ministry of Health, Labor and Welfare, Japan; Japan Biliary Association. Clinical diagnostic criteria of IgG4-related sclerosing cholangitis 2012. J Hepatobiliary Pancreat Sci. 2012;19:536–542. doi: 10.1007/s00534-012-0521-y. [DOI] [PubMed] [Google Scholar]
- 8.Chiba K, Kamisawa T, Tabata T, Hara S, Kuruma S, Fujiwara T, Kuwata G, Egashira H, Koizumi K, Koizumi S, et al. Clinical features of 10 patients with IgG4-related retroperitoneal fibrosis. Intern Med. 2013;52:1545–1551. doi: 10.2169/internalmedicine.52.0306. [DOI] [PubMed] [Google Scholar]
- 9.Kawano M, Saeki T, Nakashima H, Nishi S, Yamaguchi Y, Hisano S, Yamanaka N, Inoue D, Yamamoto M, Takahashi H, et al. Proposal for diagnostic criteria for IgG4-related kidney disease. Clin Exp Nephrol. 2011;15:615–626. doi: 10.1007/s10157-011-0521-2. [DOI] [PubMed] [Google Scholar]
- 10.Ohara H, Nakazawa T, Sano H, Ando T, Okamoto T, Takada H, Hayashi K, Kitajima Y, Nakao H, Joh T. Systemic extrapancreatic lesions associated with autoimmune pancreatitis. Pancreas. 2005;31:232–237. doi: 10.1097/01.mpa.0000175178.85786.1d. [DOI] [PubMed] [Google Scholar]
- 11.Okazaki K. Autoimmune pancreatitis: etiology, pathogenesis, clinical findings and treatment. The Japanese experience. JOP. 2005;6:89–96. [PubMed] [Google Scholar]
- 12.Hamano H, Arakura N, Muraki T, Ozaki Y, Kiyosawa K, Kawa S. Prevalence and distribution of extrapancreatic lesions complicating autoimmune pancreatitis. J Gastroenterol. 2006;41:1197–1205. doi: 10.1007/s00535-006-1908-9. [DOI] [PubMed] [Google Scholar]
- 13.Kanno A, Nishimori I, Masamune A, Kikuta K, Hirota M, Kuriyama S, Tsuji I, Shimosegawa T Research Committee on Intractable Diseases of Pancreas. Nationwide epidemiological survey of autoimmune pancreatitis in Japan. Pancreas. 2012;41:835–839. doi: 10.1097/MPA.0b013e3182480c99. [DOI] [PubMed] [Google Scholar]
- 14.Moriyama M, Tanaka A, Maehara T, Ohyama Y, Shimizu M, Nakashima H, Hayashida JN, Shinozaki S, Kubo Y, Furukawa S, et al. Clinical characteristics of Mikulicz's disease as an IgG4-related disease. Clin Oral Investig. 2013;17:1995–2002. doi: 10.1007/s00784-012-0905-z. [DOI] [PubMed] [Google Scholar]
- 15.Sato Y, Kojima M, Takata K, Morito T, Asaoku H, Takeuchi T, Mizobuchi K, Fujihara M, Kuraoka K, Nakai T, et al. Systemic IgG4-related lymphadenopathy: a clinical and pathologic comparison to multicentric Castleman's disease. Mod Pathol. 2009;22:589–599. doi: 10.1038/modpathol.2009.17. [DOI] [PubMed] [Google Scholar]
- 16.Cheuk W, Yuen HK, Chu SY, Chiu EK, Lam LK, Chan JK. Lymphadenopathy of IgG4-related sclerosing disease. Am J Surg Pathol. 2008;32:671–681. doi: 10.1097/PAS.0b013e318157c068. [DOI] [PubMed] [Google Scholar]
- 17.Zen Y, Onodera M, Inoue D, Kitao A, Matsui O, Nohara T, Namiki M, Kasashima S, Kawashima A, Matsumoto Y, et al. Retroperitoneal fibrosis: a clinicopathologic study with respect to immunoglobulin G4. Am J Surg Pathol. 2009;33:1833–1839. doi: 10.1097/pas.0b013e3181b72882. [DOI] [PubMed] [Google Scholar]
- 18.Saeki T, Kawano M, Mizushima I, Yamamoto M, Wada Y, Nakashima H, Homma N, Tsubata Y, Takahashi H, Ito T, et al. The clinical course of patients with IgG4-related kidney disease. Kidney Int. 2013;84:826–833. doi: 10.1038/ki.2013.191. [DOI] [PubMed] [Google Scholar]
- 19.Ozaki Y, Oguchi K, Hamano H, Arakura N, Muraki T, Kiyosawa K, Momose M, Kadoya M, Miyata K, Aizawa T, et al. Differentiation of autoimmune pancreatitis from suspected pancreatic cancer by fluorine-18 fluorodeoxyglucose positron emission tomography. J Gastroenterol. 2008;43:144–151. doi: 10.1007/s00535-007-2132-y. [DOI] [PubMed] [Google Scholar]
- 20.Zhang J, Chen H, Ma Y, Xiao Y, Niu N, Lin W, Wang X, Liang Z, Zhang F, Li F, et al. Characterizing IgG4-related disease with 18F-FDG PET/CT: a prospective cohort study. Eur J Nucl Med Mol Imaging. 2014;41:1624–1634. doi: 10.1007/s00259-014-2729-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamamoto M, Takahashi H, Ohara M, Suzuki C, Naishiro Y, Yamamoto H, Shinomura Y, Imai K. A new conceptualization for Mikulicz's disease as an IgG4-related plasmacytic disease. Mod Rheumatol. 2006;16:335–340. doi: 10.1007/s10165-006-0518-Y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kubota K, Wada T, Kato S, Mozaki Y, Yoneda M, Fujita K, Takahashi H, Inamori M, Abe Y, Kobayashi N, et al. Highly active state of autoimmune pancreatitis with mikulicz disease. Pancreas. 2010;39:e6–e10. doi: 10.1097/MPA.0b013e3181bc119d. [DOI] [PubMed] [Google Scholar]