Abstract
Controversies persist regarding the effect of Helicobacter pylori eradication on the development of metachronous gastric cancer after endoscopic resection of early gastric cancer (EGC). The aim of this study was to assess the efficacy of Helicobacter pylori eradication after endoscopic resection of EGC for the prevention of metachronous gastric cancer. A systematic literature review and meta-analysis were conducted using the core databases PubMed, EMBASE, and the Cochrane Library. The rates of development of metachronous gastric cancer between the Helicobacter pylori eradication group vs. the non-eradication group were extracted and analyzed using risk ratios (RRs). A random effect model was applied. The methodological quality of the enrolled studies was assessed by the Risk of Bias table and by the Newcastle-Ottawa Scale. Publication bias was evaluated through the funnel plot with trim and fill method, Egger's test, and by the rank correlation test. Ten studies (2 randomized and 8 non-randomized/5,914 patients with EGC or dysplasia) were identified and analyzed. Overall, the Helicobacter pylori eradication group showed a RR of 0.467 (95% CI: 0.362-0.602, P < 0.001) for the development of metachronous gastric cancer after endoscopic resection of EGC. Subgroup analyses showed consistent results. Publication bias was not detected. Helicobacter pylori eradication after endoscopic resection of EGC reduces the occurrence of metachronous gastric cancer.
Keywords: Stomach Neoplasms, Helicobacter pylori, Metachronous Neoplasms
INTRODUCTION
Helicobacter pylori is regarded as the most important pathogen in the development of gastric cancer (1, 2). According to the hypothesis of Correa, infection with H. pylori causes chronic atrophic gastritis, intestinal metaplasia, dysplasia, and gastric cancer in sequence (3). Based on previous studies, the elimination of H. pylori is considered the most promising strategy for the prevention of gastric cancer and is recommended in current guidelines (4, 5, 6). However, there have been conflicting results, especially in patients with high-risk lesions. Some studies have indicated that the beneficial effects of H. pylori eradication are limited to patients without preneoplastic lesions (7). However, other studies have suggested that the prophylactic effect is also valid in individuals with neoplastic or preneoplastic lesions (8, 9).
Currently, endoscopic resection, including endoscopic mucosal resection (EMR) or endoscopic submucosal dissection (ESD), is the most widely accepted treatment modality in a specific subset of patients for the treatment of early gastric cancer (EGC) in East Asia (1, 2). This procedure can preserve nearly the entire gastric mucosa. Therefore, metachronous recurrence could develop in the abnormal background mucosa. However, data with regard to the prophylactic effect of H. pylori eradication after endoscopic resection of EGC remain unclear. Thus, a meta-analysis was conducted to assess the effect of H. pylori eradication on the development of metachronous gastric cancer after endoscopic resection of EGC.
MATERIALS AND METHODS
Literature search
MEDLINE (through PubMed), EMBASE, and the Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library were searched using common keywords related to H. pylori eradication after endoscopic resection of EGC in the development of metachronous gastric cancer (from inception to July 2014). Medical Subject Headings (MeSH) were used because all 3 databases permit searches using MeSH terminology. The keywords included 'Helicobacter' and 'metachronous neoplasms' using Boolean operators. Only publications on human subjects were sought, and the bibliographies of relevant articles were also reviewed to identify additional studies. The language of the publications was not restricted.
Selection criteria
We included randomized or non-randomized studies that met the following criteria: 1) the study was designed to evaluate the occurrence of metachronous gastric cancer after endoscopic resection of EGC in the target or control group, or 2) the study included a group that was given medicine for the eradication of H. pylori and a comparison group that was given placebo or no antibiotics. The exclusion criteria were as follows: 1) incomplete data, 2) review articles, or 3) abstract-only studies.
Selection of relevant studies
Two of the authors (C.S.B. and G.H.B.) independently evaluated the eligibility of all studies retrieved from the databases based on the predetermined selection criteria. The abstracts of all identified studies were reviewed to exclude irrelevant articles. Full-text reviews were performed to determine whether the inclusion criteria were satisfied by the remaining studies. Disagreements between the two evaluators were resolved by discussion or by consultation with a third author (D.J.K.).
Assessment of methodological quality
The methodological quality of the enrolled studies was assessed using the Risk of Bias table (RoB) for randomized studies and the Newcastle-Ottawa Scale (NOS) for non-randomized studies. The RoB was assessed as described in the Cochrane handbook by recording the method used to generate the randomization sequence, allocation concealment, the determination of whether blinding was implemented for participants or staff, and whether there was evidence of selective reporting of the outcomes (10). Review Manager version 5.3.3 (Revman for Windows 7, the Nordic Cochrane Centre, Copenhagen, Denmark) was used to generate the RoB table. The NOS is categorized into three parameters: the selection of the study population, the comparability of the groups, and the ascertainment of the exposure or outcome. Each parameter consists of subcategorized questions: selection (n=4), comparability (n=1), and exposure or outcome (n=3) (11, 12). Stars awarded for each item serve as a quick visual assessment for the methodological quality of the studies. A study can be awarded a maximum of nine stars, which indicates the highest quality. Two of the authors (C.S.B. and G. H.B.) independently evaluated the methodological quality of all studies, and any disagreements between the two evaluators were resolved by discussion or by consultation with a third author (D.J.K.). Subgroup analyses were performed according to the results of the methodological quality assessment.
Primary and modifier-based analyses
We investigated the efficacy of H. pylori eradication on the development of metachronous gastric cancer after endoscopic resection of EGC. Efficacy was defined as non-occurrence of metachronous gastric cancer (non-development of new gastric cancer at a previously uninvolved site in the stomach) in the follow-up endoscopic examinations. We also performed subgroup analyses based on the study design (randomized/non-randomized/non-randomized, prospective/non-randomized, retrospective studies), study setting (multicenter vs. single center studies), location of the studies (Korea vs. Japan), the definition of metachronous recurrence, and the methodological quality of the enrolled studies. In randomized studies (9, 13), which enabled both intention-to-treat (ITT) and per-protocol (PP) analysis, a meta-analysis based on the ITT analysis was performed, whenever possible. However, a separate meta-analysis was also performed, and both a cumulative analysis and a one-study-removed analysis were performed.
Statistics
Comprehensive Meta-Analysis (CMA) software (version 2.2.064, Biostat; Borenstein M, Hedges L, Higgins J and Rothstein H. Englewood, NJ, USA) was used for this meta-analysis. We calculated the RRs with 95% confidence intervals (CIs) using 2×2 tables based on ITT analysis, and whenever possible, from the original articles in order to compare the efficacy of H. pylori eradication in the prevention of metachronous recurrence after endoscopic resection of EGC. Heterogeneity was determined using the I2 test developed by Higgins, which measures the percentage of total variation across studies (14). I2 was calculated as follows: I2 (%)=100×(Q-df)/Q, where Q is Cochrane's heterogeneity statistic and df signifies the degree of freedom. Negative values for I2 were set to zero, and an I2 value over 50% was considered to be of substantial heterogeneity (range: 0%-100%) (15). Pooled-effect sizes with 95% confidence intervals (CIs) were calculated using a random effects model and the method of DerSimonian and Laird because of methodological heterogeneity (16). These results were confirmed by the I2 test. A fixed effects model using the inverse variance-weighted (Woolf's) method was used in the subgroup analyses, including cumulative and one-study-removed analyses, based on the assumption of a common effect size shared by the studies within each subgroup (17, 18). Significance was set at P=0.05 in both models. Publication bias was evaluated using Begg's funnel plot, Egger's test of the intercept, Duval and Tweedie's trim and fill, and Begg and Mazumdar's rank correlation test (19, 20, 21, 22, 23).
Ethics statement
This study was approved by the institutional review board of Chuncheon Sacred Heart hospital (2015-14). Informed consent was waived by the board.
RESULTS
Identification of relevant studies
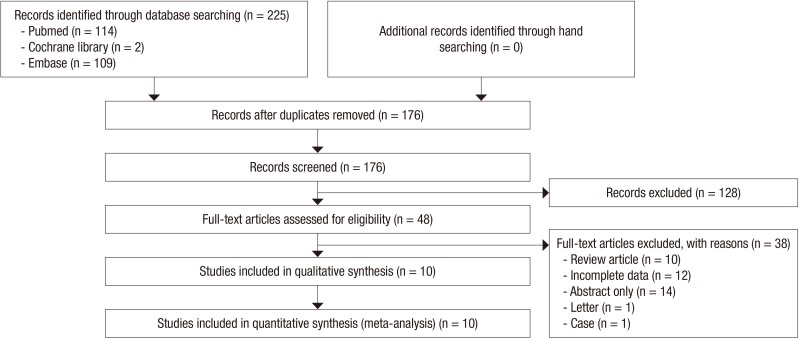
Fig. 1 illustrates a flow diagram of how relevant studies were identified. A total of 225 articles were identified by a search of 3 core databases and a manual search of relevant bibliographies. In all, 49 duplicate studies and an additional 128 studies were excluded during the initial screening through a review of the titles and abstracts. The full texts of the remaining 48 studies were then thoroughly reviewed. Among these studies, 38 were excluded from the final analysis. The reasons for study exclusion during the final review were as follows: review article (n=10), incomplete data (n=12), abstract-only article (n=14), letter (n=1), or case study (n=1). The remaining 10 studies (2 randomized and 8 non-randomized studies) were included in the final analysis.
Fig. 1. Flow diagram for identification of relevant studies.
Characteristics of studies
Within the 10 studies (9, 13, 24, 25, 26, 27, 28, 29, 30, 31), we identified a total of 5,914 patients with EGC or dysplasia. The clinical characteristics of the patients in the included studies are shown in Tables 1 and 2. The included studies were published between 1997 and 2014. Two randomized studies (9, 13) and 8 non-randomized studies, 2 prospective (24, 26) and 6 retrospective studies (25, 27, 28, 29, 30, 31) were included. All the studies were conducted in Asia, 5 studies (13, 28, 29, 30, 31) in Korea and 5 studies (9, 24, 25, 26, 27) in Japan. Four studies were conducted in a multicenter setting (9, 25, 26, 27), whereas the other studies were conducted in a single center setting (13, 24, 28, 29, 30, 31). All the included studies were written in English.
Table 1. Clinical data of included studies.
| Studies | Study design | Total No. of patients (M/F) | Age (eradication/non-eradication group) | Disease | Endoscopic treatment | Duration of follow up | Location, language |
|---|---|---|---|---|---|---|---|
| Uemura et al., 1997 (24) | Non-randomized trial (S) | 132 (97/35) | Mean 69 (44-85 yr ranged) | EGC | Endoscopic resection (strip biopsy) | 3-4 yr (unclear) | Japan (English) |
| Nakagawa et al., 2006 (25) | Retrospective study (M) | 2,825 | EGC | EMR | Median 2 yr (0.5-12 yr) | Japan (English) | |
| Fukase et al., 2008 (9) | Open-label RCT (M) | 544 (ITT), 505 (PP) (386/119) | Median 68/69 | EGC | Endoscopic resection | All 3 yr | Japan (English) |
| Shiotani et al., 2008 (26) | Prospective study (M) | 91 (82/18)* | 66±8 | EGC | ESD | Median 33 months | Japan (English) |
| Maehata et al., 2012 (27) | Retrospective study (M) | 268 (194/74) | Median 68/72 | EGC | Endoscopic resection | Median 3 yr (1.1-11.1 yr) | Japan (English) |
| Seo et al., 2013 (28) | Retrospective study (S) | 47 (61/13)† | Mean 61.92±11.07/62.08±12.55 | EGC | EMR, ESD | Mean 27.2±18.7 months | Korea (English) |
| Choi et al., 2014 (13) | Open-label RCT (S) | 901 (596/284)‡ | Mean 59.8±8.2/61±8.2 | EGC or dysplasia | EMR, ESD, Ablation | Median 3 yr (1.1-11.1 yr) | Korea (English) |
| Bae et al., 2014 (29) | Retrospective study (S) | 667 (525/142) | Median 62/64 | EGC or dysplasia | Endoscopic resection | Median 60 months (24-137 months) | Korea (English) |
| Kwon et al., 2014 (30) | Retrospective study (S) | 283 (190/93) | Mean 61.1±9.2/60±7.3 | EGC§ | ESD | Median 41 months | Korea (English) |
| Kim et al., 2014 (31) | Retrospective study (S) | 156 (112/44) | Median 59/64 | EGC | Endoscopic resection | Median 4.3 yr (1.0-11.3 yr) | Korea (English) |
*Included 9 H. pylori negative patients (Total 100 patients); †A total of 74 patients were enrolled, and 47 of them were followed-up for more than 18 months; ‡A total of 901 patients were enrolled, but 21 patients were lost to follow-up; §Metachronous recurrence was checked for not only EGC, but also dysplasia. S, single center study; M, multicenter study.
Table 2. Clinical data of included studies (continued).
| Studies | H. pylori eradication group | H. pylori non-eradication group | Definition of metachronous recurrence | Eradication regimen | ||
|---|---|---|---|---|---|---|
| Metachronous recurrence | Total | Metachronous recurrence | Total | |||
| Uemura et al., 1997 (24) | 0 | 65 | 6 | 67 | No* | OMP 20 mg 4 weeks, CM 400 mg 2 weeks → 2nd: OMP 20 mg, AMX 1,500 mg, MZ 500 mg 2 weeks |
| Nakagawa et al., 2006 (25) | 8 | 356 | 129 | 2,469 | No | Mainly LANS 60 mg, CM 400 mg, AMX 1,500 mg 1 week** |
| Fukase et al., 2008 (9) | 9 | 272 (ITT), 255 (PP) | 24 | 272 (ITT), 250 (PP) | No | LANS 60 mg, CM 400 mg, AMX 1,500 mg 1 week. |
| Shiotani et al., 2008 (26) | 9 | 80 | 1 | 11 | No | (OMP 40 mg or LANS 60 mg or RABE 20 mg), CM 400 mg, AMX 1,500 mg 1 week → 2nd PPI, AMX, METRO 750 mg |
| Maehata et al., 2012 (27) | 15 | 177 | 13 | 91 | Yes∥ | (OMP 40 mg or LANS 60 mg or RABE 20 mg), CM 400 mg, AMX 1,500 mg 1 week → 2nd PPI, AMX, METRO 250 mg |
| Seo et al., 2013 (28) | 4 | 41 | 3 | 6 | No | (OMP 40 mg or LANS 60 mg or RABE 40 mg, ESOM 80 mg), CM 1,000 mg, AMX 2,000 mg 1 week |
| Choi et al., 2014 (13) | 10 | 444 (ITT), 439 (PP) | 17 | 457 (ITT), 441 (PP) | Yes† | OMP 40 mg, CM 1,000 mg, AMX 2,000 mg 1 week |
| Bae et al., 2014 (29) | 34 | 485 | 24 | 182 | Yes‡ | (OMP 60 mg or LANS 60 mg or PANTO 80 mg), CM 400 mg, AMX 1,500 mg 1-2 weeks |
| Kwon et al., 2014 (30) | 18 | 214 | 13 | 69 | Yes§ | (OMP 40 mg or LANS 60 mg or RABE 40 mg), CM 400 mg, AMX 1,500 mg 1 week |
| Kim et al., 2014 (31) | 2 | 49 | 16 | 107 | Yes¶ | (OMP 40 mg or RABE 20 mg or PANTO 80 mg), CM 1,000 mg, AMX 2,000 mg 1 week |
*Not defined. However, all the metachronous recurrence were developed after 12 months of endoscopic resection; †New carcinoma occurring at another site in the stomach; ‡New carcinoma occurring after 6 months of endoscopic resection; §New dysplasia or carcinoma (Vienna 3-5) developing in areas other than the site of primary gastric cancers at least 1 yr after the endoscopic resection; ¶Development of new gastric cancer at a previously uninvolved site in the stomach at least 1 yr after endoscopic resection; ∥New carcinoma developing in areas other than the site of primary gastric cancer at least 1 yr after the endoscopic resection; **Multicenter study. The regimen depended on the choice of each institution. The main regimen is described. ITT, intention-to-treat analysis; PP, per-protocol analysis; week; OMP, omeprazole; LANS, lansoprazole; RABE, rabeprazole; ESOM, esomeprazole; PANTO, pantoprazole; CM, clarithromycin; AMX, amoxicillin; METRO, metronidazole.
The age of enrolled patients ranged from 59 to 72 yr (median). Nearly all of the enrolled patients were diagnosed with EGC. However, 2 studies (13, 29) featured patients with EGC or dysplasia, and thus a separate analysis of only patients with EGC was impossible. To minimize the heterogeneity and to confirm the robustness of the main analysis, a subgroup analysis that excluded studies that included patients with dysplasia was performed. The endoscopic treatment method for EGC varied from strip biopsy and ablation to EMR or ESD. The duration of the follow-up ranged from 2 to 5 yr. Five studies (13, 27, 29, 30, 31) reported the definition of metachronous recurrence, whereas the remaining studies did not. Among the studies that reported the definition of metachronous recurrence, 3 studies (27, 30, 31) commonly defined the standard occurrence time as at least 1 yr after endoscopic resection of EGC, 1 study (29) defined the time course as at least 6 months after endoscopic resection of EGC, while the remaining 1 study (13) did not define the time standards. With respect to the H. pylori eradication regimen, the combination, dose, and duration varied. However, a proton-pump inhibitor with clarithromycin and amoxicillin for 1 or 2 weeks was the most frequently prescribed regimen (Table 2).
Efficacy of H. pylori eradication on the development of metachronous gastric cancer after endoscopic resection of EGC
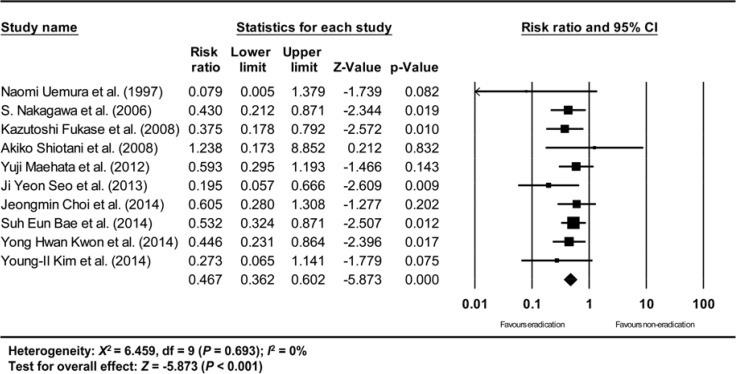
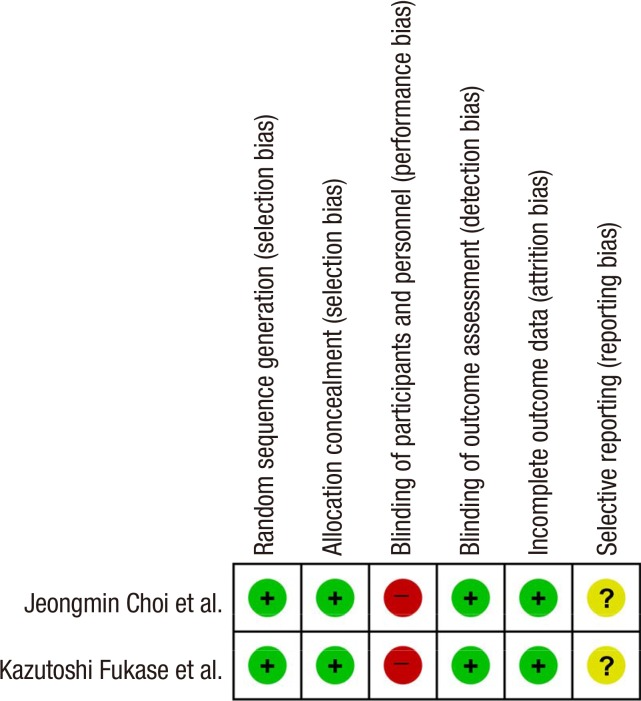
The overall efficacy of H. pylori eradication on the development of metachronous gastric cancer after endoscopic resection of EGC showed a RR of 0.467 (95% CI: 0.362-0.602, P<0.001) in a random effect model-based meta-analysis of 10 studies (Fig. 2). According to the methodological quality assessment of the randomized studies (9, 13), the risk of random sequence generation and allocation concealment was low in both of the studies. However, blinding was not accomplished (open-label studies) in either of the studies (Fig. 3). In the non-randomized studies, the mean value of the awarded star was 7.375, 6 (2 studies), 7 (2 studies), 8 (3 studies), and 9 (1 study) (Table 3).
Fig. 2. Total efficacy of H. pylori eradication for the prevention of metachronous recurrence after endoscopic resection of EGC. H. pylori, Helicobacter pylori; EGC, early gastric cancer; ITT, intention-to-treat. The size of each square is proportional to the study's weight. Diamond is the summary estimate from the pooled studies (random effect model, including studies with ITT analysis).
Fig. 3. RoB table for the assessment of methodological quality for randomized studies. RoB, risk of bias. (+) denotes low risk of bias, (-) denotes high risk of bias, (?) denotes unclear risk of bias.
Table 3. Methodological quality of included studies measured by Newcastle-Ottawa scale.
| Studies | Selection | Comparability | Exposure or outcome | Total |
|---|---|---|---|---|
| Uemura et al., 1997 (24) | ☆☆☆☆ | ☆☆ | ☆ | 7 |
| Nakagawa et al., 2006 (25) | ☆☆☆☆ | ☆ | ☆☆ | 7 |
| Fukase et al., 2008 (9) | ☆☆☆ | ☆ | ☆☆ | 6 |
| Shiotani et al., 2008 (26) | ☆☆☆ | ☆☆ | ☆☆☆ | 8 |
| Maehata et al., 2012 (27) | ☆☆☆ | ☆☆ | ☆ | 6 |
| Seo et al., 2013 (28) | ☆☆☆☆ | ☆ | ☆☆☆ | 8 |
| Choi et al., 2014 (13) | ☆☆☆☆ | ☆☆ | ☆☆☆ | 9 |
| Bae et al., 2014 (29) | ☆☆☆☆ | ☆ | ☆☆☆ | 8 |
Subgroup meta-analysis
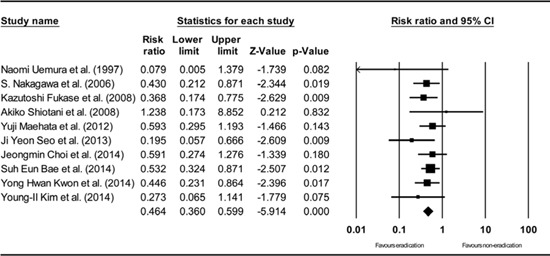
The cumulative meta-analysis of the enrolled studies in the order of the year published showed a consistent result (Appendix 1). A study by Naomi Uemura et al. (24) demonstrated a relatively low RR compared to other studies. However, the methodological quality of this study was the lowest among the included studies (Table 3) and this was the only study which did not prescribe a triple eradication regimen (omeprazole 20 mg/day with clarithromycin 400 mg/day for 2 weeks). Moreover, the definite follow-up duration was unclear (more than 3 yr and not exceeding 4 yr) (Table 2). The one-study-removed meta-analysis of the included studies in the order of the year published showed a consistent result and showed no specific outlier (Appendix 2). Among the included studies, 2 studies were randomized trials (9, 13), which enabled both an ITT and a PP analysis. The primary analysis was based on the result of the ITT analysis. Thus, a separate meta-analysis based on the size effects from the PP analysis was performed. The overall efficacy of H. pylori eradication on the development of metachronous gastric cancer after endoscopic resection of EGC showed a RR of 0.464 (95% CI: 0.360-0.599, P<0.001) in a random effect model-based meta-analysis of 10 studies (Appendix 3), which was not different from the result based on the ITT analysis (Fig. 2).
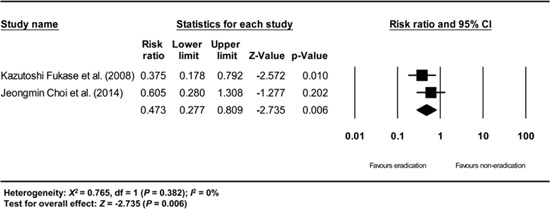
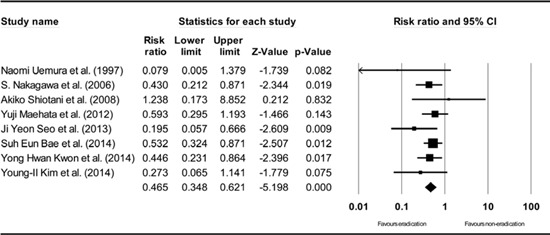
The subgroup analysis based on the study design showed consistent results (Appendix 5, 7, and 8), except for the analysis of the non-randomized prospective studies (Appendix 6). The subgroup analysis of the randomized studies showed a RR of 0.473 (95% CI: 0.277-0.809, P=0.006) (Appendix 5), and the subgroup analysis of the non-randomized retrospective studies showed a RR of 0.463 (95% CI: 0.346-0.622, P<0.001) (Appendix 7). The subgroup analysis of all non-randomized studies (prospective and retrospective studies) showed a RR of 0.465 (95% CI: 0.348-0.621, P<0.001) (Appendix 8). However, the analysis of non-randomized prospective studies showed a RR of 0.511 (95% CI: 0.101-2.583, P=0.417), which is not statistically significant (Appendix 6). However, non-randomized prospective studies, including the studies by Naomi Uemura et al. (24) and Akiko Shiotani et al. (26) showed the lowest methodological quality among the included studies (Table 3). In addition, a relatively small number of patients was evaluated in these studies (Table 1).
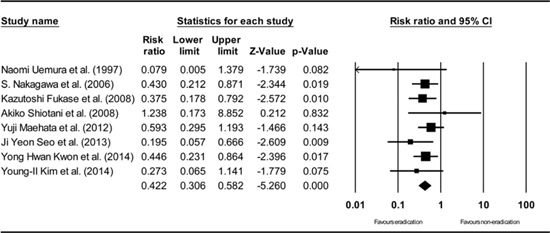
Among all of the included studies, 2 (13, 29) did not feature patients with only EGC but instead had a combined population with EGC or dysplasia (low grade or high grade). However, a subgroup analysis that excluded these studies that featured patients with dysplasia demonstrated a consistent result (RR: 0.422, 95% CI: 0.306-0.582, P<0.001) (Appendix 9).
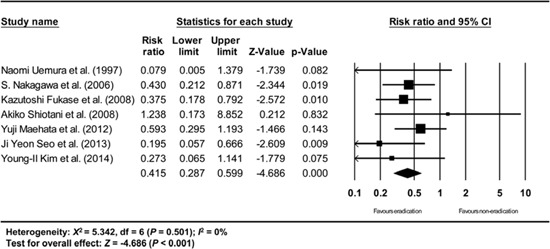
Among the included studies, 3 studies (13, 29, 30) reported metachronous recurrence as not only metachronous cancer but also as metachronous dysplasia. However, a subgroup analysis that excluded these studies gave a consistent result (RR: 0.415, 95% CI: 0.287-0.599, P<0.001) (Appendix 10).
A subgroup analysis based on the study setting (multicenter vs. single center studies) also demonstrated consistent results (multicenter study: RR: 0.481, 95% CI: 0.321-0.721, P<0.001; single center study: RR: 0.457, 95% CI: 0.330-0.634, P<0.001) (Appendix 11 and 12).
Furthermore, a subgroup analysis based on where the studies were performed (Korea vs. Japan) showed consistent results (Japanese studies: RR: 0.465, 95% CI: 0.311-0.693, P<0.001; Korean studies: RR: 0.468, 95% CI: 0.337-0.651, P<0.001) (Appendix 13 and 14).
A subgroup analysis based on the definition of metachronous recurrence (studies that gave a definition/studies that did not give a definition/studies that did not define the time standard of at least 1 yr after endoscopic resection of EGC) showed consistent results (RR: 0.503, 95% CI: 0.360-0.702, P<0.001/RR: 0.422, 95% CI: 0.285-0.624, P<0.001/RR: 0.479, 95% CI: 0.304-0.755, P=0.002) (Appendix 15, 16, and 17).
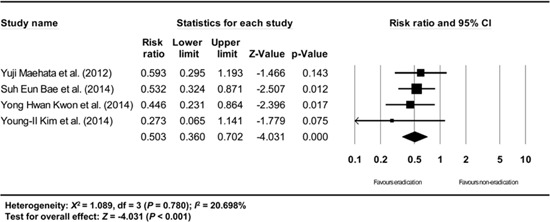
A subgroup analysis based on the methodological quality (among the non-randomized studies, defined as high quality by NOS >7 vs. low quality for NOS ≤7) showed consistent results (RR: 0.503, 95% CI: 0.360-0.702, P<0.001 / RR: 0.370, 95% CI: 0.209-0.656, P=0.001) (Appendix 18 and 19).
Publication bias
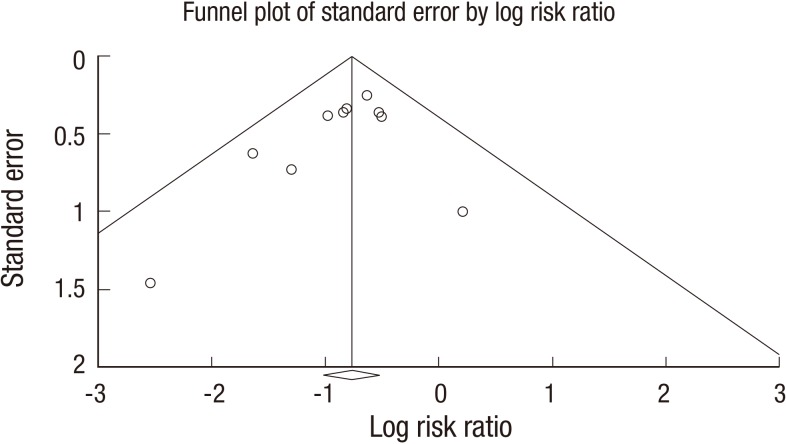
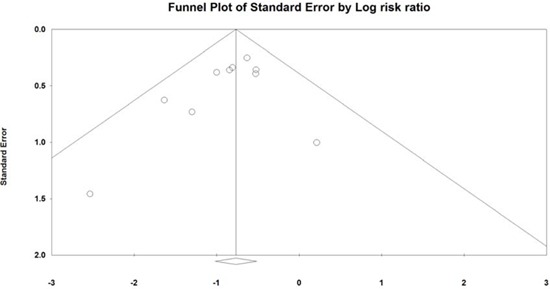
A funnel plot for the included studies is illustrated in Fig. 4 (including studies that comprised the ITT analysis) and in Appendix 4 (including studies that comprised the PP analysis). These plots show a symmetrical shape. In the publication bias analysis that consisted of studies included in the ITT analysis, Egger's regression test revealed that the intercept was -0.91 (95% CI: -2.37-0.55, t-value: 1.43, df: 8, P=0.09 (1-tailed) and P=0.19 [2-tailed]). A trim and fill analysis showed that no study was missed or trimmed. The rank correlation test indicated a Kendall's tau of -0.27 with a continuity correction (P=0.14 [1-tailed] and P=0.28 [2-tailed]).
Fig. 4. Funnel plot for publication bias (including studies with ITT analysis). ITT, Intention-to-treat. Funnel plot of studies. The line in center is the natural logarithm of pooled RR, and 2 oblique lines are pseudo 95% confidence limits. RR: risk ratio.
In the analysis that consisted of the studies included in the PP analysis, Egger's regression test revealed that the intercept was -0.91 (95% CI: -2.37-0.55, t-value: 1.44, df: 8, P=0.09 [1-tailed] and P=0.19 [2-tailed]). A trim and fill analysis showed that no study was missed or trimmed. The rank correlation test indicated a Kendall's tau of -0.27 with a continuity correction (P= 0.14 [1-tailed] and P=0.28 [2-tailed]).
Overall, there was no evidence of publication bias in this analysis.
DISCUSSION
According to this meta-analysis, H. pylori eradication after endoscopic resection of EGC reduced the occurrence of metachronous gastric cancer. This finding was confirmed by the subgroup analyses. This result is not consistent with the findings of some of the included studies (13, 26, 27).
In the study by Akiko shiotani et al. (26), the number of enrolled patients was relatively small, and the incidence of metachronous recurrence after H. pylori eradication was higher compared to that in previous Japanese studies (11.2%). Moreover, selection bias was suspected, and the methodological quality among the non-randomized studies was relatively low, although they were prospective in nature (Table 3). In the study by Maehata et al. (27), selection bias was suspected, and the metachronous recurrence after H. pylori eradication was also higher (8.5%). In the study by Choi et al. (13), patients with dysplasia were included in addition to patients with EGC. The time standard definition for metachronous recurrence was not indicated. Thus, metachronous tumors that recurred within 1 yr accounted for about half of all metachronous cancers. It is possible that these tumors were synchronous lesions that were missed at the initial examination. Moreover, after the rapid urease test and histologic examination, positivity by only 1 test indicated H. pylori infection, and false positive cases were suspected. These 3 studies commonly indicated that H. pylori eradication seemed to reduce the incidence of metachronous recurrence during the early follow-up period. However, the incidence increased after a long-term follow-up period, and there seems to be no prophylactic effect on the late metachronous recurrence of gastric cancer (13, 26, 27). However, the study that demonstrated the biggest effect size (29) showed a decreased incidence of metachronous recurrence in the eradication group. This was consistent with what was observed during the long-term follow-up period (median follow-up: 60 months, range: 24-137 months). The median time to metachronous recurrence was 18 months (range: 7-75 months) in this study.
In the present study, however, substantial methodological heterogeneity was observed among the included studies, which had a potential effect on the risk estimates. This phenomenon was evaluated by subgroup analyses for confirmation of the robustness of this meta-analysis. The most noticeable modifier was the study design. Non-randomized studies tend to exaggerate the effects of an intervention, and this type of study is known to have inherent bias (32). Our meta-analysis included non-randomized studies due to the lack of randomized studies relevant to this topic. However, we also included randomized studies because of the high-quality evidence typically presented in this type of study. This could be the cause of significant heterogeneity, although subgroup analyses revealed consistent results which were not different from those of the main analysis (Appendix 5, 6, 7, and 8).
Another consideration involves the inclusion of dysplastic lesions. The majority of studies enrolled patients with EGC. However, 2 studies (13, 29) also enrolled patients with dysplasia in addition to the patients with EGC. Moreover, one study (30) enrolled only EGC patients but reported metachronous dysplasia in addition to metachronous cancer. These points were evaluated by subgroup analyses and showed results consistent with those of the main analysis (Appendix 9 and 10).
Considering the conflicting results regarding the durability of the effect of H. pylori eradication on the prevention of metachronous recurrence as stated above, the various follow-up duration times could result in bias. However, no established time span relevant to this topic exists that would minimize the bias. Therefore, a subgroup analysis was not performed due to the uncertain time standards between the long-term vs. the short-term follow-up times.
Another issue is the unclear definition of metachronous recurrence. Three studies (27, 30, 31) set the time standard as 1 yr (new carcinoma that develops in areas other than the site of the primary gastric cancer at least 1 yr after the endoscopic resection). One study set the time standard as 6 months (29). However, the other studies did not report definite time standards.
Other sources of heterogeneity could be uneven study populations (such as the inclusion of patients with a history of endoscopic resection), different diagnostic methods of H. pylori infection, and various eradication regimens among the studies. Although the I2 test showed no heterogeneity, and subgroup analyses demonstrated consistent results, these factors could not be totally controlled for in this analysis.
This study is the meta-analysis of the effect of H. pylori eradication on the development of metachronous recurrence after endoscopic resection of EGC. The strength of this study is the rigorous literature search, although there is a lack of data from western countries. When possible, potential modifiers were detected within the articles, and subgroup analyses were performed to confirm the robustness of the results.
Despite the strengths, there are several limitations in the present study. First, a fundamental limitation exists with regard to the interpretation of the results. The risk of development of gastric cancer is known to dependent on the background mucosal inflammation or atrophy, even among patients with H. pylori infection (25). This was progressed to the 'point of no return' theory, which means that only low-risk lesions without intestinal metaplasia or mucosal atrophy are reversible and that the prevention of gastric cancer is possible with H. pylori eradication (7, 33). However, conflicting results regarding this theory still continue to subsist (9, 34). In this meta-analysis, mucosal inflammation and atrophy were not measurable. Genetic or molecular biologic markers including CDX1, CDX2, or spasmolytic polypeptide-expressing metaplasia also was not measurable (35, 36).
The limitations described above could be a cause of heterogeneity and bias. Due to the lack of prospective or randomized studies on this topic, large-scale, well-organized, long-term follow-up studies are needed to confirm these findings. If the development of biomarkers reflects mucosal inflammation or atrophy, longer-term follow-up periods without invasive procedures might be preferred.
In conclusion, H. pylori eradication after endoscopic resection of EGC reduces the occurrence of metachronous gastric cancer.
Appendix 1
Cumulative meta-analysis of enrolled studies for the efficacy of H. pylori eradication for the development of metachronous recurrence after endoscopic resection of EGC. H. pylori, Helicobacter pylori; EGC, early gastric cancer. Diamond is the summary estimate from the pooled studies (Fixed effect model).
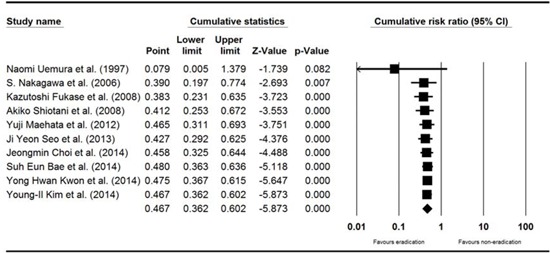
Appendix 2
One study removed meta-analysis of enrolled studies for the efficacy of H. pylori eradication for the development of metachronous recurrence after endoscopic resection of EGC. H. pylori, Helicobacter pylori; EGC, early gastric cancer. Diamond is the summary estimate from the pooled studies (Fixed effect model).

Appendix 3
Total efficacy of H. pylori eradication for the development of metachronous recurrence after endoscopic resection of EGC (including studies with PP analysis). H. pylori, Helicobacter pylori; EGC, early gastric cancer; PP, per-protocol. The size of each square is proportional to the study's weight. Diamond is the summary estimate from the pooled studies (random effect model).
Appendix 4
Funnel plot for publication bias (including studies with PP analysis). PP, per-protocol. Funnel plot of studies. The line in center is the natural logarithm of pooled RR, and 2 oblique lines are pseudo 95% confidence limits. RR: risk ratio.
Appendix 5
Subgroup analysis of randomized studies. The size of each square is proportional to the study's weight. Diamond is the summary estimate from the pooled studies (fixed effect model).
Appendix 6
Subgroup analysis of non-randomized prospective studies. The size of each square is proportional to the study's weight. Diamond is the summary estimate from the pooled studies (fixed effect model).
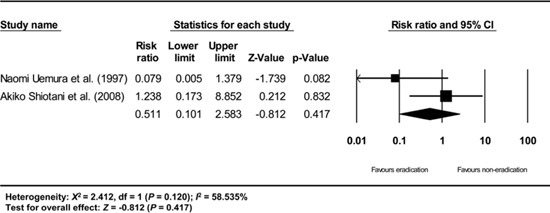
Appendix 7
Subgroup analysis of non-randomized retrospective studies. The size of each square is proportional to the study's weight. Diamond is the summary estimate from the pooled studies (fixed effect model).

Appendix 8
Subgroup analysis of total non-randomized studies. The size of each square is proportional to the study's weight. Diamond is the summary estimate from the pooled studies (fixed effect model).
Appendix 9
Subgroup analysis excluding studies having patients with dysplasia. The size of each square is proportional to the study's weight. Diamond is the summary estimate from the pooled studies (fixed effect model).
Appendix 10
Subgroup analysis excluding studies having patients with metachronous dysplasia other than metachronous cancer. The size of each square is proportional to the study's weight. Diamond is the summary estimate from the pooled studies (fixed effect model).
Appendix 11
Subgroup analysis of multicenter studies. The size of each square is proportional to the study's weight. Diamond is the summary estimate from the pooled studies (fixed effect model).
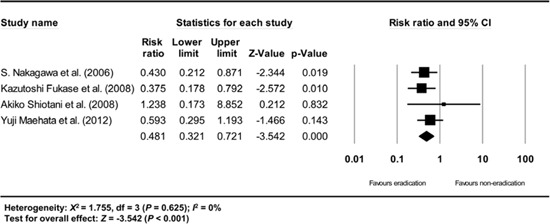
Appendix 12
Subgroup analysis of single center studies. The size of each square is proportional to the study's weight. Diamond is the summary estimate from the pooled studies (fixed effect model).
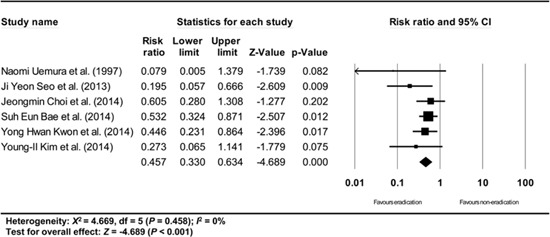
Appendix 13
Subgroup analysis of Japanese studies. The size of each square is proportional to the study's weight. Diamond is the summary estimate from the pooled studies (fixed effect model).
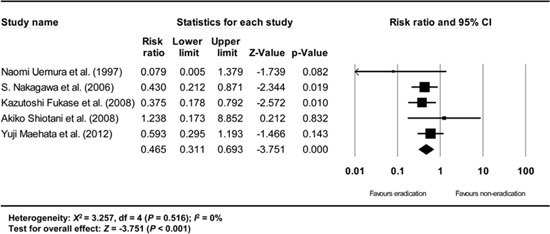
Appendix 14
Subgroup analysis of Korean studies. The size of each square is proportional to the study's weight. Diamond is the summary estimate from the pooled studies (fixed effect model).
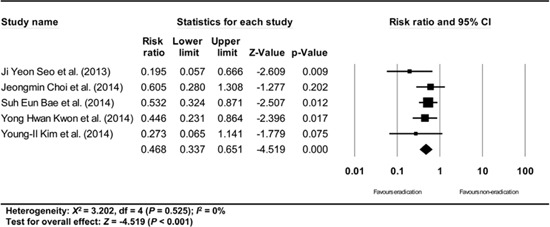
Appendix 15
Subgroup analysis of studies having definition of metachronous recurrence. The size of each square is proportional to the study's weight. Diamond is the summary estimate from the pooled studies (fixed effect model).
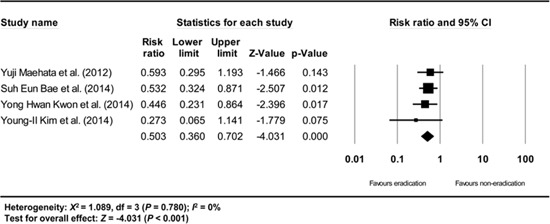
Appendix 16
Subgroup analysis of studies having no definition of metachronous recurrence. The size of each square is proportional to the study's weight. Diamond is the summary estimate from the pooled studies (fixed effect model).
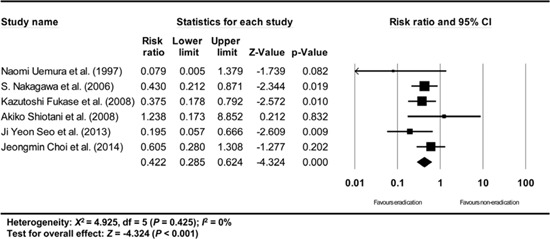
Appendix 17
Subgroup analysis of studies having definition of metachronous recurrence (new carcinoma developing in areas other than the site of primary gastric cancer at least 1 year after endoscopic resection of EGC). The size of each square is proportional to the study's weight. Diamond is the summary estimate from the pooled studies (fixed effect model).
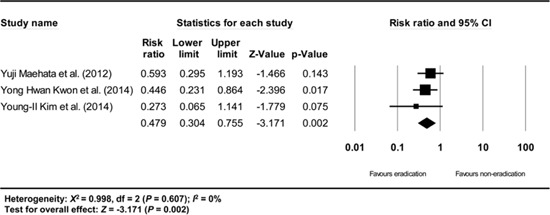
Appendix 18
Subgroup analysis of high quality studies (among the non-randomized studies). The size of each square is proportional to the study's weight. Diamond is the summary estimate from the pooled studies (fixed effect model).
Appendix 19
Subgroup analysis of low quality studies (among the non-randomized studies). The size of each square is proportional to the study's weight. Diamond is the summary estimate from the pooled studies (fixed effect model).

Footnotes
DISCLOSURE: The authors have no potential conflicts of interest to disclose.
AUTHOR CONTRIBUTION: Conception and design of the study: Bang CS, Baik GH. Data collection, analysis, interpretation: Bang CS, Baik GH, Shin IS, Kim JB, Suk KT, Yoon JH, Kim YS, Kim DJ. Drafting: Bang CS. Revision of manuscript and approval of submission: all authors.
References
- 1.Schistosomes, liver flukes and Helicobacter pylori. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Lyon, 7-14 June 1994. IARC Monogr Eval Carcinog Risks Hum. 1994;61:1–241. [PMC free article] [PubMed] [Google Scholar]
- 2.Huang JQ, Sridhar S, Chen Y, Hunt RH. Meta-analysis of the relationship between Helicobacter pylori seropositivity and gastric cancer. Gastroenterology. 1998;114:1169–1179. doi: 10.1016/s0016-5085(98)70422-6. [DOI] [PubMed] [Google Scholar]
- 3.Correa P. A human model of gastric carcinogenesis. Cancer Res. 1988;48:3554–3560. [PubMed] [Google Scholar]
- 4.Fuccio L, Zagari RM, Eusebi LH, Laterza L, Cennamo V, Ceroni L, Grilli D, Bazzoli F. Meta-analysis: can Helicobacter pylori eradication treatment reduce the risk for gastric cancer? Ann Intern Med. 2009;151:121–128. doi: 10.7326/0003-4819-151-2-200907210-00009. [DOI] [PubMed] [Google Scholar]
- 5.Rokkas T, Pistiolas D, Sechopoulos P, Robotis I, Margantinis G. The long-term impact of Helicobacter pylori eradication on gastric histology: a systematic review and meta-analysis. Helicobacter. 2007;12:32–38. doi: 10.1111/j.1523-5378.2007.00563.x. [DOI] [PubMed] [Google Scholar]
- 6.Malfertheiner P, Megraud F, O'Morain CA, Atherton J, Axon AT, Bazzoli F, Gensini GF, Gisbert JP, Graham DY, Rokkas T, et al. European Helicobacter Study Group. Management of Helicobacter pylori infection--the Maastricht IV/ Florence Consensus Report. Gut. 2012;61:646–646. doi: 10.1136/gutjnl-2012-302084. [DOI] [PubMed] [Google Scholar]
- 7.Wong BC, Lam SK, Wong WM, Chen JS, Zheng TT, Feng RE, Lai KC, Hu WH, Yuen ST, Leung SY, et al. China Gastric Cancer Study Group. Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of China: a randomized controlled trial. JAMA. 2004;291:187–194. doi: 10.1001/jama.291.2.187. [DOI] [PubMed] [Google Scholar]
- 8.Takenaka R, Okada H, Kato J, Makidono C, Hori S, Kawahara Y, Miyoshi M, Yumoto E, Imagawa A, Toyokawa T, et al. Helicobacter pylori eradication reduced the incidence of gastric cancer, especially of the intestinal type. Aliment Pharmacol Ther. 2007;25:805–812. doi: 10.1111/j.1365-2036.2007.03268.x. [DOI] [PubMed] [Google Scholar]
- 9.Fukase K, Kato M, Kikuchi S, Inoue K, Uemura N, Okamoto S, Terao S, Amagai K, Hayashi S, Asaka M Japan Gast Study Group. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open-label, randomised controlled trial. Lancet. 2008;372:392–397. doi: 10.1016/S0140-6736(08)61159-9. [DOI] [PubMed] [Google Scholar]
- 10.Higgins J, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions 5.1.0. [updated 2013] The Cochrane Collaboration; 2013. [Google Scholar]
- 11.Deeks JJ, Dinnes J, D'Amico R, Sowden AJ, Sakarovitch C, Song F, Petticrew M, Altman DG International Stroke Trial Collaborative Group. Evaluating non-randomised intervention studies. Health Technol Assess. 2003;7:iii–iix. 1–173. doi: 10.3310/hta7270. [DOI] [PubMed] [Google Scholar]
- 12.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 13.Choi J, Kim SG, Yoon H, Im JP, Kim JS, Kim WH, Jung HC. Eradication of Helicobacter pylori after endoscopic resection of gastric tumors does not reduce incidence of metachronous gastric carcinoma. Clin Gastroenterol Hepatol. 2014;12:793–800.e1. doi: 10.1016/j.cgh.2013.09.057. [DOI] [PubMed] [Google Scholar]
- 14.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 15.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 17.Borenstein M, Hedges LV, Higgins JP, Rothstein HR. Introduction to meta-analysis. Chichester, U.K.: John Wiley & Sons; 2009. [Google Scholar]
- 18.Hedges LV, Olkin I. Statistical methods for meta-analysis. Orlando: Academic Press; 1985. [Google Scholar]
- 19.Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 20.Sutton AJ. Methods for meta-analysis in medical research. Chichester: Wiley; 2000. [Google Scholar]
- 21.Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. 2001;54:1046–1055. doi: 10.1016/s0895-4356(01)00377-8. [DOI] [PubMed] [Google Scholar]
- 22.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 23.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uemura N, Mukai T, Okamoto S, Yamaguchi S, Mashiba H, Taniyama K, Sasaki N, Haruma K, Sumii K, Kajiyama G. Effect of Helicobacter pylori eradication on subsequent development of cancer after endoscopic resection of early gastric cancer. Cancer Epidemiol Biomarkers Prev. 1997;6:639–642. [PubMed] [Google Scholar]
- 25.Nakagawa S, Asaka M, Kato M, Nakamura T, Kato C, Fujioka T, Tatsuta M, Keida K, Terao S, Takahashi S, et al. Helicobacter pylori eradication and metachronous gastric cancer after endoscopic mucosal resection of early gastric cancer. Alimentary Pharmacology & Therapeutics. 2006;24:214–218. [Google Scholar]
- 26.Shiotani A, Uedo N, Iishi H, Yoshiyuki Y, Ishii M, Manabe N, Kamada T, Kusunoki H, Hata J, Haruma K. Predictive factors for metachronous gastric cancer in high-risk patients after successful Helicobacter pylori eradication. Digestion. 2008;78:113–119. doi: 10.1159/000173719. [DOI] [PubMed] [Google Scholar]
- 27.Maehata Y, Nakamura S, Fujisawa K, Esaki M, Moriyama T, Asano K, Fuyuno Y, Yamaguchi K, Egashira I, Kim H, et al. Long-term effect of Helicobacter pylori eradication on the development of metachronous gastric cancer after endoscopic resection of early gastric cancer. Gastrointest Endosc. 2012;75:39–46. doi: 10.1016/j.gie.2011.08.030. [DOI] [PubMed] [Google Scholar]
- 28.Seo JY, Lee DH, Cho Y, Lee DH, Oh HS, Jo HJ, Shin CM, Lee SH, Park YS, Hwang JH, et al. Eradication of Helicobacter pylori reduces metachronous gastric cancer after endoscopic resection of early gastric cancer. Hepatogastroenterology. 2013;60:776–780. doi: 10.5754/hge12929. [DOI] [PubMed] [Google Scholar]
- 29.Bae SE, Jung HY, Kang J, Park YS, Baek S, Jung JH, Choi JY, Kim MY, Ahn JY, Choi KS, et al. Effect of Helicobacter pylori eradication on metachronous recurrence after endoscopic resection of gastric neoplasm. Am J Gastroenterol. 2014;109:60–67. doi: 10.1038/ajg.2013.404. [DOI] [PubMed] [Google Scholar]
- 30.Kwon YH, Heo J, Lee HS, Cho CM, Jeon SW. Failure of Helicobacter pylori eradication and age are independent risk factors for recurrent neoplasia after endoscopic resection of early gastric cancer in 283 patients. Aliment Pharmacol Ther. 2014;39:609–618. doi: 10.1111/apt.12633. [DOI] [PubMed] [Google Scholar]
- 31.Kim YI, Choi IJ, Kook MC, Cho SJ, Lee JY, Kim CG, Ryu KW, Kim YW. The association between Helicobacter pylori status and incidence of metachronous gastric cancer after endoscopic resection of early gastric cancer. Helicobacter. 2014;19:194–201. doi: 10.1111/hel.12116. [DOI] [PubMed] [Google Scholar]
- 32.Ellenberg JH. Selection bias in observational and experimental studies. Stat Med. 1994;13:557–567. doi: 10.1002/sim.4780130518. [DOI] [PubMed] [Google Scholar]
- 33.Zhou LY, Lin SR, Ding SG, Huang XB, Zhang L, Meng LM, Cui RL, Zhu J. The changing trends of the incidence of gastric cancer after Helicobacter pylori eradication in Shandong area. Chin J Dig Dis. 2005;6:114–115. doi: 10.1111/j.1443-9573.2005.00204.x. [DOI] [PubMed] [Google Scholar]
- 34.Kato M, Asaka M, Ono S, Nakagawa M, Nakagawa S, Shimizu Y, Chuma M, Kawakami H, Komatsu Y, Hige S, et al. Eradication of Helicobacter pylori for primary gastric cancer and secondary gastric cancer after endoscopic mucosal resection. J Gastroenterol. 2007;42:16–20. doi: 10.1007/s00535-006-1928-5. [DOI] [PubMed] [Google Scholar]
- 35.Kang JM, Lee BH, Kim N, Lee HS, Lee HE, Park JH, Kim JS, Jung HC, Song IS. CDX1 and CDX2 expression in intestinal metaplasia, dysplasia and gastric cancer. J Korean Med Sci. 2011;26:647–653. doi: 10.3346/jkms.2011.26.5.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nam KT, O'Neal RL, Coffey RJ, Finke PE, Barker N, Goldenring JR. Spasmolytic polypeptide-expressing metaplasia (SPEM) in the gastric oxyntic mucosa does not arise from Lgr5-expressing cells. Gut. 2012;61:1678–1685. doi: 10.1136/gutjnl-2011-301193. [DOI] [PMC free article] [PubMed] [Google Scholar]